Synthesis,Structure and Fungicidal Activity of Organotin 1H-Tetrazolyl-1-acetates
GAN Xian-XueTANG Liang-Fu(Department of Chemistry and Chemical Engineering,Yibin University,Yibin,Sichuan 644007,China)(Department of Chemistry,State Key Laboratory of Elemento-Organic Chemistry,Nankai University,Tianjin 30007,China)
Synthesis,Structure and Fungicidal Activity of Organotin 1H-Tetrazolyl-1-acetates
GAN Xian-Xue1TANG Liang-Fu*,2
(1Department of Chemistry and Chemical Engineering,Yibin University,Yibin,Sichuan 644007,China)(2Department of Chemistry,State Key Laboratory of Elemento-Organic Chemistry,Nankai University,Tianjin 300071,China)
Four new organotin 1H-tetrazolyl-1-acetates,namely{[(CHN4)CH2CO2Sn(n-Bu)2]2O}2(1)and{[(CHN4)CH2CO2SnEt2]2O}2·0.5C6H6(2)as well as((CHN4)CH2CO2)2SnR2(R=n-Bu(3)or Et(4),(CHN4)CH2CO2=1H-tetrazol-1-acetate),have been synthesized by the reaction of R2SnO with 1H-tetrazolyl-1-acetic acid in a 1∶1 or 1∶2 molar ratio.These complexes have been characterized by IR and NMR spectroscopy,and their structures have been further confirmed by X-ray crystal diffraction.Preliminary in vitro tests for fungicidal activity show that these complexes displaysomedegreeofantifungalactivitiesto GibberellazeaeandRhizoctoniacerealis.CCDC:783970,2;783971,3.
organotin carboxylate;1H-tetrazolyl-1-acetic acid;X-ray structure;fungicidal activity
0 Introduction
In spite of the toxicity and environmental effects partially limiting their application, organotin carboxylateshave been extensively used in the industrial,agricultural and pharmaceutical fields owing to their remarkable structural diversity[1-2],catalytic activity[3]as well as significant biological activity[4-7],for example as pesticidal,antibacterial,antitumor agents and wood preservatives.Carboxylic acids containing heteroatoms have proved their value in the capability of affecting the coordination modes of tin atom as well as decent bioactivities,and therefore attracted a great deal of attention.A large number of organotin carboxylates containing heteroatoms have been synthesized and characterized in recent years[7-11].Furthermore,organotin derivatives from S-or N-functionalized carboxylic acids have displayed fascinating structural features and excellent antibacterial activities[12-15].Taking into consideration of the important bioactivity of tetrazolyl derivatives and their variable coordination modes[15-18],four new organotin 1H-tetrazolyl-1-acetates,namely{[(CHN4)CH2CO2Sn(n-Bu)2]2O}2(1)and{[(CHN4)CH2CO2SnEt2]2O}2·0.5C6H6(2)as well as((CHN4)CH2CO2)2SnR2(R=n-Bu(3)or Et(4),(CHN4)CH2CO2=1H-tetrazol-1-acetate),were synthesized in this paper by the reaction of R2SnO (R=n-Bu or Et)with 1H-tetrazolyl-1-acetic acid,and their antifungal activities were tested in vitro.
1 Experimental
NMR spectra were recorded on a Bruker 400 spectrometer,and the chemical shifts are reported in ppm with respect to the reference(internal SiMe4for1H NMR and13C NMR spectra,external SnMe4for119Sn NMR).IR spectroscopic data were obtained from a Shimadzu FTIR 8400S spectrometer as KBr pellets.Elemental analyses were carried out on an Elementar Vairo EL analyzer.Melting points were measured with an X-4 digital micro melting-point apparatus and were uncorrected.All the chemicals used are commercially available and were used as received without further purification.
1.1 Synthesis of complex 1
The mixture of 1H-tetrazolyl-1-acetic acid(0.26 g,2 mmol)and(n-Bu)2SnO(0.50 g,2 mmol)in anhydrous benzene(50 mL)was stirred and heated at reflux for 8 h.After cooling to room temperature,a white solid precipitated out, which was filtered off and recrystallized from acetone/benzene to yield white needle crystals of 1.Yield:0.52 g(71%),m.p.180~182℃.1H NMR(DMSO-d6,ppm),δ:0.84(t,J=7.2 Hz,3H,CH3),0.92(t,J=7.3 Hz,3H,CH3),1.26~1.60(m,12H,CH2CH2CH2),5.19 (s,2H,CH2),9.30 (s,1H,CHN4).13C NMR (DMSO-d6,ppm), δ:13.3,13.4,25.7,26.2,26.5,26.6,26.9,27.0(butyl carbons),49.6(CH2),144.6(CHN4),169.6(COO).119Sn NMR (DMSO-d6,ppm),δ:-178.9,-214.3.IR (cm-1):νas(COO)1674.2,1612.5,νs(COO)1 404.2,1 375.3.Anal.calc.for C44H84N16O10Sn4(%):C,35.90;H,5.75;N,15.22.Found(%):C,35.69;H,5.68;N,15.66.
1.2 Synthesis of complex 2
This complex was obtained similarly using Et2SnO instead of(n-Bu)2SnO as described above for 1.Yield:83%,m.p.215 ℃ (dec.).1H NMR(DMSO-d6,ppm),δ:1.12~1.42(m,10H,CH2CH3),5.20(s,2H,CH2),9.32(s,1H,CHN4).IR(cm-1):νas(COO)1 647.2,1 616.4,νs(COO)1 406.1,1 383.0.Anal.calc.for C31H55N16O10Sn4(%):C,28.94;H,4.31;N,17.42.Found(%):C,28.47;H,3.83;N,17.57.
1.3 Synthesis of complex 3
This complex was obtained similarly as described above for 1,but in a 2∶1 (acid:tin)molar ratio.Yield:76%,m.p.199~201℃.1H NMR (DMSO-d6,ppm),δ:0.84(t,J=7.3 Hz,3H,CH3),1.21~1.26,1.39~1.48(m,m,2H,4H,CH2CH2CH2),5.23 (s,2H,CH2),9.31(s,1H,CHN4).13C NMR (DMSO-d6,ppm),δ:13.5,25.7,26.5,29.6(butyl carbons),49.5 (CH2),144.7(CHN4),169.6(COO).IR(cm-1): νas(COO)1 620.2, νs(COO)1 398.4.Anal.calc.for C14H24N8O4Sn(%):C,34.52;H,4.97;N,23.00.Found(%):C,34.59;H,4.58;N,23.36.
1.4 Synthesis of complex 4
This complex was obtained similarly using Et2SnO instead of(n-Bu)2SnO as described above for 1,but in a 2∶1(acid∶tin)molar ratio.Yield:86%,m.p.188~190 ℃.1H NMR (DMSO-d6,ppm),δ:1.13(t,J=7.8 Hz,6H,CH3),1.42 (q,J=7.8 Hz,4H,CH2CH3),5.22 (s,4H,CH2),9.32(s,2H,CHN4).13C NMR(DMSO-d6,ppm),δ:9.5(CH2CH3),23.5(CH2CH3),49.5(CH2),144.7(CHN4),170.0(COO).119Sn NMR (DMSO-d6,ppm),δ:-304.2.IR(cm-1):νas(COO)1 614.4, νs(COO)1 392.6.Anal.calc.for C10H16N8O4Sn(%):C,27.87;H,3.74;N,26.00.Found(%):C,27.52;H,3.80;N,26.28.
1.5 Structure determination of complexes 2 and 3
Colorless crystals of complexes 2 and 3 suitable for X-ray analyses were obtained by slowly cooling their hot acetone/benzene solutions.In complex 2,0.5 molecule of benzene was observed.Intensity data were collected on a Bruker SMART CCD using graphite monochromated Mo Kα radiation (λ=0.071 03 nm by the ω/2θ scan technique,and a semi-empirical absorption correction was applied.The structures were solved by direct methods and refined by full-matrix least-squares on F2.All non-hydrogen atomswere refined with anisotropic displacement parameters.The highest peak in complex 3 is located near the Sn(1)center by a distance of 0.081 nm.A summary of the fundamental crystal data is listed in Table 1.
CCDC:783970,2;783971,3.

Table 1 Crystallographic data and refinement parameters of complexes 2 and 3
2 Results and discussion
2.1 Synthesis and characterization
Reaction of R2SnO (R=n-Bu or Et)with 1H-tetrazolyl-1-acetic acid(CHN4CH2CO2H)in a 1:1 molar ratio yielded dimeric tetranuclear complexes{[(CHN4)CH2CO2Sn(n-Bu)2]2O}2(1)and{[(CHN4)CH2CO2SnEt2]2O}2·0.5C6H6(2).While monomeric complexes((CHN4)CH2CO2)2SnR2(R=n-Bu (3)and Et(4),respectively)were obtained by the reaction of R2SnO with 1H-tetrazolyl-1-acetic acid in a 1∶2 molar ratio(Scheme 1).These four complexes have been characterized by IR and NMR spectroscopy as well as elemental analyses.

Scheme 1 Reaction of R2SnO(R=n-Bu or Et)with 1H-tetrazolyl-1-acetic acid
The IR spectra of complexes 1 and 2 display two types of carbonyl absorption bands,implying that the carboxylate groups possibly coordinate to the tin atom in different manners[19-20].The corresponding differences Δ[νas(COO-)-νsCOO-)](298.9 and 208.2 cm-1in 1 as well as 264.2 and 208.3 cm-1in 2,respectively)reflect the monodentate and bidentate coordination modes of the carboxylate groups[20].The NMR spectra of complexes 1 and 2 support the suggested centrosymmetric dimeric structure.For example,two sets of butyl signals of1H and13C NMR spectra were observed in complex 1,suggesting them attached to different tin atoms.At the same time,its119Sn spectrum has also confirmed the presence of endo-and exo-cyclic tin atoms.A pair of resonances of equal intensities were observed at-178.9 and-214.3 ppm in this complex,which are comparable with the previously reported valuesfordimericdistannoxanes[12].On the other hand,the IR spectra of complexes 3 and 4 show that the difference between asymmetric and symmetric stretching vibrations of the carboxylate groups is 221.8 cm-1,very close to the corresponding value of sodium 1H-tetrazolyl-1-acetate(228 cm-1)[15],indicating that the carboxylate groups in these two complexes possibly act as bidentate ligands[20].
2.2 Crystal structures of complexes 2 and 3
The molecular structures of complexes 2 and 3 have also been confirmed further by X-ray crystallography.As shown in Fig.1,complex 2 has a tetranuclear distannoxane structure,similar with that of{[(2-PySCH2CO2)SnEt2]2O}2[12].Unlike those in triorganotin derivatives[15],the tetrazolyl nitrogen atoms do not coordinate to the tin atoms in complex 2.Each tin atom adopts a five-coordinate distorted trigonal bipyramidal geometry with two oxygen atoms occupying the axial positions.The axial O-Sn-O angles(O(1)-Sn(1)-O(3)173.8(2)°and O(4)-Sn(2)-O(5A)168.9(2)°,Table 2)deviate from the linearity.The crystallographically unique carboxylic ligands show different coordination modes.One carboxylic ligand acts as a monodentate ligand by the carboxylate oxygen,while the other is a bridging bidentate ligand by two oxygen atoms of the carboxylgroup to two tin atoms.Some weak intramolecular Sn…O interactions are observed in this complex.The intramolecular distances of Sn(1)…O(2)and Sn(2)…O(1A)are 0.274 5(8)and 0.286 0(6)nm,significantly shorter than the sum of the van der Waal′s radii for the Sn and O atoms of 0.357 nm[21],but comparable to the corresponding Sn…O distances in{[(2-PySCH2CO2)SnEt2]2O}2[12].In addition,some weak intermolecular C-H…N hydrogen bonding interactions have been observed in the crystal packing,such as C(1)-H(1)…N(4B)and C(4)-H(4)…N(8B)(H(1)…N(4B)/C(1)…N(4B)distances:0.259 2(11)/0.342 2(16)nm,and H(4)… N(8B)/C(4)… N(8B)distances:0.236(1)/0.3226(16)nm;symmetry operation B:x,1.5-y,-0.5+z).These weak interactions play important roles in stabilizing the crystal framework.

Fig.1 Molecular structure of complex 2
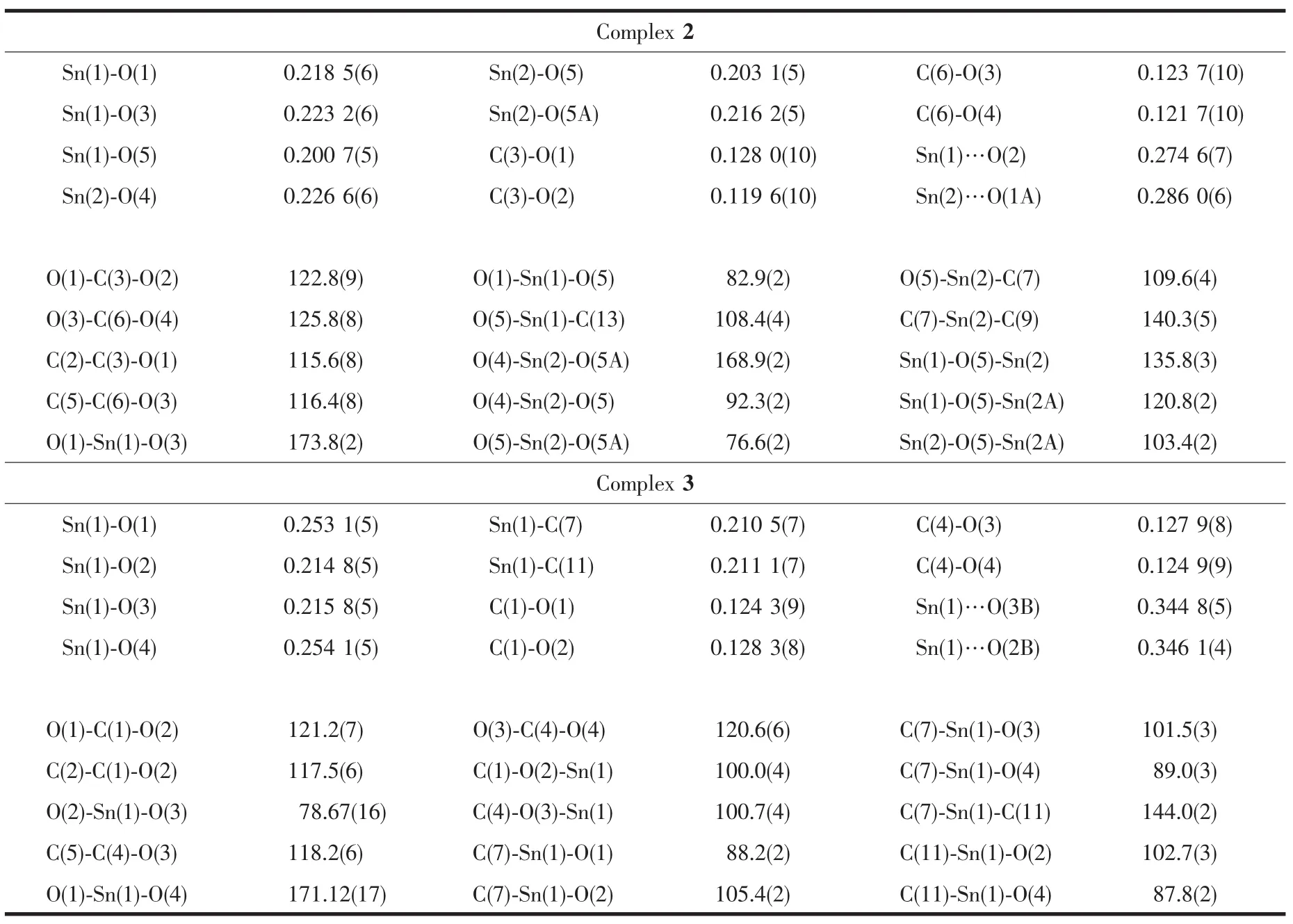
Table 2 Selected bond length(nm)and angles(°)of complexes 2 and 3
The molecular structure of 3 is presented in Fig.2.The tin atom adopts a six-coordinate distorted octahedral geometry.The Sn(1)-O(1)(0.253 1(5)nm)and Sn(1)-O(4)(0.254 1(5)nm)bond distances are significantly longer than the Sn(1)-O(2)(0.214 8(5)nm)and Sn(1)-O(3)(0.215 8(5)nm)bond distances,suggesting that the carboxylate groups act as anisobidentate ligands,consistent with the results of the IR analyses.Like that in complex 2,there is no direct interaction between the tetrazolyl nitrogen atoms and thetin atom in complex3.A seriesofweak intermolecular C-H…N hydrogen bonding interactions still exist in the crystal packing of complex 3(Fig.3).The C(3)-H(3)… N(6A)and C(6)-H(6)… N(2A)distances(symmetry operation A:0.5-x,0.5+y,0.5+z)are 0.253 5(7)/0.333 6(10)nm(H(3)…N(6A)/C(3)…N(6A))and 0.264 3(7)/0.346 9(11)nm(H(6)…N(2A)/C(6)…N(2A)),respectively.Furthermore,the non-bond Sn(1)…O(3B)and Sn(1)…O(2B)distances(symmetry operation B:x,-1+y,z) are 0.344 8(5)nm and 0.346 1(4)nm,respectively,shorter than the sum of the van der Waal′s radii for the Sn and O atoms[21],indicating the presence of some weak interactions among these atoms.This complex forms a supermolecular structure through these weak intermolecular C-H…N and Sn…O interactions.

Fig.2 Molecular structure of complex 3

Fig.3 Crystal packing diagram of complex 3
2.3 Antifungal activity
The fungicidal activities in vitro of four complexes were evaluated according to the fungi growth inhibition method[15],and the data are summarized in Table 3.Although these complexes show relatively lower activities than triorganotin 1H-tetrazolyl-1-acetates[15],they exhibit some degree of antifungal activities to Gibberella zeae and Rhizoctonia cerealis.Moreover,the activities of the butyltin derivatives(complexes 1 and 3)seem higher than those of the ethyltin derivatives(complexes 2 and 4).Similar results have been observed previously[12].

Table 3 Fungicidal activities of complexes

Continued Table 3
[1]Chandrasekhar V,Nagendran S,Baskar V.Coord.Chem.Rev.,2002,235:1-52
[2]Tiekink E R T.Appl.Organometal.Chem.,1991,5:1-23
[3]DU Zhi-Ping(杜治平),LIU Liang(劉亮),WANG Gong-Ying(王公應(yīng)),etal.ChineseJ.Inorg.Chem.(WujiHuaxueXuebao),2009,25:2225-2228
[4]Hadjikakou S K,Hadjiliadis N.Coord.Chem.Rev.,2009,253:235-249
[5]Baul T S B.Appl.Organometal.Chem.,2008,22:195-204
[6]WANG Yan-Hua(王艷華),YE Zhang-Ji(葉章基),JIN Xiao-Hong(金曉鴻),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2008,24:145-148
[7]DENGYi-Fang(鄧奕芳),CHEN Man-Sheng(陳滿生),ZHANG Chun-Hua(張春華),etal.ChineseJ.Inorg.Chem.(WujiHuaxue Xuebao),2009,25:2229-2232
[8]Ma C,Wang Q,Zhang R.Inorg.Chem.,2008,47:7060-7061
[9]Chandrasekhar V,Thirumoorthi R.Organometallics,2009,28:2096-2106
[10]Hong M,Hin H D,Chen S W,et al.J.Organomet.Chem.,2010,695:653-662
[11]Ruisi G,Canfora L,Bruno G,et al.J.Organomet.Chem.,2010,695:546-551
[12]GAN Xian-Xue(甘賢雪),WANG Xi(王希),ZHANG Hai-Ke(張海科),etal.ChineseJ.Inorg.Chem.(WujiHuaxueXuebao),2008,24:1504-1509
[13]Li F L,Dai B,Song H B,et al.Heteroatom Chem.,2009,20:411-417
[14]ZHANG Xiao-Yan(張 曉 燕 ),YANG Guang(楊 光),ZHANG Jun(張俊),et al.Chem.J.Chinese Universities(Gaodeng Xuexiao Huaxue Xuebao),2010,31:1162-1166
[15]Xie Y F,Yu Y,Fan Z J,et al.Appl.Organometal.Chem.,2010,24:1-7
[16]He F,Tong M L,Yu X L,et al.Inorg.Chem.,2005,44:559-565
[17]Dong W W,Zhao J,Xu L.Cryst.Growth Des.,2008,8:2882-2886
[18]Yu Q,Zhang X,Bian H,et al.Cryst.Growth Des.,2008,8:1140-1146
[19]Khan M I,Baloch M K,Ashfaq M.J.Organomet.Chem.,2004,689:3370-3378
[20]Szorcsik A,Nagy L,Sletten J,et al.J.Organomet.Chem.,2004,689:1145-1154
[21]Szorcsik A,Nagy L,Deák A,et al.J.Organomet.Chem.,2004,689:2762-2769
有機(jī)錫四唑乙酸酯的合成、結(jié)構(gòu)與抗真菌活性
甘賢雪1唐良富*,2
(1宜賓學(xué)院化學(xué)化工系,宜賓 644007)(2南開大學(xué)化學(xué)系,元素有機(jī)化學(xué)國家重點(diǎn)實(shí)驗(yàn)室,天津 300071)
通過四唑乙酸與二丁基氧化錫(或二乙基氧化錫)反應(yīng),合成了4個(gè)新的有機(jī)錫四唑乙酸酯。它們的結(jié)構(gòu)通過紅外,核磁以及X-射線單晶衍射分析得到確證。生物活性測試表明,它們對小麥赤霉病菌以及禾谷絲核菌等具有一定的抑制活性。
有機(jī)錫羧酸酯;四唑乙酸;晶體結(jié)構(gòu);抗真菌活性
O614.43+2
:A
:1001-4861(2011)02-0387-06
2010-07-16。收修改稿日期:2010-09-13。
國家自然科學(xué)基金資助項(xiàng)目(No.20721062)。
*通訊聯(lián)系人。 E-mail:lftang@nankai.edu.cn;會(huì)員登記號:S060015703M。
 無機(jī)化學(xué)學(xué)報(bào)2013年12期
無機(jī)化學(xué)學(xué)報(bào)2013年12期
- 無機(jī)化學(xué)學(xué)報(bào)的其它文章
- Synthesis,Crystal Structure and Antibacterial Activity of 2D Hydrogen-bonds Layered Magnesium(Ⅱ)Complex
- The Uptake and Membrane Transport of Cesium in Human Erythrocytes
- Preparation of Functionalized Graphene Sheets via Microwave-Assisted Solid-State Process and Their Electrochemical Capacitive Behaviors
- A New 2D Layer Manganese(Ⅱ)Complex Assembled by Flexible 1,4-Benzenebis(thioacetic acid)Ligand:Synthesis,Crystal Structure and Magnetic Property
- Synthesis of a New Cu(Ⅱ)Complex with in situ Generated 3-Hydroxy-2,4,6-pyridinetricarboxylate Ligand and Analysis of the Reaction Mechanism
- A New Nickel(Ⅱ)Complex Based on Novel Pyridyl-carboxylate Schiff-Base Ligand:Synthesis and Crystal Structure
