Hydrothermal Synthesis and Properties of CoFe2O4 Magnetic Nanoparticles?
Wubulikasimu Kadier,Beysen Sadeh?,Bahat Duamet,Mutila Aman
(1.College of Physics Science and Technology,Xinjiang University,Urumqi,Xinjiang 830046,China;2.Kazakh National Technical University named after Satpayev,Almaty,050013,Kazakhstan)
Abstract: We present a survey of properties of CoFe2O4 nanoparticles synthesized by a poly ethane glycol(PEG)-assisted hydrothermal method.XRD,TEM and VSM were used to characterize the structural,morphological and magnetic properties of the samples.The XRD analysis confirmed the formation of pure spinel phase and the crystallite size was found to be in the range of 10-32 nm.It was also observed that the mean size of CoFe2O4nanoparticles increases with the increasing of hydrothermal temperature.The hydrothermal temperature not only affects the size of nanoparticles but also affects its morphology,the morphology of CoFe2O4 nanoparticles changed from spherical shape to octahedral with increasing of the hydrothermal temperature.Magnetization measurement shows that the saturation magnetization and coercivities of synthesized samples increased with the increasing of nanoparticles’mean size.
Key words:nanoparticles;hydrothermal method;magnetic measurement
0 Introduction
Synthesis of spinel ferrite nanomaterials has been attracted a great deal of interest during the last two decades,due to their novel physico-chemical properties that are different from their bulk form[1,2].Nanocrystalline spinel ferrites can be used in many areas,such as ferrofluids[3],microwave absorber[4],magnetic drug delivery[5],permanent magnets,hard disk recording media,sensors[6],and catalysis[7],etc.Among spinel ferrites cobalt ferrite is a well-known spinel ferrite and is a hard magnetic material with high coercivity[8],moderate saturation magnetization[9],remarkable mechanical hardness and chemical stability.CoFe2O4nanocrystals are widely used in various applications,such as lithium batteries[10],ferrofluids[11],electronic devices[12],detoxification of magnetic hyperthermia[13],magnetically controlled transport of anti-cancer drugs[14],magnetic resonance imaging(MRI)contrast[15],and antitumor applications[16].
However,properties of CoFe2O4strongly depend on the preparation method.In recent years,cobalt ferrite nanocrystals have been prepared using various methods,such as chemical copercipitation[17],sol-gel method[18],combustion method[19],reverse micelle methods[20],thermal decomposition[21],solvothermal method[22]and hydrothermalmethod[23].Among these methods hydrothermal method has been known as one of the most promising synthetic strategy for production of various nanoparticles.
As a surfactant,PEG is one of polymers commonly used,due to its nontoxic,non-flammable and easy to handle.It has been reported that PEG with uniform and ordered chain structure is easily absorbed to at the surface of metal oxide colloid[24].When the surface of the colloid adsorbs PEG,the activities of colloid greatly decreases and growth rate of the colloid in some facet will be confined[25].Therefore,PEG has been widely used in synthesis of series nanoparticles in homogenous solution[26,27].As far as we know,there is no systematic investigation of the effect of reaction temperature on the formation of CoFe2O4in a homogenous solution using the hydrothermal method assisted by PEG.The main purposes of this work are to investigate the temperature effect on the size of CoFe2O4nanoparticles prepared in alkaline aqueous media and characterize their magnetic properties.
1 Experimental
1.1 Synthesis
All reagents were in the analytical grade and used without further purification.In a typical procedure,1.35 g of FeCl2·6H2O and 0.595 g of CoCl2·6H2O were first dissolved in 15 ml of deionized water which contains 8 g of poly ethane glycol(PEG,Mw=6000)under magnetic stirring at room temperature,then 20 ml of NaOH(0.2mol)aqueous solution was added to the above solution under fierce stirring for 15 min.The mixed solution was then sealed into a 50 ml of Teflon-lined stainless-steel autoclave and kept at certain temperature,after which,the autoclave was allowed to cool to the room temperature naturally.Then the product was washed alternatively with deionized water and absolute ethanol several times until the filtrate pH value was neutral,and finally dried in a vacuum oven at 60?C for 6h.The possible chemical reactions occurred during the experiment are described below,
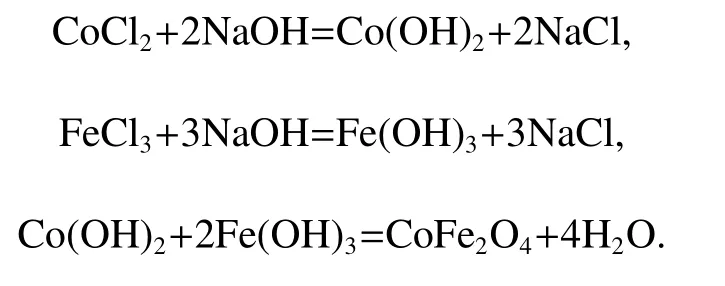
1.2 Characterization
The X-ray diffraction(XRD)spectrum of the products was acquired by a Japan Mac Science applying 18kW X-ray diffractometer with Cu καradiation(λ=0.154056 nm).Transmission electron microscopy(TEM)images of the products were obtained by Japan Hitachi-600.Magnetic hysteresis loops of the samples were measured using a vibrating sample magnetometer(USA,Lakeshore 7404).
2 Results and discussion
2.1 XRD analysis
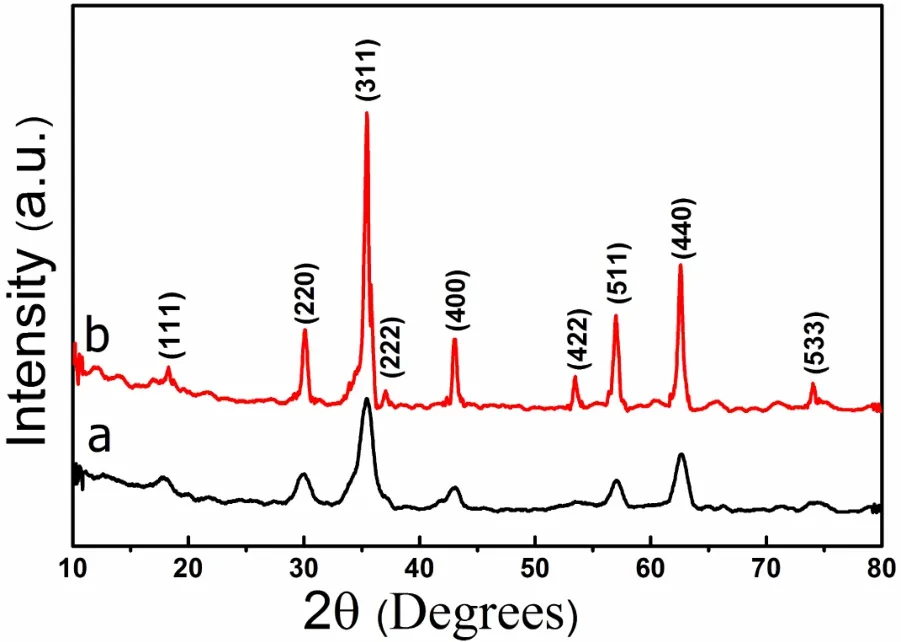
Fig1 XRD patterns of the products prepared by hydrothermal method at various temperatures for 24 h(a)160?C;(b)220?C
The XRD pattern of the as-prepared CoFe2O4nanoparticles prepared at 160?C and 220?C is shown in Fig.1.It can be seen that the peaks of XRD pattern are compatible with Cubic crystal of JCDPS card Number of 22-1086.Obviously,the all samples are pure CoFe2O4crystals without impurities such as oxides of iron and cobalt.It can be also observed from Fig.1a that the X-ray pattern shows a considerable broadening at lower temperature indicating the small-size particle nature of cobalt ferrite powder.The intensities of the major characteristic peaks become sharper and stronger with the increase of hydrothermal temperature,meanwhile we can see that there is no corresponding characteristic peaks for some planes such as(222)and(422)at lower reaction temperatures,but at the higher reaction temperature these peaks appear in X-ray diffraction pattern,indicating that the crystallinity tends to be improved.The peak broadening is purely due to the reduced particle size.The particle sizes of synthesized products were calculated from Scherer formula:

where,Dis the average grain size,k is a constant equal to 0.89,λ is the wavelength of X-ray 0.154056 nm,β is full width at half maximum(FWHM)of the diffraction peaks and θ is the Bragg’s angle in radian.According Scherer formula:the results shown in Table1.

Table1 The effect reaction temperature on the particle size and magnetic properties of the CoFe2O4 nanoparticles
It is distinct that the average crystallite size increases with the reaction temperature from 10 nm at 160?C to 32 nm at 220?C.And these results well match to the direct observation of TEM images of as synthesized CoFe2O4nanoparticles.
2.2 TEM analysis
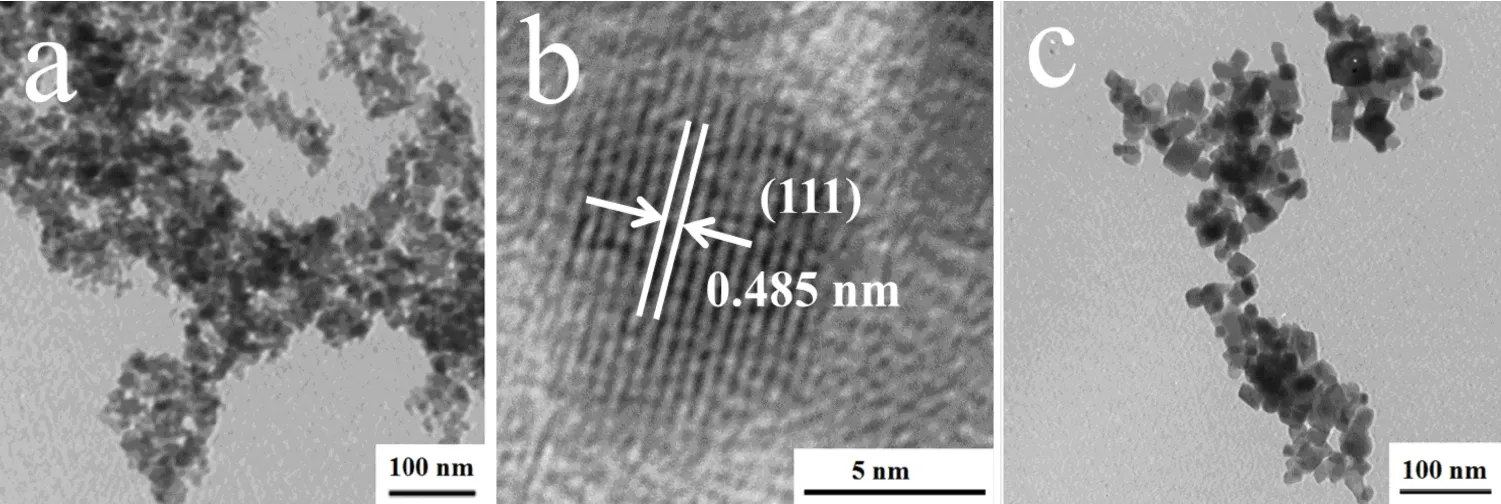
Fig2 TEM images of the products synthesis at(a)160?C for 24h,(b)HRTEM images of sample a,(c)220?C for 24h
The morphologies of as-prepared CoFe2O4nanoparticles prepared at 160?C and 220?C is given in Fig.2,from which it can be seen that with the increase of reaction temperature the particle sizes tend to be larger.Samples were uniform in both morphology and crystal size when the hydrothermal temperature was 160?C.As shown in Fig.2b,the HRTEM image of sample shows clear and perfect lattice fringes with spacing of 0.485 nm,which is in agreement with the spacing of(111)planes of CoFe2O4(JCDPS PDF No.22-1086).The HRTEM image reveals single crystal nature of the nanoparticles.When hydrothermal temperature is 220?C,the products were octahedral shape and have a broader size distribution.
2.3 VSM analysis

Fig3 Hysteresis loops of CoFe2O4 nanoparticles synthesized at different temperatures(a)160?C,(b)220?C
Magnetic properties of as synthesized nanoparticles were measured under an increasing magnetic field up to±19 kOe at room temperature.Fig.3 shows the hysteresis loops of samples prepared at 160?C and 220?C,their sizes were respectively 10 nm and 32 nm.The hysteresis loops of the samples measured at room temperature show ferromagnetic characteristic of samples.For better understanding the magnetic behavior of as synthesized products,we have also measured other two samples with the sizes of 14 nm and 19 nm,and displayed their magnetic properties in Table1.The magnetic saturation values of the samples increased with the increase of mean size as shown in Fig.4a,this result accordance with earlier report[28].Our products get their maximum saturation magnetization value of 72.9 emu/g when the sample size is 32 nm,which is smaller than Ms value of bulk CoFe2O4(80 emu/g)[29].The lower magnetization value observed in this study should be resulted from the small size effect,pinning of surface spins and existence of the nonmagnetic surfactant on the surface of cobalt ferrite nanoparticles[28].As shown Fig.4b the coercivities of as synthesized samples increased with the increase of nanoparticles’mean size,which can be attributed to the increased mean particle size and single domain size,and aspect ratio of nanoparticles prepared at lower temperature.In this study,the morphology of CoFe2O4nanoparticles,prepared by a simple hydrothermal changed from spherical shape to octahedral with increase of the hydrothermal temperature.It has been reported that various morphology can lead to very different magnetic property of cobalt ferrite[30].
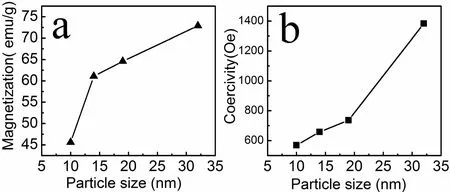
Fig4 The variations of the magnetization(a)and the coercivity with particle size
Therefore the higher hydrothermal temperature evaluates the crystallinity of samples and changes the morphology of product,which causes increase in magnetic saturation.Because of the good ferromagnetic properties of the samples,it is a promising candidate for high-density magnetic storage,medical diagnostics,etc.
3 Conclusion
A facile hydrothermal method has been adopted for the synthesis of cobalt ferrite nanoparticles at different hydrothermal temperature.The mean size of CoFe2O4nanoparticles increases with the increase of hydrothermal temperature.The hydrothermal temperature not only affects the size of nanoparticles but also affects its morphology,themorphology of CoFe2O4nanoparticles changed from spherical shape to octahedral with increase of the hydrothermal temperature.The room temperature VSM studies shows that the saturation magnetization and corecivities of as synthesized samples increased with the increase of nanoparticles’mean size.

