二苯基喹啉氧基乙酰胺的La、Ce同構配合物的合成、表征及熒光性質
張照坡 王 元 吳偉娜 蔡紅新
(河南理工大學物理化學學院,焦作 454000)
0 Introduction
Owing to their good luminescent properties,such as the large stokes shifts,narrow emission profiles,long lifetimes and so on[1-2],the lanthanide complexes are attracting more and more attention and have been widely used in many aspects,such as light-emitting diode(LED),laser materials,optical signal amplification and fluoroimmunoassay[3-6].The amide type ligands have often been chosen as an sensitizer to enhance the fluorescence emission intensity of the lanthanide complexes,because they are flexible in structure and would shield the encapsulated lanthanide ion from interaction with the surroundings effectively[1-2].In our previous work,we have reported the synthesis and possible structures ofsix lanthanide complexes(lanthanide ions=La,Sm,Eu,Gd,Tb,Dy)with the ligand L,including the fluorescence properties of Euバcomplex.However,the crystal structures of such complexes are still not confirmed.Therefore,in this paper,two isostructural lanthanide (Laバ and Ceバ)complexes of the ligand L were synthesized and characterized by X-ray diffraction.Furthermore,the fluorescence spectra of the complexes in CH3CN solution were investigated.
1 Experimental
1.1 Materials and measurements
Solvents and starting materials for synthesis were purchased commercially and used as received.Elemental analysis was carried out on an Elemental Vario EL analyzer.The IR spectra(ν=4 000~400 cm-1)were determined by the KBr pressed disc method on a Bruker V70 FT-IR spectrophotometer.All conductivity measurements were performed in acetone with a DDS-11A conductometer at room temperature.The UV spectra were recorded on a Purkinje General TU-1901 spectrophotometer.Fluorescence spectra were determined on a Varian CARY Eclipse spectrophotometer.
1.2 Preparations of the complexes
The ligand L[7](0.070 4 g,2 mmol)and La(NO3)3·6H2O(0.043 3 g,1 mmol)were dissolved in acetone(20 mL).After stirring for 1 h,the mixture was filtered and set aside to crystallize at room temperature.The product 1,which was collected by filtration,washed with Et2O and dried in air.Some of the obtained crystals were suitable for a single crystal X-ray analysis.The synthesis of 2 is similar to that of 1,while Ce(NO3)3·6H2O instead of La(NO3)3·6H2O.
1:Colorless blocks.Yield 41%.Anal.Calcd.for C46H36N7O13La(%):C,53.45;H,3.51;N,9.48.Found(%):C,53.54;H,3.23;N,9.17.IR(KBr,cm-1):ν(C=O)1 667,ν(C=N)1 593,ν(Ar-O-C)1258,ν4(NO3)1 493,ν1(NO3)1 318.Λm(S·cm2·mol-1):3.95.
2:Colorless blocks.Yield 57%.Anal.Calcd.for C46H36N7O13Ce(%):C,53.38;H,3.51;N,9.47.Found(%):C,53.49;H,3.82;N,9.21.IR(KBr,cm-1):ν(C=O)1 667,ν(C=N)1 594,ν(Ar-O-C)1 256,ν4(NO3)1 494,ν1(NO3)1 319.Λm(S·cm2·mol-1):4.83.
1.3.1 X-ray crystallography
The X-ray diffraction measurementsfortwo complexes were performed on a Bruker SMART APEXⅡ CCD diffractometer equipped with a graphite monochromatized Mo Kα radiation(λ=0.071 073 nm)by using φ-ω scan mode.Semi-empirical absorption correction was applied to the intensity data using the SADABS program[8].The structures were solved by direct methods and refined by full matrix least-square on F2using the SHELXTL-97 program[9].All nonhydrogen atoms were refined anisotropically.All H atoms were generated geometrically and refined isotropically using the riding mode.Details of the crystal parameters,data collection and refinements for 1 and 2 are summarized in Table 1.
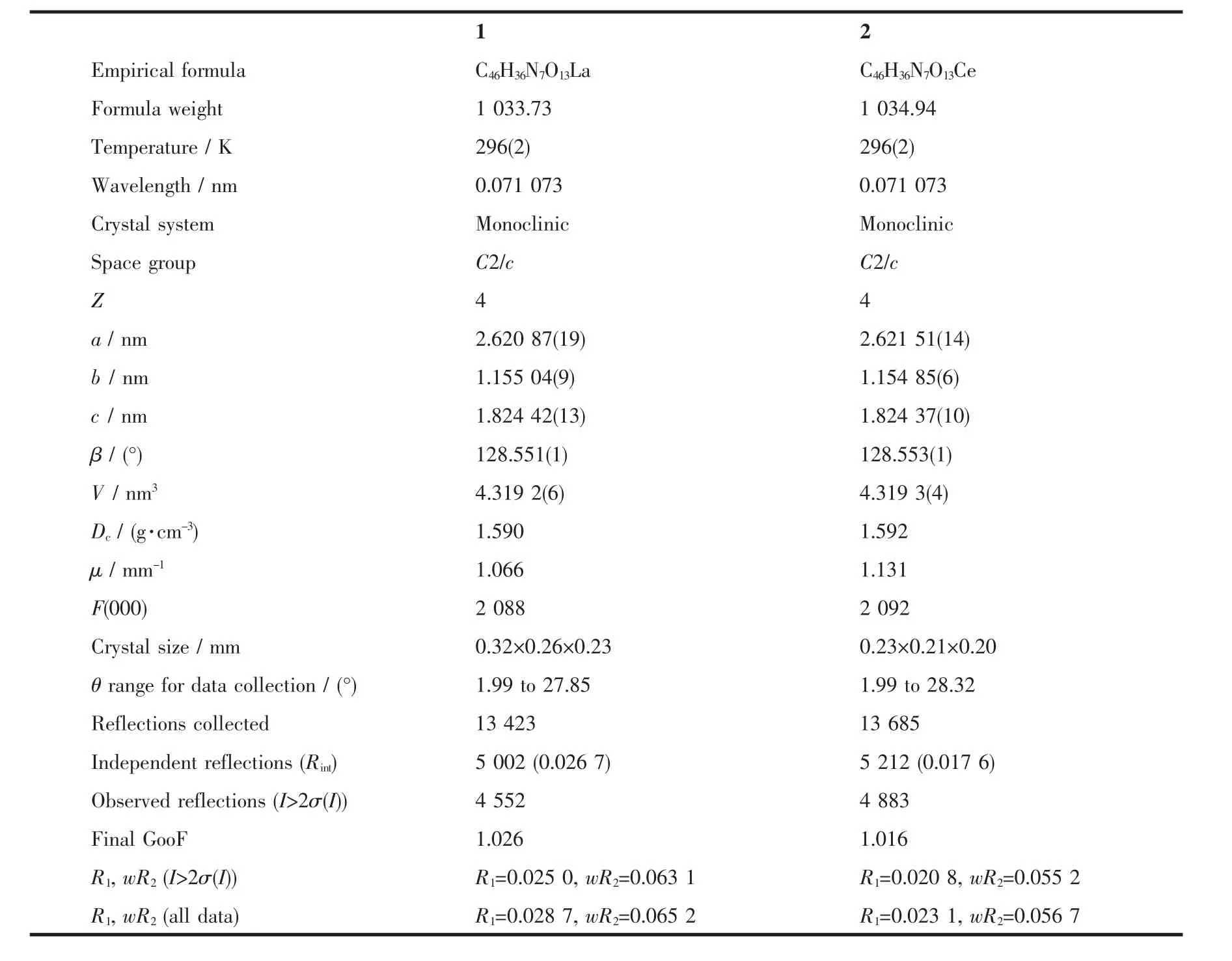
Table 1 Crystal data and structure refinement for the complexes
CCDC:941145,1;941146,2.
2 Results and discussion
2.1 Crystal structure of the complexes
As show in Fig.1a,the asymmetric unit of the complex 1 contains a half of the molecule with N4 and O7 atoms from one nitrate anion lying on the two fold rotational axis.In complex 1,the twelve-coordinated La atom is in a distorted icosahedron geometry(Fig.2a)with the donor centers of four oxygen and two nitrogen atomsfrom two independentacetamide ligands,and six oxygen atoms from three nitrate anions.Selected bond lengths and angles are summarized in Table 2.The La-N and La-O bond lengths are 0.278 98(17)and 0.257 53(13)~0.285 94(14)nm,respectively,comparable to the La complexes with similar donor sets[10].In complex 1,mostly bond angles are highly deviated from those of ideal icosahedron geometry.It is obvious that the crystal structure of 1 isdifferentfrom itssuggested structure in our previous work[2],in which the coordination number for La atom is 8,and there is a free nitrate group.
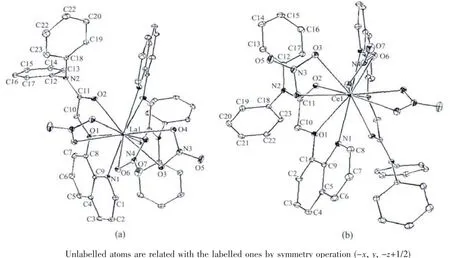
Fig.1 Molecular structures of the complex 1(a)and 2(b)shown with 10%probability displacement ellipsoids
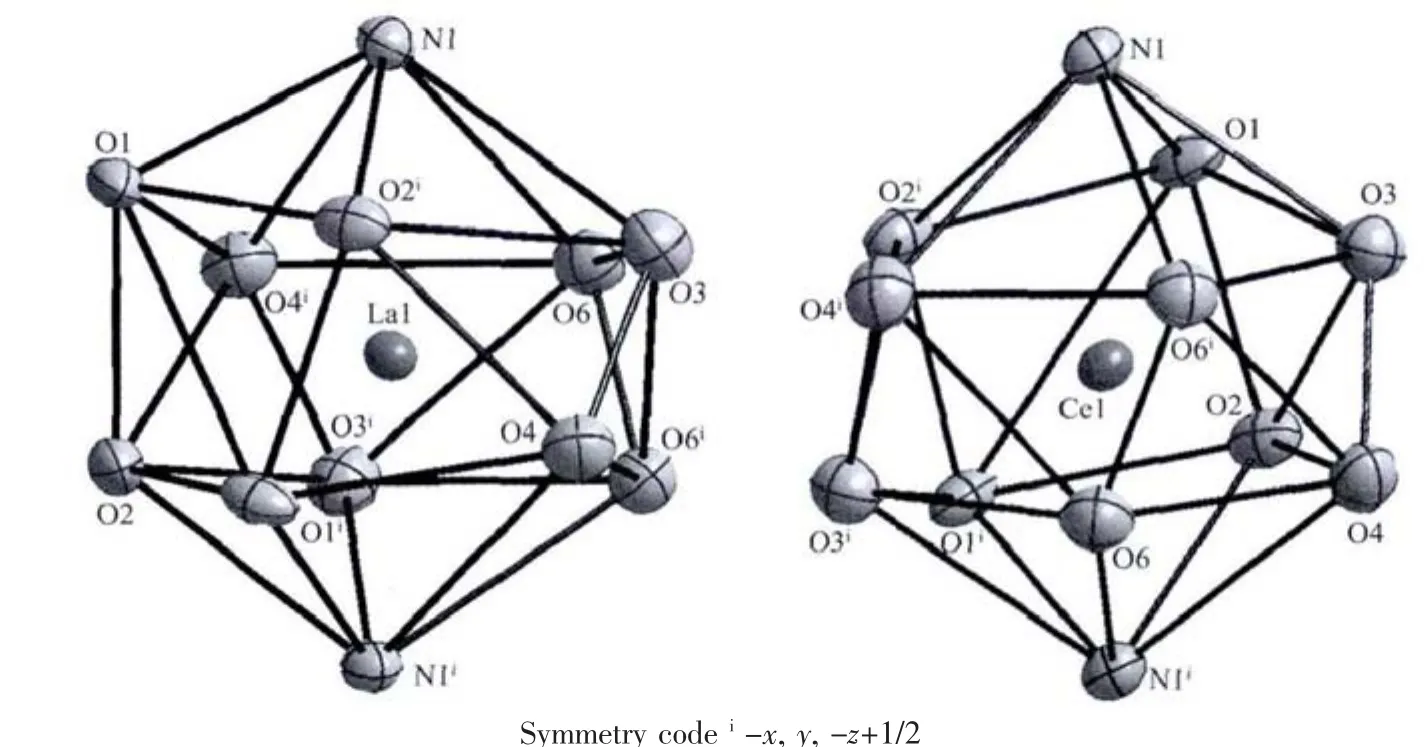
Fig.2 Coordination geometries of the center lanthanide ions shown with 30%probability displacement ellipsoids in the complex 1(a)and 2(b)
Complex 2 (Fig.1b and 2b)is isostructural to 1.The bond lengths of Ce-N(0.277 73(14)nm)and Ce-O(0.255 97(11)~0.284 80(11)nm)are shorter than that of La-N and La-O bonds in complex 1,which may be due to lanthanide contraction[3].As expected,there are no classic hydrogen bonds in both complexes.
2.2 Molar conductivities and IR spectra
The molar conductivity values of the complexes in acetone are 3.95 and 4.83 S·cm2·mol-1,respectively,indicating that both complexes are non-electrolytes[11].In our previous work[2],the molar conductivity was determined in DMF,the different results are probably due to the coordination ability of DMF,in which the complexes may be not stable enough.
The IR spectra of L show strong band at 1 679 cm-1,which are attributable to stretch vibrations of the carbonyl group of amide(ν(C=O)).The peak at 1 618 cm-1should be assigned to the ν(C=N)[2]. Upon coordination with Lnバ ion,ν(C=O)and ν(C=N)shift about 13 and 25 cm-1in both complexes;indicating that carboxyl oxygen atom and quinoline nitrogen atom take part in coordination to the Lnバion[1-2,12-13].The similar bands of ν(Ar-O-C)at about 1 258 cm-1in the acetamide ligand[2]and the complexes are mainly due to the fact that the ligands have large sterically hindered effect,which prevents ethereal oxygen atom from tight coordinating Lnバion[12].In addition,the two intense absorption bands in the spectra of both complexes associated with the asymmetric stretching appear at around 1 318 cm-1(ν4)and 1 493 cm-1(ν1),clearly establishing that there are some coordinated NO3groups (C2v).The differences between the two bands lie at 175 cm-1,suggesting that the coordinated nitrate groups are bidentate[12].It is in accordance with the result of the crystal structure study.
2.3 UV spectra

Fig.3 UV spectra of L(a),1(b)and 2(c)in CH3CN solution at room temperature
The UV spectra of L,1 and 2 in CH3CN solution(concentration:1 ×10-5mol·L-1)were measured at room temperature (Fig.3).The spectra of L feature three main bands located around 203 (ε:96 696 L·mol-1·cm-1),239 (ε:53 091 L·mol-1·cm-1)and 294 nm (ε 1 856 L·mol-1·cm-1),respectively.The bands could be assigned to characteristic π-π*transitions centered on benzene ring,quinoline ring and the acetamide unit,respectively[13].The spectra of 1 and 2 are quite similar as that of L.The ε values of the corresponding bandsin complex 1 and 2 are 13 542,5 679,2 613 and 152 065,57 925,3 256 L·mol-1·cm-1,respectively.However,the hyperchromicities indicate that the ligand L takes part in the coordination in both complexes.
2.4 Fluorescence spectra
The fluorescence spectra of L,1 and 2 in CH3CN solution(concentration:1×10-5mol·L-1)was measured at room temperature.The excitation and emission wavelengths of three compounds are at 300 and 389 nm,respectively (Fig.4).It also can be seen that the emission intensity of the complexes are much higher than that of L,which may be explained from two aspects:first,in the complexes the molar ratios of the ligand and Ln ion are 2∶1;second,the coordination of Ln ion may enhance the π→π*electron transition of the acetamide ligand[5].
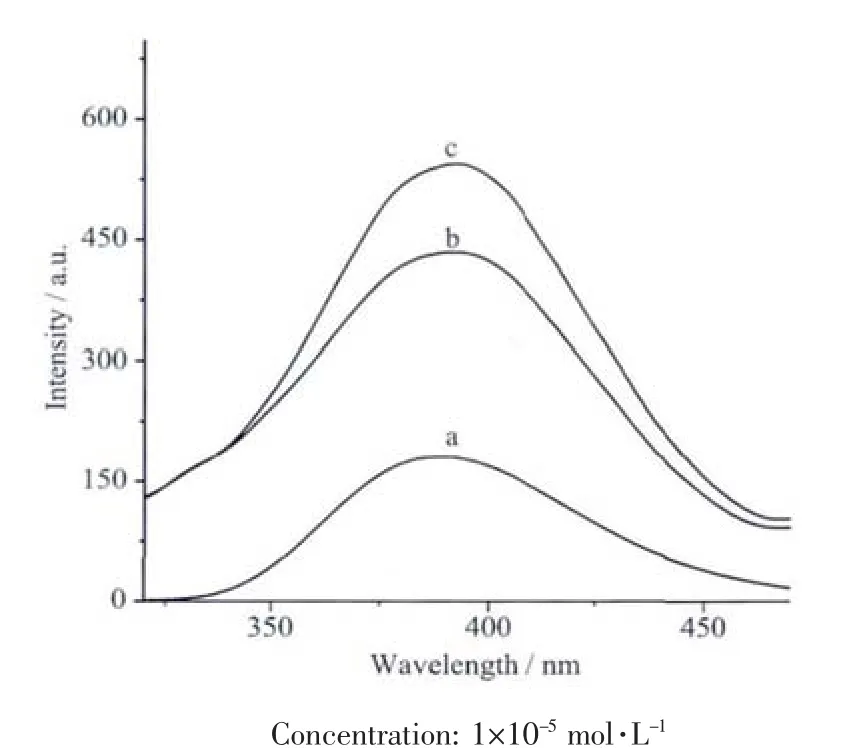
Fig.4 Fluorescence emission spectra of L(a),1(b)and 2(c)in CH3CN solution at room temperature
[1]Song X Q,Wen X G,Liu W S,et al.J.Solid State Chem.,2010,183:1-9
[2]Wu W N,Tang N,Yan L.J.Fluoresc.,2008,18:101-107
[3]LIU Mei-Juan(劉美娟),CAO Deng-Ke(曹登科),HUANG Jian(黃建),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2012,28:2017-2024
[4]LIU Jian-Fen(劉建風),CHEN Ji-Fei(陳吉妃),ZHAO Guo-Liang(趙國良),Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2011,27:100-106
[5]LI Li(李麗),CHEN Ya-Shao(陳亞芍),ZHAO Li-Fang(趙麗芳),Chem.J.Chinese Universities(Gaodeng Xuexiao Huaxue Xuebao),2006,27:199-203
[6]Liu D Y,Kou Z Q,Li Y F,et al.Inorg.Chem.Commun.,2009,12:461-464
[7]Wen Y H,Zhang S S,Li M J,et al.Acta Cryst.E,2005,61:o1807-o1809
[8]Sheldrick G M.SADABS,University of G?ttingen,Germany,1996.
[9]Sheldrick G M.SHELX-97,Program for the Solution and the Refinement of Crystal Structures,University of G?ttingen,Germany,1997.
[10]LIU Ru(劉茹),ZHU Wen-Xiang(朱文祥),ZHANG Yong-An(張永安),et al.Acta Chim.Sin.(Huaxue Xuebao),2001,59:533-537
[11]Geary W.J.Coord.Chem.Rev.,1971,7:81-121
[12]Wu W N,Cheng F X,Yan L,et al.J.Coord.Chem.,2008,61:2207-2215
[13]Song X Q,Zang Z P,Liu W S,et al.J.Solid State Chem.,2009,182:841-848

