2-丙基-1H-4,5-咪唑二酸構筑的鎘ギ配位聚合物的合成、晶體結構、形貌及熒光性質
顧金忠 朱孔陽 趙四杰
(甘肅省有色金屬化學與資源利用重點實驗室,蘭州大學化學化工學院,蘭州 730000)
0 Introduction
The design and synthesis of transition metal coordination polymers(metal-organic complexes)have achieved great progress over the past decade[1-2].These coordination polymers have shown versatile architecture[3-5],accompanied with desirable properties,like luminescent,magnetic,catalytic,and gas absorption and separation properties[2,6-9].Many multi-carboxylate or heterocylic carboxylic acids have been used for this purpose[1-2,4-5,7,9-12].In the designed synthesis of the transition metal coordination polymers,imidazole-4,5-dicarboxylic acid (H3IDC)is an excellent aromatic dicarboxylate ligand,which can afford at most two N and four O coordination sites and has strong potential to act as a bridging ligand.We and others reported many coordination polymers with intriguing architectures and interesting properties based on H3IDC[13-21].In order to extend our investigations in this field,we chose one analogue ligand,2-propyl-1H-imidazole-4,5-dicarboxylic acid (H3pimda)with a propyl substituent in the two-position of the imidazole group,because it may introduce additional structural constrain in controlling the assembly of metal-organic networks[22].In this paper,we report the synthesis and characterization of the coordination polymer[Cd(H2pimda)2]n(1).
1 Experimental
1.1 Reagents and physical measurements
All chemicals were of analytical reagent grade and used without further purification.Carbon,hydrogen and nitrogen were determined using an Elementar Vario EL elemental analyzer.IR spectra were recorded using KBr pellets and a Bruker EQUINOX 55 spectrometer.Thermogravimetric analysis(TG)data were collected on a Netzsch TG-209 instrument with a heating rate of 10℃ ·min-1.Excitation and emission spectra were recorded on an Edinburgh FLS920 fluorescence spectrometer at room temperature for the solid samples.
1.2 Synthesis of[Cd(H2pimda)2]n(1)
A mixture of CdSO4·8/3H2O (0.128 g,0.5 mmol),H3pimda(0.400 g,2.0 mmol)and Nd2O3(0.168 g,0.50 mmol)were dissolved in 10 mL distilled water.The resulting solution(pH=3.0)was stirred for about 1 h at room temperature,sealed in a 25 mL Teflon-lined stainless steel autoclave,and heated at 160℃for 5 d under autogenous pressure,followed by cooling to room temperature at a rate of 10 ℃·h-1.Colourless tubeshaped crystals of 1 were isolated manually,and washed with distilled water.Yield 0.075 g,30%(based on Cd).Found for C16H18CdN4O8(%):C,37.57;H,3.88;N,11.43.Anal.Calcd.(%):C,37.92;H,3.58;N,11.06.IR(KBr,cm-1):as(CO2)1 625 and 1 542,s(CO2)1 462 and 1 386.
1.3 Structure determinations
Single-crystal data of 1 were collected at 296(2)K on a Bruker Smart Apex 1000 CCD diffractometer with Mo Kα radiation (λ=0.071 073 nm).A summary of the crystallography data and structure refinement is given in Table 1,and selected bond lengths and angles of the complex 1 are listed in Table 2.The structure was solved using direct methods,which yielded the positions of all non-hydrogen atoms.These were refined first isotropically and then anisotropically.All the hydrogen atoms were placed in calculated positions with fixed isotropic thermal parameters and included in structure factor calculations in the final stage offull-matrix least-squaresrefinement.All calculations were performed using the SHELXTL-97 system[23].
CCDC:849059.
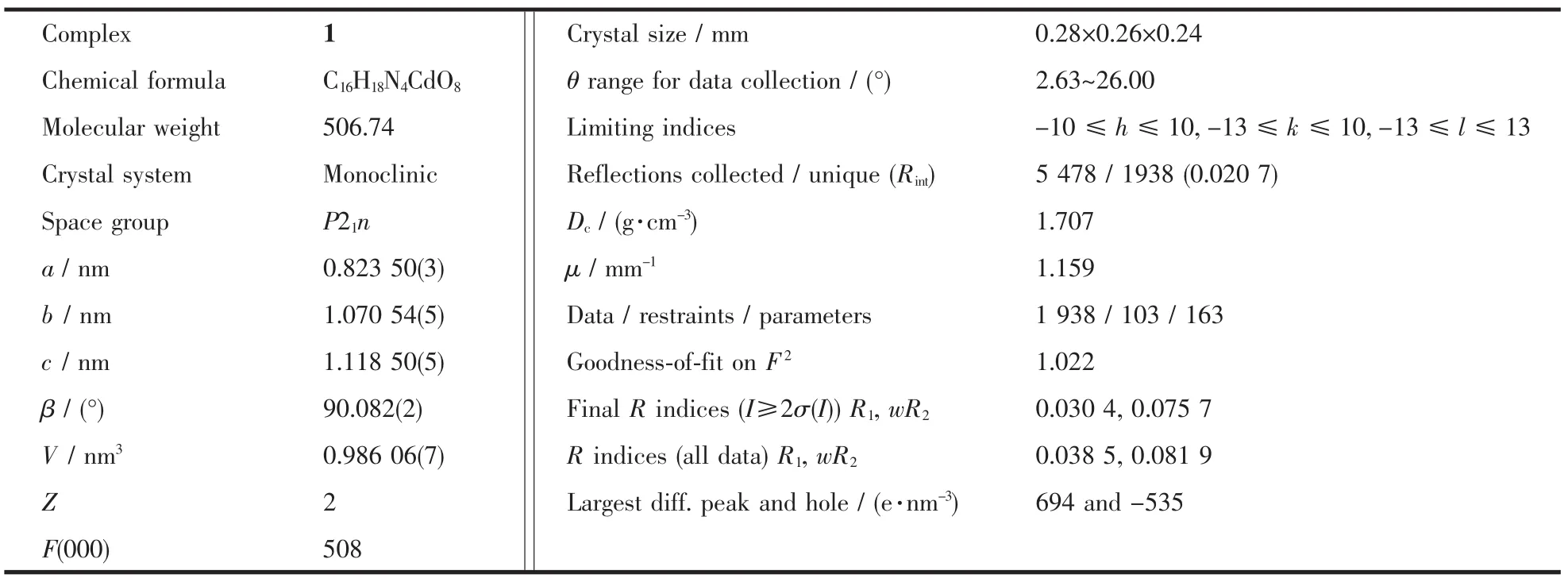
Table 1 Crystal data for complex 1
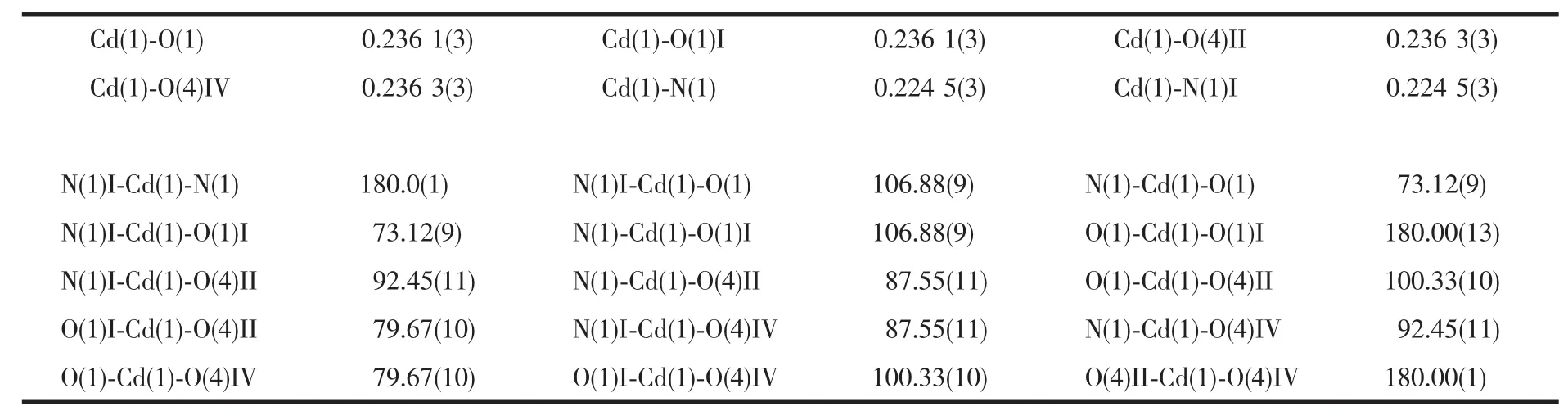
Table 2 Selected bond distances(nm)and bond angles(°)for compound 1
2 Results and discussion
2.1 Description of the structure
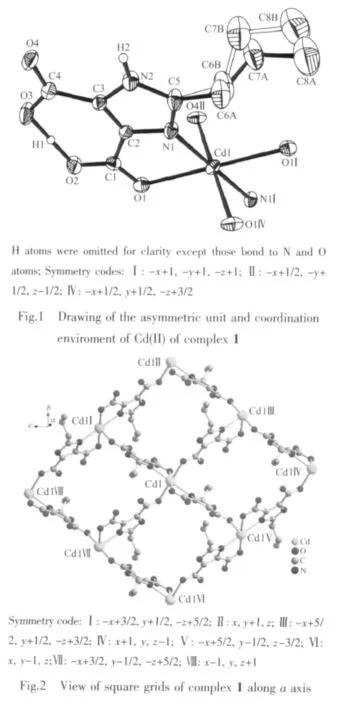
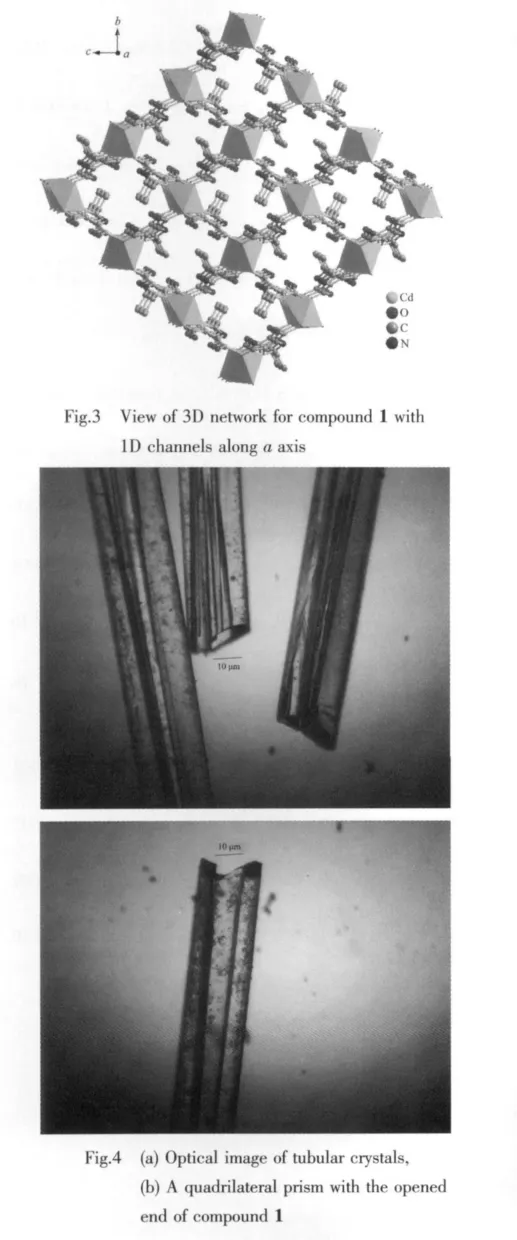
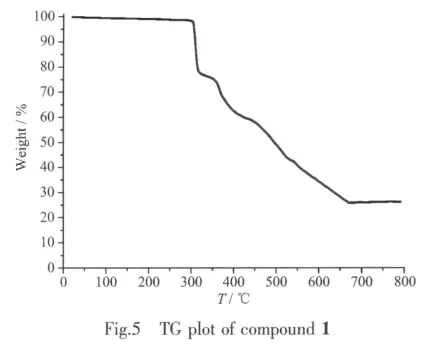
Crystallographic analysis reveals that complex 1 crystallizes in the monoclinic space group P21/n.As shown in Fig.1,the Cdギatom is six-coordinated and adopts a slightly distorted octahedral geometry.The equatorial plane is defined by two oxygen atoms(O1 and O1I)and two nitrogen atoms(N1 and N1I)of two H2pimda-ligands.The axial positions are occupied by two oxygen atoms (O4Ⅱand O4Ⅳ)from two H2pimda-ligands.H2pimda-ligands adopt μ3-tridentate bridging mode with one intramolecular hydrogen bond.As shown in Fig.2,four H2pimda-ligands bridge four Cdギions to form a square.The squares are further connected by the coordination interactions of the μ3-H2pimda-ligands and Cdギions to generate a novel MOF containing 1D open channels as shown in Fig.3.The dimension of each channel is 0.876 nm×0.838 nm measured by atom-to-atom distances(C3…C3Ⅰ0.876 nm,C2…C2Ⅰ 0.838 nm,I:-x+2,-y+1,-z+2).It is worth noting that microscopic single-crystal tubes were assembled with compound 1.Under an optical microscope,the quadrilateral prisms are found to be with one opened terminus and one closed terminus,that is,they have quadrilateral cavities(Fig.4).Most crystal tubes were grown with a length of several millimeters,diameter of ten of micrometres,and thickness of several of micrometres.Although many complexes with hollow tubular architectures have been reported,most of the reports focused on the crystalline nanotubes.Microscopic tubularmetalorganic complexes are very rare[24-26].
In order to probe the factors which may affect the formation of the tubular crystals,we varied the length of heating time,temperature and the pH.Tubular crystals can be produced with temperatures vary from 150 to170℃and heating time longer than 94 h with pH between 2 and 4.If heating time is less than 72 h,only block-shaped crystals with the same structure as complex 1 but no tubular structures were observed.Neither crystals nor tubular architectures were formed if pH is higher than 4 or below 2.
2.2 TG analysis
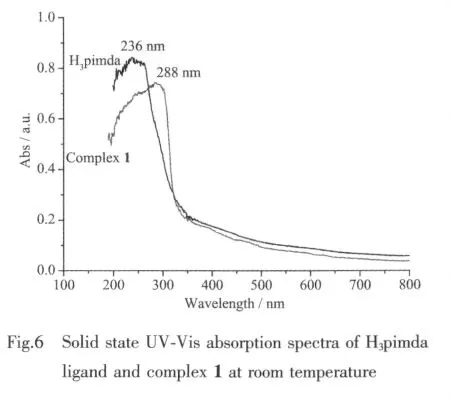
In order to examine the thermal stability of the network,thermal gravimertric analyses (TG)were carried out for crystalline samples of compound 1 in the temperature range 20~800 ℃.As shown in Fig.5,complex 1 was stable up to 300℃,and then began to decompose.
2.2 UV-Vis absorbance and photoluminescence properties
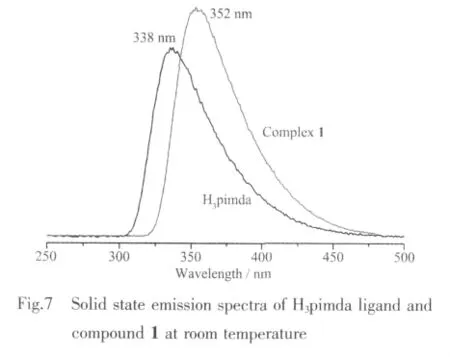
The solid state UV-Vis spectra of H3pimda ligand and complex 1 are displayed in Fig.6.The free H3pimda ligand displays a strong absorption band at 236 nm,arising from the π-π*transition of the ligands.The absorption of complex 1 is observed at 288 nm.The fluorescence emission spectra of free H3pimda ligand and compound 1 were investigated in the solid state at room temperature (Fig.7).The maxima of emission band is observed at 338 nm(λex=239 nm)for free H3pimda ligand.For complex 1,the peak is shifted to 352 nm (λex=290 nm).Since the profile and location of the emission band in the spectrum of the complex 1 are similar to those observed in the free ligand,they can be assigned mainly to intraligand transitions[27].Further studies are ongoing to explore the potential applications of the single-crystal tubes.
[1]Zhang F W,Li Z F,Ge T Z,et al.Inorg.Chem.,2010,49:3776-3788
[2]Li L N,Luo J H,Wang S Y,et al.Cryst.Growth Des.,2011,11:3744-3747
[3]Chang Y J,Liu D,Ren Z G,et al.Inorg.Chem.Commun.,2011,14:403-406
[4]Wang H L,Zhang D P,Sun D F,et al.CrystEngComm,2010,12:1096-1102
[5]Chen S C,Zhang Z H,Zhou Y S,et al.Cryst.Growth Des.,2011,11:4190-4197
[6]Song Y S,Yan B,Chen Z X.Inorg.Chem.Commun.,2005,8:1165-1168
[7]Wang H L,Zhang D P,Sun D F,et al.Cryst.Growth Des.,2009,9:5273-5282
[8]Corma A,Iglesias M,Llabres i Xamena F X,et al.Chem.Eur.J.,2010,16:9789-9795
[9]Jiang G Y,Wu T,Zheng S T,et al.Cryst.Growth Des.,2011,11:3713-3716
[10]Schnobrich J K,Lebel O,Cychosz K A,et al.J.Am.Chem.Soc.,2010,132:13941-13948
[11]Xu C Y,Li L K,Wang Y P,et al.Cryst.Growth Des.,2011,11:4667-4675
[12]Gu J Z,Lü D Y,Gao Z Q,et al.J.Solid State Chem.,2011,184:675-683
[13]Caudle M T,Kampf J W,Kirk M L,et al.J.Am.Chem.Soc.,1997,119:9297-9298
[14]Rajendiran T M,Kirk M L,Setyawati I A,et al.Chem.Commun.,2003:824-825
[15]Liu Y L,Kravtsov V,Walsh R D,et al.Chem.Commun.,2004:2806-2807
[16]Wang Y L,Yuan D Q,Bi W H,et al.Cryst.Growth Des.,2005,5:1849-1855
[17]Panagiotis A,Jeff W K,Vincent L P.Inorg.Chem.,2005,44:3626-3635
[18]Lu W G,Jiang L,Feng X L,et al.Cryst.Growth Des.,2006,6:564-571
[19]Lu W G,Su C Y,Lu T B,et al.J.Am.Chem.Soc.,2006,128:34-35
[20]Gu J Z,Lu W G,Jiang L,et al.Inorg.Chem.,2007,46:5835-5837
[21]Lu W G,Gu J Z,Jiang L,et al.Cryst.Growth Des.,2008,8:192-199
[22]Feng X,Zhao J S,Liu B,et al.Cryst.Growth Des.,2010,10:1399-1408
[23]Sheldrick G M.SHELXL NT Version 5.1,Program for Solution and Refinement of Crystal Structures,University of G?ttingen,Germany,1997.
[24]Zhang X L,Guo C P,Yang Q Y,et al.Chem.Mater.,2007,19:4630-4632
[25]Feng S S,Zhu M L,Lu L P,et al.Chem.Comm.,2007:4785-4787
[26]Liao W P,Li Y L,Wang X F,et al.Chem.Comm.,2009:1861-1863
[27]Ma L F,Meng O L,Li C P,et al.Cryst.Growth Des.,2010,10:3036-3046