一氧化氮對植物重金屬脅迫抗性的影響研究進展
夏海威,施國新,黃 敏,吳 娟
南京師范大學生命科學學院 江蘇省生物多樣性與生物技術重點實驗室, 南京 210023
一氧化氮對植物重金屬脅迫抗性的影響研究進展
夏海威,施國新*,黃 敏,吳 娟
南京師范大學生命科學學院 江蘇省生物多樣性與生物技術重點實驗室, 南京 210023
一氧化氮(NO)作為一種重要的信號分子,在調節植物重金屬脅迫抗性方面上起著非常重要的作用。綜述了NO在植物體內的產生途徑,重金屬脅迫下植物體內內源NO含量的變化以及外源NO與內源NO對植物重金屬脅迫抗性的影響。大量研究表明外源NO能夠增強植物對重金屬脅迫的抗性,一方面是通過增強植物細胞的抗氧化系統或直接清除活性氧,另一方面是通過影響植物對重金屬的吸收以及重金屬在植物細胞內的分布。然而內源NO在調節植物重金屬脅迫抗性上的功能角色仍存在爭議。有些研究表明內源NO是有益的,能夠緩解重金屬脅迫誘導的毒性;但是也有證據表明內源NO是有害的,能夠通過促進植物對重金屬的吸收以及對植物螯合素進行S-亞硝基化弱化其解毒功能,從而參與重金屬誘導的毒害反應和細胞凋亡過程。
外源NO; 內源NO; 重金屬脅迫; 抗性
一氧化氮(Nitric Oxide, NO)是一種廣泛存在于生物體內的水溶性和脂溶性氣體小分子信號物質,在植物體內參與多種生理過程,如誘導種子萌發[1]、抑制種子休眠[2], 調節植物光合作用[3]、花的形成[4]以及植物的根向地性生長[5],延緩植物的衰老過程[6]等。同時,NO也參與調節植物對一系列非生物和生物脅迫的抗性,在非生物脅迫抗性方面,NO能夠增強植物抗鹽性,抗旱性,抗澇性,抗極端溫度,抗紫外線輻等[7];在生物脅迫抗性方面,NO能通過增加3′5′-環-磷酸鳥苷(cGMP)和水楊酸(SA)的水平來增強植物的抗病性[8]。
對重金屬脅迫抗性而言,已有大量文獻表明NO能夠調節植物對重金屬脅迫抗性,但對NO在植物重金屬脅迫抗性方面的具體機制做系統總結和概括的研究較少并且對內源NO在調節植物重金屬脅迫抗性上的功能角色也很少涉及。本文結合國內外最新的研究進展,綜述了NO調節植物重金屬脅迫抗性的相關機制,為研究植物重金屬脅迫抗性機制提供了參考。
1 植物體內NO的產生途徑
1.1 酶促反應途徑
1.1.1 一氧化氮合酶(NOS)途徑
在動物體內,NO主要通過NOS以L-精氨酸、O2及NADPH為底物催化而成,FAD、FMN、血紅素、四氫葉酸、Ca2+/CaM和Zn2+為NOS的輔基[9]。在植物體內也有類似的NOS,Neill等[7]發現植物能夠通過氧化L-精氨酸形成瓜氨酸而產生NO,并且動物NOS抑制劑L-硝基精氨酸甲酯(L-NAME)能夠抑制擬南芥NOS活性并減少NO的產生[10],另外人們已經在植物組織如豌豆的根、莖、葉中[11]以及各種細胞器如過氧化物酶體[12]和葉綠體[13]中都檢測到了NOS活性。然而植物NOS的基因和蛋白序列與動物卻并不相同,如最近發現的綠藻(Ostreococcustauri)中的NOS蛋白序列已經被鑒定出來,結果其與動物的NOS蛋白序列僅有45%的相似性,而其結構模型與動物的反應區域卻高等相似[14],另外Guo等發現擬南芥中AtNOS1基因編碼的蛋白與蝸牛中參與NO合成的蛋白有相似序列,但這一蛋白與典型的動物NOS蛋白序列沒有相似性[15],并且體外重組的AtNOS1也沒有NOS活性[16],實際上AtNOS1是一種GTPase而不是NOS,因而命名為AtNOA1[17],該蛋白可能參與線粒體核糖體的生物合成以及翻譯過程,并在此過程中間接參與NO的合成[16]。
1.1.2 硝酸還原酶(NR)和亞硝酸還原酶(Ni-NOR)途徑

1.1.3 其他酶促途徑
另外,植物體內還有其他的酶也參與NO的產生,如辣根過氧化物酶[21]、黃嘌呤氧化酶(XOR)以及黃嘌呤脫氫酶(XDH)[22]、細胞色素P450[23]等。
1.2 非酶促途徑

2 重金屬脅迫下植物體內內源NO含量的變化
重金屬脅迫下,植物細胞內源NO含量往往會發生顯著變化,然而內源NO含量的變化卻有眾多的影響因素。
如100 μmol/L的銅(Cu)、鎘(Cd)、鋅(Zn)短期處理24 h以后,豌豆根內的內源NO含量升高[27],而100 μmol/L的Cu、Cd、Zn長期處理14 d以后,豌豆根內的內源NO含量卻下降[28],說明重金屬處理時間的長短能夠影響植物體內內源NO含量的變化。
Leterier等用不同濃度的砷(As)對擬南芥處理7 d以后發現,當砷濃度小于250 μmol/L時,擬南芥根內的NO含量沒有增加,甚至在250 μmol/L時出現了下降;而當砷濃度超過500 μmol/L時,擬南芥根內的NO含量大幅增加,并且在500 μmol/L時達到最大[29],表明植物體內內源NO含量的變化與重金屬的濃度有關。
100 μmol/L的Cd處理24 h以后,水稻根內的NO含量下降[30],而在同樣的100 μmol/L的Cd處理24 h以后豌豆根內的NO卻增加[27];另外,Chen等在研究大麥的兩種基因型weishuobuzhi和dong17對5 μmol/L的Cd脅迫反應時發現,兩種基因型大麥體內的NO含量變化不同,其中weishuobuzhi型大麥根內的NO含量在第1天達到最大值,而后隨著處理時間的延長,NO含量迅速下降,而對Cd敏感的dong17型大麥根內的NO含量在第10天達到最大,另外,weishuobuzhi和dong17葉內的NO含量都在第1天達到最大[31]。這些發現說明重金屬脅迫下,植物體內內源NO含量的變化與植物的種類以及基因型和植物組織類型有關。
盡管大量研究證明植物在重金屬脅迫下內源NO含量的變化受植物種類與基因型,重金屬濃度以及處理時間等因素的影響,然而對植物懸浮細胞而言,重金屬脅迫下其內源NO含量總會增加[32- 35],出現這一現象的原因可能是植物懸浮細胞缺少細胞與細胞之間的網絡調控。
3 外源NO增強植物對重金屬脅迫的抗性
3.1 外源NO緩解重金屬對植物細胞造成的氧化脅迫
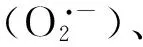


金屬硫蛋白(Metallothioneins, MTs)是一種小分子的富含半胱氨酸的金屬結合蛋白,通過巰基與重金屬結合形成無毒或低毒絡合物,從而避免有害重金屬對植物體的潛在毒性[59]。在動物細胞中,有研究發現在肝細胞中添加NO的供體V-PYRRO/NO,能夠大幅增強MTs基因的表達,從而減弱Cd的毒性,增強肝細胞對Cd脅迫的抗性[60]。原因可能是NO能夠替換與MTs結合的重金屬[61- 62],并且釋放的重金屬能夠進一步促進MTs基因的表達[62]。在植物細胞中可能也存在類似的機制,Wang等研究發現外源NO能夠提高MTs的含量并增強番茄對Cu脅迫的抗性,而對MTs敏感型番茄而言,外源NO并不能明顯地增強番茄對Cu脅迫的抗性,表明MTs在介導NO緩解重金屬脅迫上起著至關重要的作用[42]。
3.2 外源NO影響植物對重金屬的吸收以及重金屬在植物體內的分布
研究發現外源NO能夠影響植物對重金屬的吸收,從而調節植物對重金屬脅迫的抗性。如外源NO能夠減少苜蓿對Cd的吸收[47],減少小麥和豌豆對Zn的過量積累[63],抑制決明對鋁(Al)的吸收[64]。但也有研究表明外源NO緩解重金屬脅迫并不是通過抑制植物對重金屬的吸收。如外源NO緩解過量Cu對小麥種子萌發的抑制作用,并且通過增強SOD和CAT的活性緩解過量Cu對小麥種子造成的氧化脅迫,然而外源NO并不能抑制小麥種子對Cu的吸收[65];外源NO能夠緩解過量Cu對人參造成的毒害作用,但并不能顯著降低人參根細胞對Cu的吸收[46];外源NO供體硝普鈉(SNP)處理能夠緩解鉛(Pb)對擬南芥的毒害,但不能減少擬南芥對Pb的積累[66]。Xu等在研究中發現,對Cd的超富集植物龍葵而言,Cd脅迫能夠誘導顯著的生長抑制,促進H2O2的積累并破壞膜的完整性,而添加外源的NO供體亞硝基谷胱甘肽(GSNO)能夠增強SOD和CAT的活性,增加Pro含量,抑制H2O2的積累,提高膜的完整性,從而緩解Cd對龍葵的毒害,并且添加NO清除劑c-PTIO后能夠加重Cd對龍葵的毒害作用,這表明外源NO能夠緩解Cd脅迫對龍葵造成的傷害,增強龍葵對Cd脅迫的抗性。但另一方面添加NO清除劑c-PTIO能夠減少龍葵對Cd的吸收,添加外源的NO供體GSNO能夠增加龍葵對Cd的吸收,說明NO促進龍葵根部對Cd的吸收[67],這些研究暗示了外源NO可能存在相應的調節機制調節重金屬在植物體內的分布。
面對重金屬脅迫,植物細胞也有一系列的防御機制,以最大程度減少重金屬可能造成的傷害,其中作為抗重金屬脅迫的第一道屏障,細胞壁對吸收的重金屬具有束縛作用[68]。研究表明進入水稻細胞的Al 80%—85%分布在細胞壁,從而有效地阻止Al進入水稻細胞的細胞質[69],因此重金屬在植物細胞壁的沉積是一種十分重要的重金屬抗性機制。纖維素、半纖維素和果膠等是細胞壁的主要組成成分,研究發現外源NO能夠調節植物細胞細胞壁成分的代謝,如低濃度的NO供體SNP能夠促進番茄根部細胞壁纖維素的合成[70],增加土豆嫩葉胼胝質的積累和沉積,而高濃度的NO卻起相反的效果[71];另外外源NO能夠減少煙草BY- 2懸浮細胞細胞壁果膠、半纖維素和纖維素的含量[72],這些研究都暗示了外源NO可能通過調節植物細胞壁成分含量進而調節植物細胞對重金屬脅迫的抗性。實際上,Xiong等證實了在Cd脅迫下,外源NO能夠增加水稻根細胞細胞壁果膠和半纖維素的含量,增加Cd在根和莖細胞壁中的積累,減少Cd在葉片可溶性成分中的分布,從而增強水稻對Cd脅迫的抗性[73];Zhang等也發現外源NO能夠逆轉Al脅迫誘導的水稻根細胞細胞壁果膠和半纖維素含量的增加,并且顯著降低Al在水稻根尖和水稻幼苗細胞壁中的積累,從而減少Al脅迫誘導的根的生長抑制和膜脂過氧化,增強水稻對Al脅迫的抗性[74];另外外源NO能夠降低小麥和黑麥根尖細胞細胞壁對Al的吸附,增強Al脅迫抗性[75]。因此上述的外源NO緩解重金屬脅迫,但并不減少植物對重金屬的吸收可能是因為外源NO能夠調節植物細胞細胞壁成分,促進了重金屬在細胞壁的分布,減少了在細胞可溶性成分中的分布,但總體上并沒有減少植物體內的重金屬,但也可能是因為存在其他的調節機制。
4 內源NO參與調節植物重金屬脅迫抗性
盡管大量的研究表明低濃度的外源NO能夠緩解重金屬脅迫對植物造成的傷害,增強植物對重金屬脅迫的抗性,然而內源NO在調節植物重金屬脅迫抗性上的功能角色仍存在爭議。有研究發現內源NO是有益的,能夠緩解重金屬脅迫誘導的毒性,如Tian等發現Al脅迫能夠減少芙蓉葵體內的內源NO含量,并且抑制芙蓉葵根的伸長,添加NO的供體SNP能夠緩解Al對根的伸長抑制,而NO清除劑c-PTIO、NOS抑制劑L-NAME以及NR抑制劑鎢酸鹽(Tungstate)卻能夠加重Al的抑制作用或者抑制外源NO的緩解作用,表明內源NO能夠促進根的伸長,增強Al脅迫抗性[76];Qiu等研究發現微波處理能夠提高小麥抗氧化酶SOD、POD、CAT、APX、GPX和GR活性以及GSH、ASA和Car含量,緩解Cd脅迫對小麥的氧化傷害,而NO清除劑c-PTIO則能夠逆轉微波的緩解效果,表明內源NO能夠增強小麥體內的抗氧化系統,參與微波增強植物對重金屬脅迫的抗性反應[77];Talukdar等發現內源NO與外源NO都能緩解As對菜豆造成的生長抑制和氧化脅迫,增強菜豆As脅迫抗性[78];在薺菜中也有類似的發現,即外源NO與內源NO都能夠緩解Cd誘導的膜質過氧化,增強薺菜對Cd脅迫的抗性[79]。

植物螯合素(Phytochelatins, PCs)是一種由半胱氨酸、谷氨酸和甘氨酸組成的含巰基螯合多肽,GSH是植物螯合素的前體,在植物螯合素合成酶催化下合成植物螯合素,植物螯合素能夠與進入細胞內的重金屬離子螯合,并把重金屬隔離在液泡內,從而減輕重金屬對細胞質中活性物質的毒害,增強植物對重金屬脅迫的抗性[59]。NO能夠促進γ-ecs和gshs基因的表達,從而增加苜蓿根內GSH的含量[82],而GSH是植物螯合素的前體,因此可以推斷出NO也可能促進植物螯合素的合成,但事實并非如此,在100 μmol/L和150 μmol/L Cd脅迫下,擬南芥懸浮細胞內的植物螯合素含量大幅升高,而添加NOS抑制劑L-NAME后,植物螯合素含量進一步升高,表明內源NO抑制植物螯合素含量的升高,進一步研究發現是因為Cd誘導的內源NO能夠通過s-亞硝基化作用與植物螯合素的半胱氨酸殘基(Cys)結合形成NO-PC2、NO-PC3和NO-PC4,從而弱化植物螯合素對Cd的解毒作用,促進擬南芥懸浮細胞的細胞凋亡[33],Elviri等也證實了NO與植物螯合素的s-亞硝基化結合[83]。由此可見,內源NO能夠使植物螯合素s-亞硝基化,減弱植物螯合素對重金屬脅迫的解毒功能,從而促進重金屬對植物的毒害。
5 研究展望
盡管國內外已對NO在調節植物重金屬脅迫抗性方面做了大量研究,但是NO的具體作用機制仍然不是很清楚。建議未來相關研究應該加強以下幾個方面的工作:
(1) 重視NO與其他信號分子如Ca2+、茉莉酸(JA)、水楊酸(SA)和乙烯(ET)等存在的交叉調控。研究表明其他的信號分子也參與調節植物重金屬脅迫抗[84],并且NO可能與其他信號分子共同作用來調節植物重金屬脅迫抗性,因此加強NO與其他信號分子的交叉調控尤為必要。
(2) 加強對NO靶標分子的研究。NO作為一種信號分子,必然通過刺激靶標分子而發揮作用,而NO正是通過靶標分子進而調節植物對重金屬脅迫的抗性,因此加強對NO靶標分子的研究很有意義。
(3) 加強對內源NO作用機制的探討。重金屬脅迫下,內源NO對植物重金屬脅迫抗性起著更為重要的作用,并且研究發現內源NO對重金屬脅迫具有雙重作用,因此內源NO在重金屬脅迫抗性上具體的功能角色有待研究。
[1] Zheng C F, Jiang D, Liu F L, Dai T B, Liu W C, Jing Q, Cao W X. Exogenous nitric oxide improves seed germination in wheat against mitochondrial oxidative damage induced by high salinity. Environmental and Experimental Botany, 2009, 67(1): 222- 227.
[2] Bethke P C, Libourel I G L, Jones R L. Nitric oxide reduces seed dormancy inArabidopsis. Journal of Experimental Botany, 2006, 57(3): 517- 526.
[3] Takahashi S, Yamasaki H. Reversible inhibition of photophosphorylation in chloroplasts by nitric oxide. FEBS Letters, 2002, 512(1/3): 145- 148.
[4] He Y K, Tang R H, Yi H, Stevens R D, Cook C W, Ahn S M, Jing L, Yang Z, Chen L, Guo F, Fiorani F, Jackson R B, Crawford N M, Pei Z M. Nitric oxide represses theArabidopsisfloral transition. Science, 2004, 305(5692): 1968- 1971.
[5] Hu X, Neill S J, Tang Z, Cai W. Nitric oxide mediates gravitropic bending in soybean roots. Plant Physiology, 2005, 137(2): 663- 670.
[6] Guo F Q, Crawford N M.Arabidopsisnitric oxide synthase1 is targeted to mitochondria and protects against oxidative damage and dark-induced senescence. The Plant Cell, 2005, 17(12): 3436- 3450.
[7] Neill S J, Desikan R, Hancock J T. Nitric oxide signalling in plants. New Phytologist, 2003, 159(1): 11- 35.
[8] Hong J K, Yun B W, Kang J G, Raja M U, Kwon E, Sorhagen K, Chu C, Wang Y, Loake G J. Nitric oxide function and signalling in plant disease resistance. Journal of Experimental Botany, 2008, 59(2): 147- 154.
[9] Nathan C, Xie Q W. Nitric oxide synthases: roles, tolls, and controls. Cell, 1994, 78(6): 915- 918.
[10] Asai S, Ohta K, Yoshioka H. MAPK signaling regulates nitric oxide and NADPH oxidase-dependent oxidative bursts inNicotianabenthamiana. The Plant Cell, 2008, 20(5): 1390- 1406.
[11] Corpas F J, Barroso J, Carreras A, Valderrama R, Palma J, León A, Sandalio L, Del Río L A. Constitutive arginine-dependent nitric oxide synthase activity in different organs of pea seedlings during plant development. Planta, 2006, 224(2): 246- 254
[12] Del Rio L A, Corpas F J, Sandalio L M, Palma J M, Gomez M, Barroso J B. Reactive oxygen species, antioxidant systems and nitric oxide in peroxisomes. Journal of Experimental Botany, 2002, 53(372): 1255- 1272.
[13] Jasid S, Simontacchi M, Bartoli C G, Puntarulo S. Chloroplasts as a nitric oxide cellular source. Effect of reactive nitrogen species on chloroplastic lipids and proteins. Plant Physiology, 2006, 142(3): 1246- 1255.
[14] Eckardt N A. A functional nitric oxide synthase inOstreococcustauri. The Plant Cell, 2010, 22(11): 3507- 3507.
[15] Guo F Q, Okamoto M, Crawford N M. Identification of a plant nitric oxide synthase gene involved in hormonal signaling. Science, 2003, 302(5642): 100- 103.
[16] Zemojtel T, Frohlich A, Palmieri M C, Kolanczyk M, Mikula I, Wyrwicz L S, Wanker E E, Mundlos S, Vingron M, Martasek P, Durner J. Plant nitric oxide synthase: a never-ending story?. Trends in Plant Science, 2006, 11(11): 524- 525.
[17] Moreau M, Lee G I, Wang Y, Crane B R, Klessig D F. AtNOS/AtNOA1 is a functionalArabidopsisthalianacGTPase and not a nitric-oxide synthase. The Journal of Biological Chemistry, 2008, 283(47): 32957- 32967.
[18] de la Haba P, Agüera E, Benitez L, Maldonado J M. Modulation of nitrate reductase activity in cucumber (Cucumissativus) roots. Plant Science, 2001, 161(2): 231- 237.
[19] Rockel P, Strube F, Rockel A, Wildt J, Kaiser W M. Regulation of nitric oxide (NO) production by plant nitrate reductaseinvivoandinvitro. Journal of Experimental Botany, 2002, 53(366):103- 110.
[20] St?hr C, Ullrich W R. Generation and possible roles of NO in plant roots and their apoplastic space. Journal of Experimental Botany, 2002, 53(379): 2293- 2303.
[21] Huang J, Sommer E M, Kim-Shapiro D B, King S B. Horseradish peroxidase catalyzed nitric oxide formation from hydroxyurea. Journal of the American Chemical Society, 2002, 124(13): 34733480.
[22] Harrison R. Structure and function of xanthine oxidoreductase: Where are we now? Free Radical Biology and Medicine, 2002, 33(6): 774- 797.
[23] Mansuy D, Boucher J L. Oxidation of N-hydroxy-guanidines by cytochromes P450 and NO-synthases and formation of nitric oxide. Drug Metabolism Reviews, 2002, 34(3): 593- 606.
[24] Cooney R V, Harwood P J, Custer L J, Franke A A. Light-mediated conversion of nitrogen dioxide to nitric oxide by carotenoids. Environmental Health Perspectives, 1994, 102(5): 460- 462.
[25] Wendehenne D, Durner J, Klessig D F. Nitric oxide: a new player in plant signalling and defence responses. Current Opinion in Plant Biology, 2004, 7(4): 449- 455.
[26] Tun N N, Santa-Catarina C, Begum T, Silveira V, Handro W, Floh E I S, Scherer G F E. Polyamines induce rapid biosynthesis of nitric oxide (NO) inArabidopsisthalianaseedlings. Plant and Cell Physiology, 2006, 47(3): 346- 354.
[27] Bartha B, Kolbert Z, Erdei L. Nitric oxide production induced by heavy metals inBrassicajunceaL. Czern. andPisumsativumL.. Acta Biologica Szegediensis, 2005, 49(1/2): 9- 12.
[28] Rodríguez-Serrano M, Romero-Puertas M C, Pazmin? D M, Testillano P S, Risueo M C, Del Río L A, Sandalio L M. Cellular response of pea plants to cadmium toxicity: cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiology, 2009, 150(1): 229- 243.
[29] Leterrier M, Airaki M, Palma J M, Chaki M, Barroso J B, Corpas F J. Arsenic triggers the nitric oxide (NO) andS-nitrosoglutathione (GSNO) metabolism inArabidopsis. Environmental Pollution, 2012, 166: 136- 143.
[30] Besson-Bard A, Gravot A, Richaud P, Auroy P, Duc C, Gaymard F, Taconnat L, Renou J P, Pugin A, Wendehenne D. Nitric oxide contributes to cadmium toxicity inArabidopsisby promoting cadmium accumulation in roots and by up-regulating genes related to iron uptake. Plant Physiology, 2009, 149(3): 1302- 1315.
[31] Chen F, Wang F, Sun H Y, Cai Y, Mao W H, Zhang G P, Vincze E, Wu F B. Genotype-dependent effect of exogenous nitric oxide on Cd-induced changes in antioxidative metabolism, ultrastructure, and photosynthetic performance in barley seedlings (Hordeumvulgare). Journal of Plant Growth Regulation, 2010, 29(4): 394- 408.
[32] Kopyra M, Stachoń-Wilk M, Gwóz′dz′ E A. Effects of exogenous nitric oxide on the anti-oxidant capacity of cadmium-treated soybean cell suspension. Acta Physiologiae Plantarum, 2006, 28(6):525- 536.
[33] Michele R D, Vurro E, Rigo C, Elviri L, Valentin M D, Careri M, Zottini M, Sanità di Toppi L, Schiavo F L. Nitric oxide is involved in cadmium-induced programmed cell death inArabidopsissuspension cultures. Plant Physiology, 2009, 150(1): 217- 228.
[34] Balestrazzi A, Macovei A, Testoni C, Raimondi E, Donà M, Carbonera D. Nitric oxide biosynthesis in white poplar (PopulusalbaL.) suspension cultures challenged with heavy metals. Plant Stress, 2009, 3(1): 1- 6.
[35] Ma W W, Xu W Z, Xu H, Chen Y S, He Z Y, Ma M. Nitric oxide modulates cadmium influx during cadmium-induced programmed cell death in tobacco BY- 2 cells. Planta, 2010, 232(2): 325- 335.
[36] Mittler R, Vanderauwera S, Gollery M, Breusegem F V. Reactive oxygen gene network of plants. Trends in Plant Science, 2004, 9(10): 490- 498.
[37] Ammar W B, Nauairi I, Zarrouk M, Jamel F. Cadmium stress induces changes in the lipid composition and biosynthesis in tomato (LycopersiconesculentumMill.) leaves. Plant Growth Regulation, 2007, 53(2): 75- 85.
[38] Romero-Puertas M C, Palma J M, Gomez M, Del Rio L A, Sandalio L M. Cadmium causes the oxidative modification of proteins in pea plants. Plant Cell and Environment, 2002, 25(5): 677- 686.
[39] Britt A B. Molecular genetics of DNA repair in higher plants. Trends in Plant Science, 1999, 4(1): 20- 25.
[40] Gill S S, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry, 2010, 48(12): 909- 930.
[41] Papadakis A K, Roubelakis-Angelakis K A. Polyamines inhibit NADPH oxidase-mediated superoxide generation and putrescine prevents programmed cell death induced by polyamine oxidase-generated hydrogen peroxide. Planta, 2005, 220(6): 826- 837.
[42] Wang L N, Yang L M, Yang G J, Li X G, Song Y P, Wang X F, Hu X Y. Involvements of H2O2and metallothionein in NO-mediated tomato tolerance to copper toxicity. Journal of Plant Physiology, 2010, 167(15): 1298- 1306.
[43] Panda P, Nath S, Chanu T T, Sharma G D, Panda S K. Cadmium stress-induced oxidative stress and role of nitric oxide in rice (OryzasativaL.). Acta Physiologiae Plantarum, 2011, 33(15): 1737- 1747.
[44] Laspina N V, Groppa M D, Tomaro M L, Benavides M P. Nitric oxide protects sunflower leaves against Cd-induced oxidative stress. Plant Science, 2005, 169(2): 323- 330.
[45] Kazemi N, Khavari-Nejad R A, Fahimi H, Saadatmand S, Nejad-Sattari T. Effects of exogenous salicylic acid and nitric oxide on lipid peroxidation and antioxidant enzyme activities in leaves ofBrassicanapusL. under nickel stress. Scientia Horticulturae, 2010, 126(3): 402- 407.
[46] Tewari R K, Hahn E J, Paek K Y. Modulation of copper toxicity-induced oxidative damage by nitric oxide supply in the adventitious roots ofPanaxginseng. Plant Cell Reports, 2008, 27(1): 171- 181.
[47] Xu J, Wang W Y, Yin H X, Liu X J, Sun H, Min Q. Exogenous nitric oxide improves antioxidative capacity and reduces auxin degradation in roots ofMedicagotruncatulaseedlings under cadmium stress. Plant and Soil, 2010, 326(1/2): 321- 330.
[48] Filippou P, Antoniou C, Fotopoulos V. The nitric oxide donor sodium nitroprusside regulates polyamine and proline metabolism in leaves ofMedicagotruncatulaplants. Free Radical Biology and Medicine, 2013, 56: 172- 183.
[49] Groppa M D, Rosales E P, Iannone M F, Benavides M P. Nitric oxide, polyamines and Cd-induced phytotoxicity in wheat roots. Phytochemistry, 2008, 69(14): 2609- 2615.
[50] Zhang L P, Mehta S K, Liu Z P, Yang Z M. Copper-Induced proline synthesis is associated with nitric oxide generation inChlamydomonasreinhardtii. Plant and Cell Physiology, 2008, 49(3): 411- 419.
[51] Singh H P, Batish D R, Kaur G, Arora K, Kohli R K. Nitric oxide (as sodium nitroprusside) supplementation ameliorates Cd toxicity in hydroponically grown wheat roots. Environmental and Experimental Botany, 2008, 63(1/3): 158- 167.
[52] Singh H P, Kaur S, Batish D R, Sharma V P, Sharma N, Kohli P K. Nitric oxide alleviates arsenic toxicity by reducing oxidative damage in the roots ofOryzasativa(rice). Nitric Oxide, 2009, 20(4): 289- 297.
[53] Srivastava S, Dubey R S. Nitric oxide alleviates manganese toxicity by preventing oxidative stress in excised rice leaves. Acta Physiologiae Plantarum, 2012, 34(2): 819- 825.
[54] Hsu Y T, Kao C H. Cadmium toxicity is reduced by nitric oxide in rice leaves. Plant Growth Regulation, 2004, 42(3): 227- 238.
[55] Caro A, Puntarulo S. Nitric oxide decreases superoxide anion generation by microsomes from soybean embryonic axes. Physiologia Plantarum, 1998, 104(3): 357- 364.
[56] Delledonne M, Zeier J, Marocco A, Lamb C. Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proceedings of the National Academy of Sciences of the United States of America, 2001, 98(23): 13454- 13459.
[57] Martinez G R, Mascio P D, Bonini M G, Augusto O, Briviba K, Sies H, Maurer P, R?thlisberger U, Herold S, Koppeno W H. Peroxynitrite does not decompose to singlet oxygen (1ΔgO2) and nitroxyl (NO-). Proceedings of the National Academy of Sciences of the United States of America, 2000, 97(19): 10307- 10312.
[58] Thompson J E, Legge R L, Barber R F. The role of free radicals in senescence and wounding. New Phytologist, 1987, 105(3): 1044- 1047.
[59] Zhang H Y, Xu W Z, Guo G B, He Z Y, Ma M. Coordinated responses of phytochelatins and metallothioneins to heavy metals in garlic seedlings. Plant Science, 2005, 169(6): 1059- 1065.
[60] Qu W, Liu J, Fuquay R, Shimoda R, Sakurai T, Saavedra J E, Keefer L K, Waalkes M P. The nitric oxide prodrug, V-PYRRO/NO, protects against cadmium toxicity and apoptosis at the cellular level. Nitric Oxide, 2005, 12(2): 114- 120.
[61] Misra R R, Hochadel J F, Smith G T, Cook J C, Waaalkes M P, Wink D A. Evidence that nitric oxide enhances cadmium toxicity by displacing the metal from metallothionein. Chemical Research in Toxicology, 1996, 9(1): 326- 332.
[62] Katakai K, Liu J, Nakajima K, Keefer L K, Waalkes M P. Nitric oxide induces metallothionein (MT) gene expression apparently by displacing zinc bound to MT. Toxicology Letters, 2001, 119(2): 103- 108.
[63] Abdel-Kader D E Z. Role of nitric oxide, glutathione and sulfhydryl groups in zinc homeostasis in plants. American Journal of Plant Physiology, 2007, 2(2): 59- 75.
[64] Wang Y S, Yang Z M. Nitric oxide reduces aluminum toxicity by preventing oxidative stress in the roots ofCassiatoraL.. Plant and Cell Physiology, 2005, 46(12): 1915- 1923.
[65] Hu K D, Hu L Y, Li Y H, Zhang F Q, Zhang H. Protective roles of nitric oxide on germination and antioxidant metabolism in wheat seeds under copper stress. Plant Growth Regulation, 2007, 53(3): 173- 183.
[66] Phang I C, Leung D W M, Taylor H H, Burritt D J. The protective effect of sodium nitroprusside (SNP) treatment onArabidopsisthalianaseedlings exposed to toxic level of Pb is not linked to avoidance of Pb uptake. Ecotoxicology and Environmental Safety, 2011, 74(5): 1310- 1315.
[67] Xu J, Wang W J, Sun J H, Zhang Y, Ge Q, Du L G, Yin H X, Liu X J. Involvement of auxin and nitric oxide in plant Cd-stress responses. Plant and Soil, 2011, 346(1/2): 107- 119.
[68] Lux A, Martinka M, Vaculk M, White P J. Root responses to cadmium in the rhizosphere: a review. Journal of Experimental Botany, 2011, 62(1): 21- 37.
[69] Yang J L, Li Y Y, Zhang Y J, Zhang S S, Wu Y R, Wu P, Zheng S J. Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiology, 2004, 146(2): 602- 611.
[70] Correa-Aragunde N, Lombardo C, Lamattina L. Nitric oxide: an active nitrogen molecule that modulates cellulose synthesis in tomato roots. New Phytologist, 2008, 179(2): 386- 396.
[71] París R, Lamattina L, Casalongue C A. Nitric oxide promotes the wound-healing response of potato leaflets. Plant Physiology and Biochemistry, 2007, 45(1): 80- 86.
[72] Pacoda D, Montefusco A, Piro G, Dalessandro G. Reactive oxygen species and nitric oxide affect cell wall metabolism in tobacco BY- 2 cells. Journal of Plant Physiology, 2004, 161(10): 1143- 1156.
[73] Xiong J, An L Y, Lu H, Zhu C. Exogenous nitric oxide enhances cadmium tolerance of rice by increasing pectin and hemicellulose contents in root cell wall. Planta, 2009, 230(4): 755- 765.
[74] Zhang Z Y, Wang H H, Wang X M, Bi Y R. Nitric oxide enhances aluminum tolerance by affecting cell wall polysaccharides in rice roots. Plant Cell Reports, 2011, 30(9): 1701- 1711.
[75] 何虎翼, 何龍飛, 黎曉峰, 顧明華. 鋁脅迫下硝普鈉對黑麥和小麥根尖細胞壁鋁吸附的影響. 廣西農業生物科學, 2007, 26(3): 235- 239, 249- 249.
[76] Tian Q Y, Sun D H, Zhao M G, Zhang W H. Inhibition of nitric oxide synthase (NOS) underlies aluminum-induced inhibition of root elongation inHibiscusmoscheutos. New Phytologist, 2007, 174(2): 322- 331.
[77] Qiu Z B, Guo J L, Zhang M M, Lei M Y, Li Z L. Nitric oxide acts as a signal molecule in microwave pretreatment induced cadmium tolerance in wheat seedlings. Acta Physiologiae Plantarum, 2013, 35(1): 65- 73.
[78] Talukdar D. Arsenic-induced oxidative stress in the common bean legume,PhaseolusvulgarisL. seedlings and its amelioration by exogenous nitric oxide. Physiology and Molecular Biology of Plants, 2013, 19(1): 69- 79.
[79] Verma K, Mehta S K, Shekhawat G S. Nitric oxide (NO) counteracts cadmium induced cytotoxic processes mediated by reactive oxygen species (ROS) in Brassica juncea: cross-talk between ROS, NO and antioxidant responses. Biometals, 2013, 26(2):255- 269.
[80] Ye Y, Lin Z, Xing D. Nitric oxide promotes MPK6-mediated caspase- 3-like activation in cadmium-inducedArabidopsisthalianaprogrammed cell death. Plant, Cell and Environment, 2013, 36(1): 1- 15.
[81] Arasimowicz-Jelonek M, Floryszak-Wieczorek J, Deckert J, Rucińska-Sobkowiak R, Gzyl J, Pawlak-Spradaa S, Abramowskib D, Jelonekc T, Gwóz′dz′a E A. Nitric oxide implication in cadmium-induced programmed cell death in roots and signaling response of yellow lupine plants. Plant Physiology and Biochemistry, 2012, 58: 124- 134.
[82] Innocenti G, Pucciariello C, Gleuher M L, Hopkins J, De Stefano M, Delledonne M, Puppo A, Bandouin E, Frendo P. Glutathione synthesis is regulated by nitric oxide inMedicagotruncatularoots. Planta, 2007, 225(6): 1597- 1602.
[83] Elviri L, Speroni F, Careri M, Mangia A, Sanità di Toppi L, Zottini M. Identification ofinvivonitrosylated phytochelatins inArabidopsisthalianacells by liquid chromatography-direct electrospray-linear ion trap-mass spectrometry. Journal of Chromatography A, 2010, 1217(25): 4120- 4126.
[84] Maksymiec W. Signaling responses in plants to heavy metal stress. Acta Physiologiae Plantarum, 2007, 29(3):177- 187.
Advances on effects of nitric oxide on resistances of plants to heavy metal stress
XIA Haiwei, SHI Guoxin*, HUANG Min, WU Juan
JiangsuKeyLaboratoryofBiodiversityandBiotechnology,CollegeofLifeScience,NanjingNormalUniversity,Nanjing210023,China
Heavy metal pollution has become an increasingly serious environmental problem because heavy metal can be easily taken up by plants, leading to inhibition of plant growth and development. Hence, it is necessary to investigate resistances of plants to heavy metal stress. As an important signaling molecule, nitric oxide (NO) is involved in the regulation of multiple plant responses to a variety of abiotic and biotic stresses. Recently, an increasing number of articles have reported the effects of NO on resistances of plants to heavy metal stress. However, studies which systematically summarize the molecular mechanisms of NO on resistances of plants to heavy metal stress are quite limited. This research mainly reviews the pathways of NO production, changes of endogenous NO contents under heavy metal stress and influences of exogenous and endogenous NO on resistances to heavy metal stress. The sources of NO production in plants involve not only enzymatic reaction pathways which include nitric oxide synthase (NOS), nitrate reductase (NR), nitrite reductase (Ni-NOR) pathways and etc. but also non-enzymatic reaction pathways. Many authors have noted discrepant reports on the effects of heavy metal stress on endogenous NO content in plants and the observed differences in endogenous NO accumulation are frequently ascribed to the use of different duration of treatment, heavy metal concentrations, species and genotypes of plants and varieties of plant tissues. Interestingly, all plant cell suspensions show a visible increase in endogenous NO accumulation under heavy metal stress, which is attributed to the fact that they are lacking of network regulation between cells and cells. Meanwhile, it has been demonstrated that exogenous NO could enhance antioxidant defence system of plant cells, act as an antioxidant promoting direct scavenging of reactive oxygen species, induce metallothioneins (MTs) gene expression by displacing heavy metal bound to MTs, and affect the uptake of heavy metal into plants and the distribution of heavy metal in plant cells though regulating the metabolism of cell wall composition, consequently relieve heavy metal toxicity and enhance resistances of plants to heavy metal stress. However, the functional roles of endogenous NO in regulating resistances of plants to heavy metal stress are controversial. Some research show that endogenous NO is helpful for alleviating heavy metal-induced toxicity. On the contrary, some evidences indicate that endogenous NO is harmful, and participates in heavy metal-induced cell toxicity and programmed cell death through accelerating the absorption of heavy metal andS-nitrosylation of phytochelatins. These conflicting data suggest that NO may have a dual effect on resistances to heavy metal stress, but great efforts are required in order to clarify this speculation. Finally, interrelated perspectives are also discussed to further study the relationship between NO and resistances to heavy metal stress.
exogenous NO; endogenous NO; heavy metal stress; resistances
江蘇高校優秀學科建設工程資助項目(164320H106)
2013- 06- 24;
2014- 05- 30
10.5846/stxb201306241770
*通訊作者Corresponding author.E-mail: gxshi@njnu.edu.cn
夏海威,施國新,黃敏,吳娟.一氧化氮對植物重金屬脅迫抗性的影響研究進展.生態學報,2015,35(10):3139- 3147.
Xia H W, Shi G X, Huang M, Wu J.Advances on effects of nitric oxide on resistances of plants to heavy metal stress.Acta Ecologica Sinica,2015,35(10):3139- 3147.

