基于2-苯基-4-喹啉酸及雙咪唑類配體的鈷ギ配合物的合成、表征、磁性和電化學性質
侯向陽 王 瀟 付 峰王記江 唐 龍
(延安大學化學與化工學院陜西省化學反應過程重點實驗室,延安 716000)
0 Introduction
The design and synthesisofmetal-organic frameworks (MOFs)are of great interest not only for their intriguing variety of structures[1-4],but also for their potential applications in magnetism,catalysis,adsorption,and fluorescence properties,etc[5-8].So far,a number of O and N-donor multifunction ligands such as 9-acridinecarboxylic acid, 4-quinolinecarboxylic acid,quinoline-based carboxylic acid,2-methylquinoline-3,4-dicarboxylate acid,2-methylquinoline-2,4-dicarboxylate acid have been widely employed to construct MOFs[9-11].In contrast,the use of 2-phenyl-4-quinolinecarboxylic acid ligands(Hpqba)to construct functional MOFs has been less investigated to date[12-15].In this regard,our synthetic strategy was to select Hpqba and introduce N-donor bridging ligand of biyb with different rigidity as coligandsinto the reaction system,based on the following considerations:(1)The Hpqba can adopts various coordination modes when it coordinates to metals and it have larger conjugated π-systems(three benzene rings), π-π stacking interactions may play important roles in the formation of their complexes;(2)The carboxylic groups can propagate magnetic super exchange between metal centers,and rigid ligands containing multi benzene rings and suitable metal ion form complexes having fluorescence properties;(3)The introduction of flexible long-chain N-donor bridging biyb ligand as co-ligands may helps us to assemble and explore their impact on MOFs.
Herein,to design complexes showing interesting architectures and magnetic behavior,we have chosen the Coギ,ion,Hpqba and biyb (1,4-bis(imidazol-1-ylmethyl)benzene)ligands as co-ligands to react.Then,one new complex is obtained,namely, [Co(pqba)2(biyb)] (1),and thedetailsoftheirsyntheses,structures,and magnetic properties are reported below.
1 Experimental
1.1 Materials and methods
Reagents and solvents employed were commercially available and without further purification.Elementalanalysis (C,H,N)was determined on a Perkin-Elmer 2400 type elemental analyzer.IR absorption spectra of the compound was recorded in a Bruker EQUINOX-55 spectrophotometer with the range 400~4 000 cm-1using KBr disks(6 mg of sample in 500 mg of KBr).Thermogravimetric analyses was recorded on a NETZSCH STA 449C thermal analyzer from room temperature up to 900℃using a heating rate of 10℃·min-1under an air atmosphere.Magnetic measurement was performed of 1 using a MPMS-XL-7 magnetometer under an applied field of 1000 Oe over the temperature range of 1.8~300 K.
1.2 Syntheses of[Co(pqba)2(biyb)](1)
Compound 1 was prepared as follows:a mixture of Hpqba(0.049 6 g,0.2 mmol),biyb(0.0477 g,0.2 mmol),cobalt acetate (0.074 7 g,0.3 mmol),NaOH(0.0080 g,0.2 mmol),deionised water (12 mL)and DMF (3 mL)was stirred for 30 min in air,then transferred and sealed in a 25 mL Teon reactor,which was heated at 110 ℃ for 48 h.The solution was then cooled to room temperature at rate of 4℃·h-1,a very ne deep red crystalline product 1 in 65%yield based on Co.C46H34O4N6Co(793.72):Calcd.(%):C 69.61,H 4.32,N 10.59;Found(%):C 69.57,H 4.44,N 10.62.
1.3 Crystal structure determination
X-ray crystallographic data for the compound 1 were collected at room temperature using Bruker Smart-1000CCD diffractometer. Graphite monochromated Mo Kα (λ=0.071 073 nm)radiation wasused.Empiricalabsorption correctionswere applied using the SADABS program[16].The structures of compounds 1~3 was solved by direct methods using the SHELXS-97[17]and refined on F2by the full-matrix least-squares methods using the SHELXL-97 program package[18], Atoms were located from iterative examination of difference F-maps following leastsquares renements of the earlier models.Hydrogen atoms were placed in calculated positions and included as riding atoms with isotropic displacement parameters 1.2 times Ueqof the attached C atoms.The final R=0.055 6,wR=0.136 9(ω=1/[σ2(Fo2)+(0.056 0P)2+10.752 0P],where P=(Fo2+2Fc2)/3),(Δρ)max=1 495 e·nm-3and(Δρ)min=-1 281 e·nm-3.The crystal data and refinement details of the compound are summarized in Table 1,and the selected interatomic distances are given in Table 2.
CCDC:919474.

Table 1 Crystal data for the compound 1

Table 2 Selected bond lengths(nm)and angles(°)the compound 1
2 Results and discussion
2.1 Crystal Structure of[Co(pqba)2(biyb)](1)
X-ray single crystaldiffraction reveals that complex 1 crystallizes in monoclinic C2/c space group.complex 1 is a 1D coordination polymer,and which expand 2D supramolecules via strong π…π stacking interactions. As shown in Fig.1, the asymmetric unit contains consists of one Coギion,two pqba anionics,and one biyb ligand.
The six-coordinated Co1ギadopts slight distorted[CoO4N2]octahedron geometry,where four carboxylate oxygen atoms from two pqba ligands(Co1-O1 0.222 9(2)nm,Co1-O1i0.222 9(2)nm,Co1-O2 0.217 4(2)nm,and Co1-O2i0.217 4(2)nm),and two N atoms from two biyb ligands(Co1-N2 0.2067(2)nm,and Co1-N2i0.206 7(2)nm).The O1 and O1iatoms lie at the both sides of the plane,and occupy the vertexes of the octahedral geometry. The selected interatomic distances and angles are given in Table 2.All the bond distances and angles are comparable to those observed in other Coギcompounds[19].

Fig.1 Coordination environment of the Coギion in 1 at 30%probability level
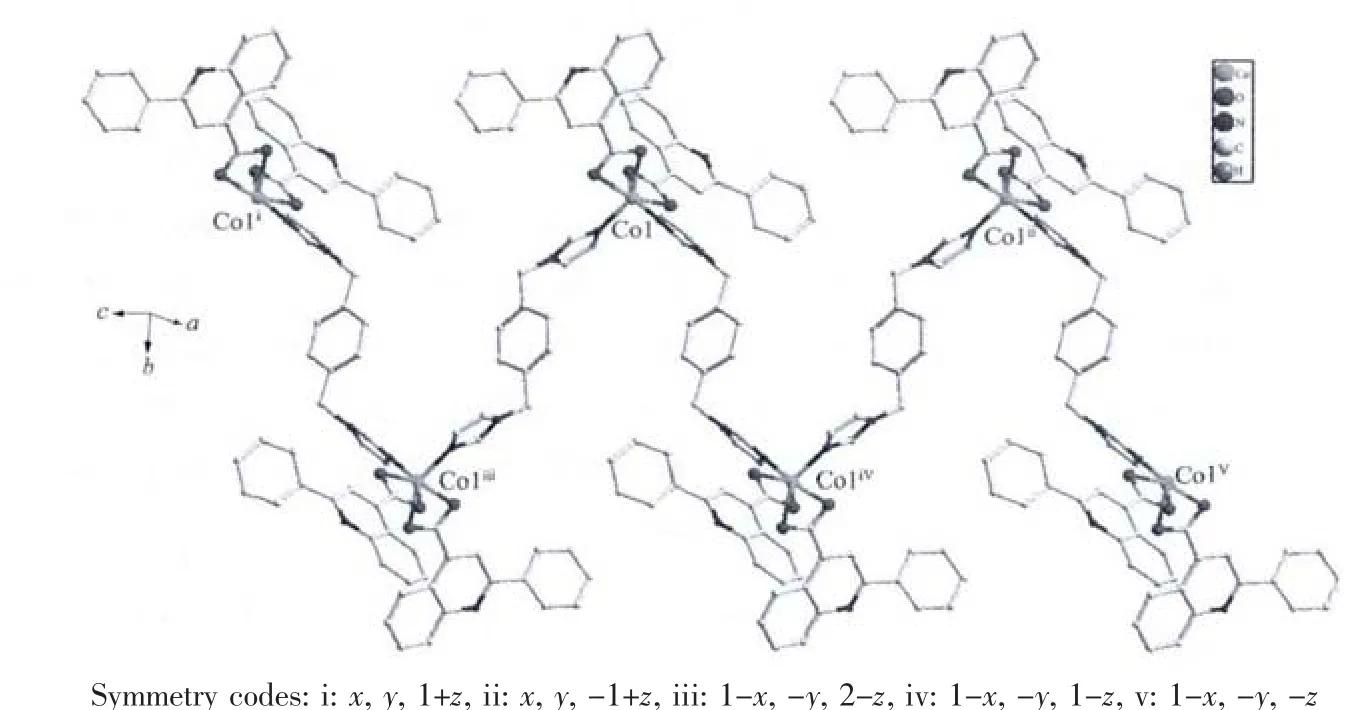
Fig.2 View of 1D chain constructed by biyb ligand along the c axis
In compound 1,each deprotonated pqba ligand adopts a μ2-chelating fashion,and merely contribute to coordination number around each Co1 ions.As shown in Fig.2,the Co1 ions were bridged by nitrogen atoms of biyb ligands to form a one-dimensional zigzag chain structure along the c axis with the neighboring Co1 distance of 1.476 9(3)nm.Where,the pqba ligands seem to act as “wings”,whereas the biyb and Znギacts as “trunk”,thus,they compose a butter-fly-like structure(Fig.2).In complex 1,the π … π stacking interaction change the framework from onedimensional zigzag chain structure to 2D supramolecules in bc plane(Fig.3),and there are π…π stacking interactions(centroid…centroid distances:0.401 3 nm,centroid …plane distances:0.3552 nm)between imidazol of biyb ligand in one-dimensional zigzag chains.
2.2 IR spectra
The IR spectra ofcomplex 1 show the characteristic vibration of carboxylic acids.The strong peaks of aromatic rings span over the range 1 330~1 620 cm-1for 1.
The presence of the characteristic bands at around 1 550~1 570 cm-1in complex 1 that attributed to the protonated carboxylic group.The vibration absorption peak of C=N at 1 540~1 570 cm-1for complex 1,compared with the IR spectra of bipy ligand the peak upfield shift.Which shows that the nitrogen atoms of bipy ligand coordinated with zinc ions.These spectral information of complex 1 is consistent with the results of the single-crystal X-ray diffraction analyses.

Fig.3 View of 2D supramolecular structure by-stacking interactions along the b axis of 1
2.3 Thermogravimetric analyses
To characterize the complex 1 more fully in terms of thermal stability,the thermal behaviors were studied by thermogravimetric analyses(Fig.4).For 1,a weight loss is observed from 140 to 490℃,which is attributed to the loss of the pqba anionic,with a weight loss of 62.1% (Calcd.62.6%);then,the ligands of bipy are removed,and a plateau of 9.2%at 790 ℃ is observed,which may be a CoO(Calcd.9.4%)residue.
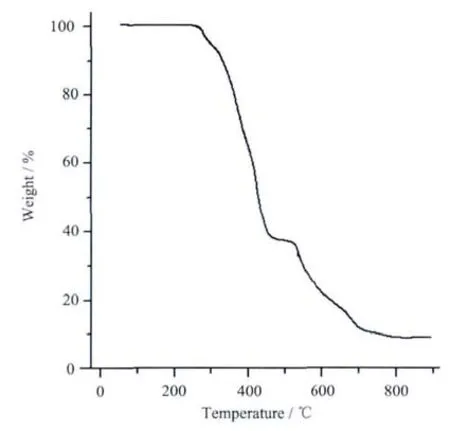
Fig.4 TGA trace of complex 1
2.4 Electrochemical property
The cyclic voltammetry of 1(Fig.5)was measured with a three electrode cell in aqueous solution with complex of 6.6×10-6mol·L-1,which with scanning range of-1.500~1.500 and scanning rate of 100 mv·s-1at room temperature.The cyclic voltammogram curve of 1 have one pair of oxidation-reduction peak,which corresponds to Coギ/Coバ redox process.Epa=-0.69 V,Epc=-0.83 V,E=0.14 V,E1/2=0.76 V,Ipa/Ipc=0.10.The results show that electron transfer of Coギbetween Coバ in electrolvsis is quasi-reversible process[20].

Fig.5 Cyclic volatmmetric of complex 1
2.5 Magnetic property of 1
The magnetic measurement was performed on polycrystalline samples of 1 using a MPMS-XL-7 magnetometer under an applied field of 1 000 Oe over the temperature range of 1.8~300 K.The temperature dependence of the magnetic susceptibility of 1 in the form of χMand χMT versus T are displayed in Fig.5.At room temperature, χMT is equal to 2.71 cm3·mol-1·K,which is much higher than the spin-only value of 1.87 cm3·mol-1K based on single Coギ ions.Upon lowering the temperature,χMT continuously decreasesand reaches 1.45 at 1.99 K.Above 140 K,the magnetic properties of 1 obey the Curie-Weiss law and give C=2.71 cm3·mol-1·K,and θ=-5.35.Which indicates an intracluster antiferromagnetic interaction between the Coギions[21].While the distance among Co ions was bridged through the long biyb ligands,it should exclude an efficient direct exchange between Co ions;the overall antiferromagnetic interaction should be attributed to the significant spin-orbit coupling,which is remarkable for the4T1gground term of Co in an octahedral ligand field,according to the preceding structure description of 1,no appropriate model could be used for tting the magnetic properties of such a system[22].
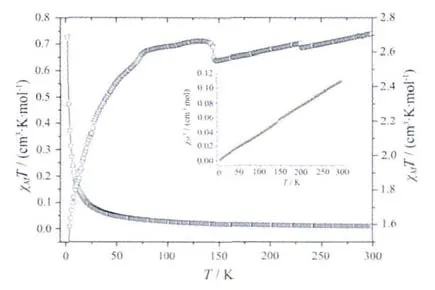
Fig.6 Temperature dependence of of magnetic susceptibility in the form χMT,χMand 1/χMT(inset)for complex 3
[1]Zhao T T,Jing X M,Wang J,et al.Cryst.Growth Des.,2012,12:5456-5461
[2]Yang W T,Guo M,Sun Z M,et al.Cryst.Growth Des.,2012,12:5529-5534
[3]Zhao F H,Che Y X,Zheng J M.Inorg.Chem.Commun.,2012,16:5560
[4]Zhang Z M,Li Y G,Wang E B,et al.Angew.Chem.Int.Ed.,2009,48:1581-1584
[5]CHEN Man-Sheng(陳滿生),ZENG Ju-Lan(曾巨瀾),ZHANG Chun-Hua(張春華),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2012,28:1775-1778
[6]Wang X Y,Wang Z M,Gao S.Chem.Commun.,2008:281294
[7]MaspochD,Ruiz-MolinaD,VecianaJ.Chem.Soc.,Rev.,2007,36:770-818
[8]Ji W J,Zhai Q G,Hu M C,et al.Chem.Commun.,2011,47:3834-3836;
[9]Bu X H,Tong M L,Chang H C,et al.Inorg.Chem.,2005,44:,9837-9846
[10]Bu X H,Tong M L,Chang H C,et al.Ange.Chem.Int.Ed.Engl.,2004,43:192-195
[11]Yuan G,Shao K Z,Wang X L,et al.Inorg.Chem.Commun.,2008,11,1246-1249
[12]Zha M Q,Li X,Bing Y.J.Coord.Chem.,2011,64:473-482
[13]Wang J J,Chang Z,Zhang A S,et al.Inorg.Chim.Acta,2010,363:13771385
[14]Qin Z Q,Jennings M C,Puddephatt R J,et al.Inorg.Chem.,2002,41:5174-5186.
[15]Shen Y C,Li Z J,Cheng J K,et al.Inorg.Chem.Commun.,2007,10:888-890
[16]Sheldrick G M.SADABS,A Program forEmpirical Absorption Correction of Area detector Data University of G?ttingen,Germany,1997.
[17]Sheldrick G M.SHELXS-97,Program for Crystal Structure Determination,University of G?ttingen,Germany,1997.
[18]Sheldrick G M.SHELXL-97,Program for Crystal Structure Refinement,University of G?ttingen,Germany,1997.
[19]HAN Wei(韓偉),CHENG Mei-Lin(程美令),LIU Qi(劉琦),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2012,28:1997-2004.
[20]LEI Jing-Bin(雷靜彬),HUANG Fu-Ping(黃富平),YU Qing(于青),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2012,28:572-578
[21]Cheng X N,Zhang W X,Chen X M.J.Am.Chem.Soc.,2007,129:15738-15739
[22]Cui J H,Li Y Z,Guo Z J,et al.Cryst.Growth Des.,2012,12:3610-3618

