Optim ization of Supercritical CO2 Extraction of Tricholoma matsutake EssentialOil via a Response Surface M ethod and Its Chem ical Com position Analysis
BIYong-xian,LIU Xiao-ying,LIU Guang-rong,HE Cong-fen* Beijing Key Laboratory of Plant Resources Research and Development,Beijing Technology and Business University,Beijing 00048,China; Infinitus(China)Company Ltd,Guangzhou 5063,China
畢永賢1,劉曉英2,劉光榮2,何聰芬1*
1北京工商大學理學院北京市植物資源研究開發重點實驗室,北京100048;2無限極(中國)有限公司,廣州510623
Introduction
Mushrooms have been widely used since ancient times not only as food or food flavorings but also formedicinal and functional purposes.Tricholoma matsutake Sing.(pinemushroom)is considered to be one of the most valuable species in the world and it exhibits a characteristic and delicate flavor,aswell as several biological activities,such as hypocholesterolemic,antioxidant,immunomodulating and antitumor effects in humans[1-3].Interestingly,its essential oilmay also have a whitening effect on the skin.T.matsutakewas widely distributed worldwide,especially in China,Korea,Japan,Canada,the United States and the Nordic countries.Themorphology,ecology andmycelial characteristics of T.matsutake from different regions differ to varying degrees.In China,T.matsutake is highly valued as its growth requires an unique environment and specific climatic conditions.
Supercritical fluid extraction(SFE)is amethod that is used for the extraction of valuable constituents from natural substances in high yield and quality.For this reason it has been widely studied as an alternative to conventional oil extraction methods[4].The combined liquid-like solvating capabilities and gas-like transport properties of supercritical fluidsmake them particularly suitable for extracting bioactive compounds from plant tissueswith a high degree of recovery in a short period of time[5,6].There are many variables in the SFE process thatmay affect the efficiency of the extraction,such as pressure,extraction time,and the bed diameter and height[7].Optimization of the experimental conditions is a critical strategy in developing a successful SC-CO2process.Response surfacemethodology(RSM)has been demonstrated as a powerful tool for determining factor effects and their interactions,which allows process optimization to be conducted effectively.RSM is a faster method for gathering research results than classic one-variable-at-a-time or full-factor experimentation[8].Three-level Box-Behnken factorial design(BBD)from RSM has been used previously to optimize the principal extraction conditions of pressure,temperature and the concentration of amodifier(ethanol)[9].The objective of this study was to obtain the essential oil of T.matsutake.Furthermore,the chemical composition of the essential oil was analyzed.This result will facilitate further research in elucidating the relationships between the chemical composition of essential oils and their activities.
M aterials and M ethods
Instruments and reagents
Tricholoma matsutake Sing.was obtained from Ganzi(Sichuan,China)and identified by Congfen He.Analytical reagent grade ethanol and CO2were purchased from Beijing Chemical Works.SC-CO2extraction equipmentwas purchased from the United States of A-merica application to the separation of company(SFEII).GC-MS was performed using a P&T-GC/MSQP2010 gas chromatography.
Supercritical fluid extraction
A laboratory-scale SFE system was used in this study.The sample of T.matsutake particles(50 g)were loaded into a 300 mL high-pressure extraction vessel.Liquid CO2was pumped into the vessel.Mono-factor analyses were made to optimize the parameters of SFE:pressure(25-35 MPa),temperature(40-50 ℃),extraction time(60 min),size ofmaterials(30 mesh),CO2flow rate(2 L/min),entrainer volume(50 mL ethanol),concentration ofmodifier(75-95 vol.%ethanol).Response surface methodology was employed to optimize the operating conditions of the SFE process with three independent variables,namely temperature(40-50 ℃,X1),pressure(25-35 MPa,X2)and the concentration of the modifier(ethanol;75-95 vol.% ,X3),to obtain the highest crude extraction yield(Table 1).
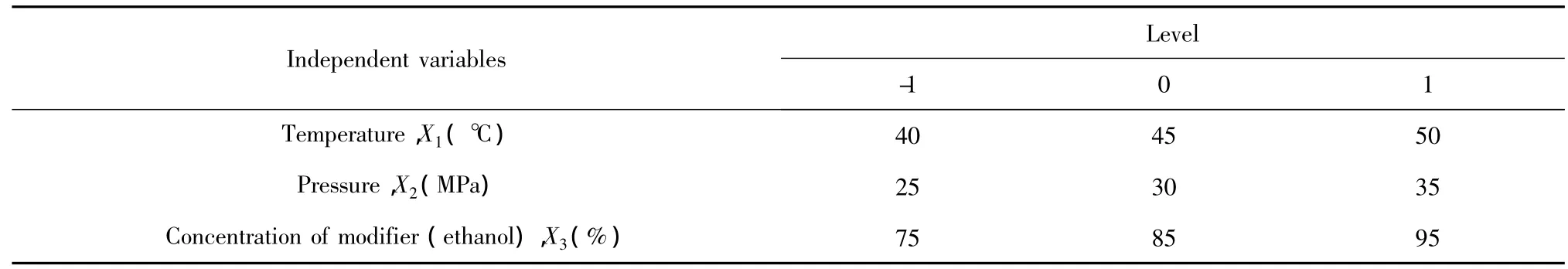
Table 1 Levels of independent variables established according to the Box-Behnken factorial design
Box-Behnken factorial design(BBD)was used to design the experiments.It generated seventeen treatments with five replicates at the center point to estimate the reproducibility of the method(Table 2).An experimental plan was designed and the results that we obtained were analyzed using Design-Expert(version 8.0)software to build models and to plot the three-dimensional response surface plots.A multiple regression model(second-order polynomial regressionmodel)was assumed to predict the Y variable.Regression equation and analysis of variance(ANOVA)were conducted to determine the regression coefficientswith statistical significance in terms of themodel and for fitting themathematicalmodels to the experimental data.The generalized polynomialmodel proposed for predicting the response variables as a function of the independent variableswas given by:
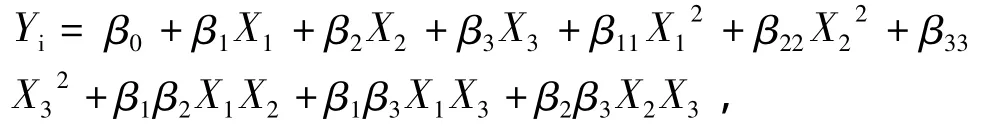
where Yiwas the predicted response;β0was the offset term;β1,β2and β3were the regression coefficients for the linear effect terms;β11,β22and β33were the quad-ratic effects;and β1β2,β1β3and β2β3were the interaction effects.In thismodel,X1,X2and X3were the independent variables.
Chem ical com position analysis
GC-MS was performed using a P&T-GC/MS-QP2010 gas chromatograph equipped with an Rxi-5MS capillary column(30 m × 0.25 mm;film thickness 0.25μm).The carrier gas was helium and the flow rate was 1.0 mL/min.The column temperature was initially 40 ℃for 2 min and then increased to 150℃at a rate of 10℃/min for 2 min,then 200℃ at a rate of 10℃/min for 2 min,then 250℃ at a rate of6 ℃/min for 2min,then 280℃ at a rate of 4℃/min for 4 min,then 300℃ at a rate of 10 ℃/min for 10 min,and finally 320℃ at a rate of 10 ℃/min for 3 min.The extractswere diluted in amethanol-dichloromethane solution and 1.0 μL of the diluted sample was injected with a split ratio of 1:10.The injector was set at 280 ℃ and the MS source at 200℃.The compounds of the essential oil were identified by comparing theirmass spectra with an MS library(NIST database).
Results and Discussion
Prelim inary analysis of response surface
The results of different sample runs were shown in Table 2.An ANOVA was conducted to determine which process variables had a significant effect on the yield.As shown in Table 3,the estimated regression coefficients,the corresponding R2,RAdj2and P values,and a lack-of-fit test for the reduced response surface model were displayed.
M odel fitting
The mathematical model representing the extraction yield of T.matsutake as a function of the independent variableswas expressed by the following equation:
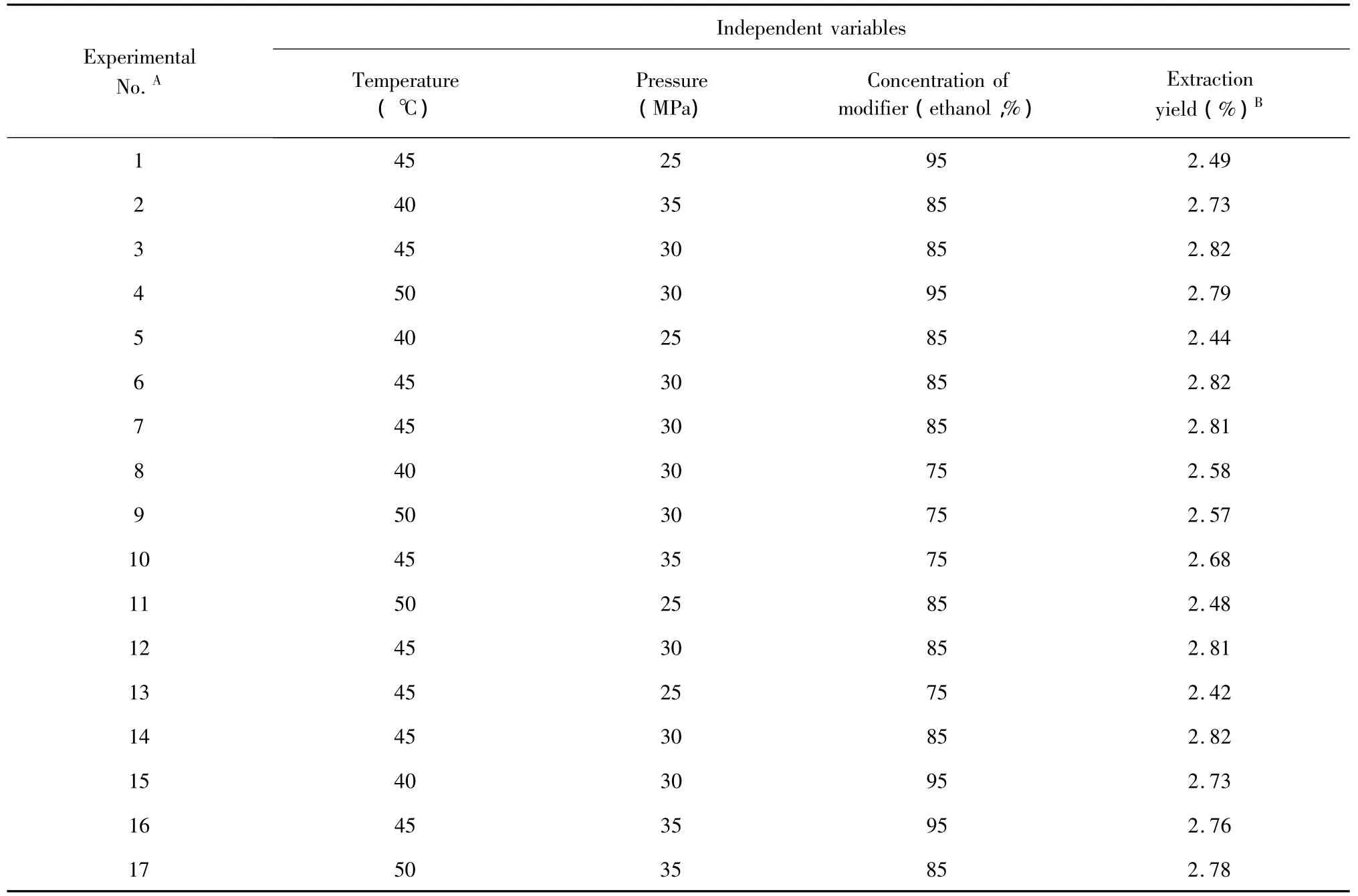
Table 2 Box-Behnken factorial design of three independent variables and the response of the crude extraction yield of T.matsutake
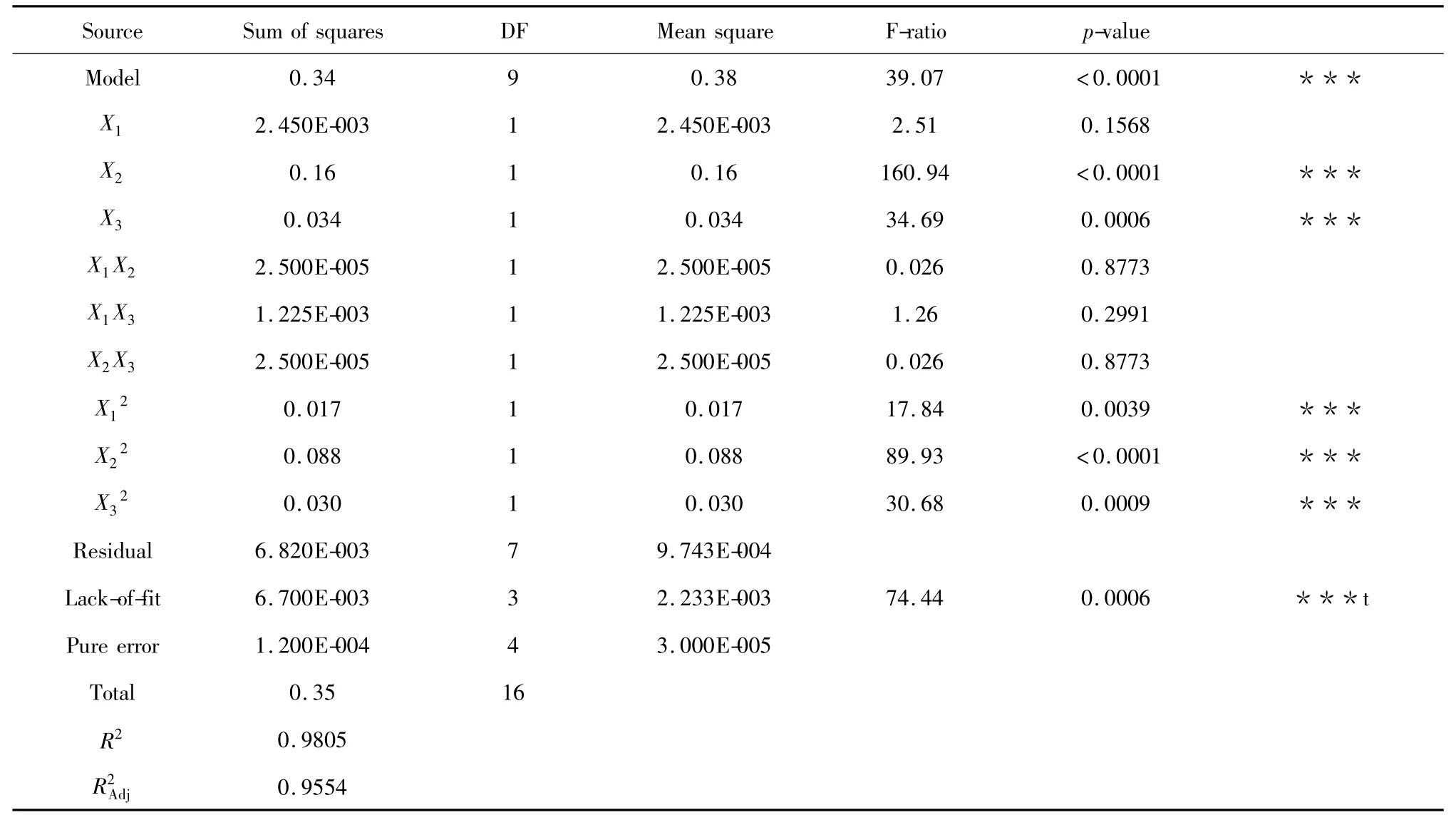
Table 3 Regression coefficients of the final regression model and ANOVA for the crude extraction yield in the Box-Behnken factorial design
Y=-13.61706 +0.20205X1+0.36545X2+0.13248X3+1.00000E-004X1X2+3.50000E-004X1X3+5.00000E-005X2X3– 2.57000E-003X12–5.77000E-003X22– 8.42500E-004X32,where Y was the extraction yield of T.matsutake,and X1,X2and X3were the non-coded variables for temperature,pressure and the concentration of the modifier(ethanol),respectively.
In general,exploration and optimization of a fitted response surface would produce poor or misleading results unless themodel exhibited a good fit,whichmade checking themodel adequacy essential.The p-value of themodelwas less than 0.05,which indicated that the model fit was sufficient(Table 3).Meanwhile,the lack-of-fit value of the model was significant(0.0006),indicating that themodel equation was adequate for predicting the crude extraction yield under any combination of values of the variables(Table 3).The coefficient(R2)of determination was defined as the ratio of the explained variation to the total variation and was ameasurement of the fitness degree.By ANOVA,the R2value of this model was determined to be 0.9805,which showed that the regression model accurately defined the true behavior of the system.Numerical optimization was carried out by response optimizer to predict the optimal conditions.The optimal conditionswere identified as 32.46 MPa pressure,46.01 ℃temperature and 89.15 vol.%ethanol.The extraction yield was 2.87%under this condition.
Effects of variables on the extraction yield
The effects of the pressure,temperature and concentration of themodifier(ethanol)on the crude extraction yield,and their interactions,were shown in Fig.1.An increasing temperature resulted in a higher extraction yield,which reached amaximum when the temperature reached 46.01 ℃,with no significant improvement thereafter(Fig.1a,b).As shown in Fig.1a and c,there was an optimum value for the pressure that resulted in the highest extraction yield of the bioactive compounds(32.46 MPa).Values lower or higher than this led to a decrease in the extraction yield.This optimum value for the pressure varied when the temperature and concentration of ethanol were varied.Fig.1b and c showed the effect of the concentration of the modifier(ethanol)on the extraction yield[10-12].An optimum value of 89.15 vol.%ethanolwas noted.
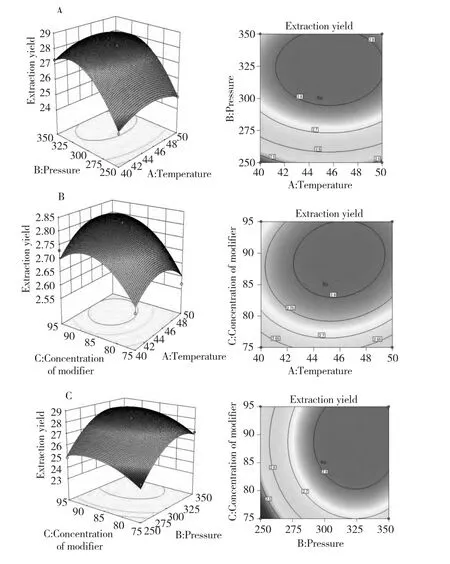
Fig.1 Response surface plots:a.yield vs.extraction temperature and pressure at 85%ethanol;b.yield vs.extraction temperature and concentration ofmodifier(ethanol)at a constant pressure of 300 bar;c.yield vs.extraction pressure and concentration ofmodifier(ethanol)at a constant tem perature of 45℃
Chem ical com position analysis
Themain extract of T.matsutake essential oil via traditional distillation method was the volatile and dissolve component,and the extraction yield was about1.12% .In contrast,the extraction yield was 2.87%via a supercritical CO2extraction[13].
The extract obtained under the optimal SC-CO2treatment conditions(pressure:32.5 MPa,temperature:46℃,ethanol concentration:89 vol.%)was analyzed by GC-MS to identify and quantify themajor bioactive flavonoids.In total,twenty two compounds were identified and quantified(Table 4)[13-16].The results showed that9,12-octadecadienoic acid,ergosterol and 2,3-dihydroxypropyl 9,12-octadecadienoate were the major constituents in the extractof T.matsutake.Many studies had shown that 9,12-octadecadienoic acid and ergosterol can inhibitmelanin synthesis.These components maybe the reason why T.matsutake.had a whitening effect on the skin.These resultswould facilitate further research in determining the efficacy of T.matsutake.essential oil in skin whitening.
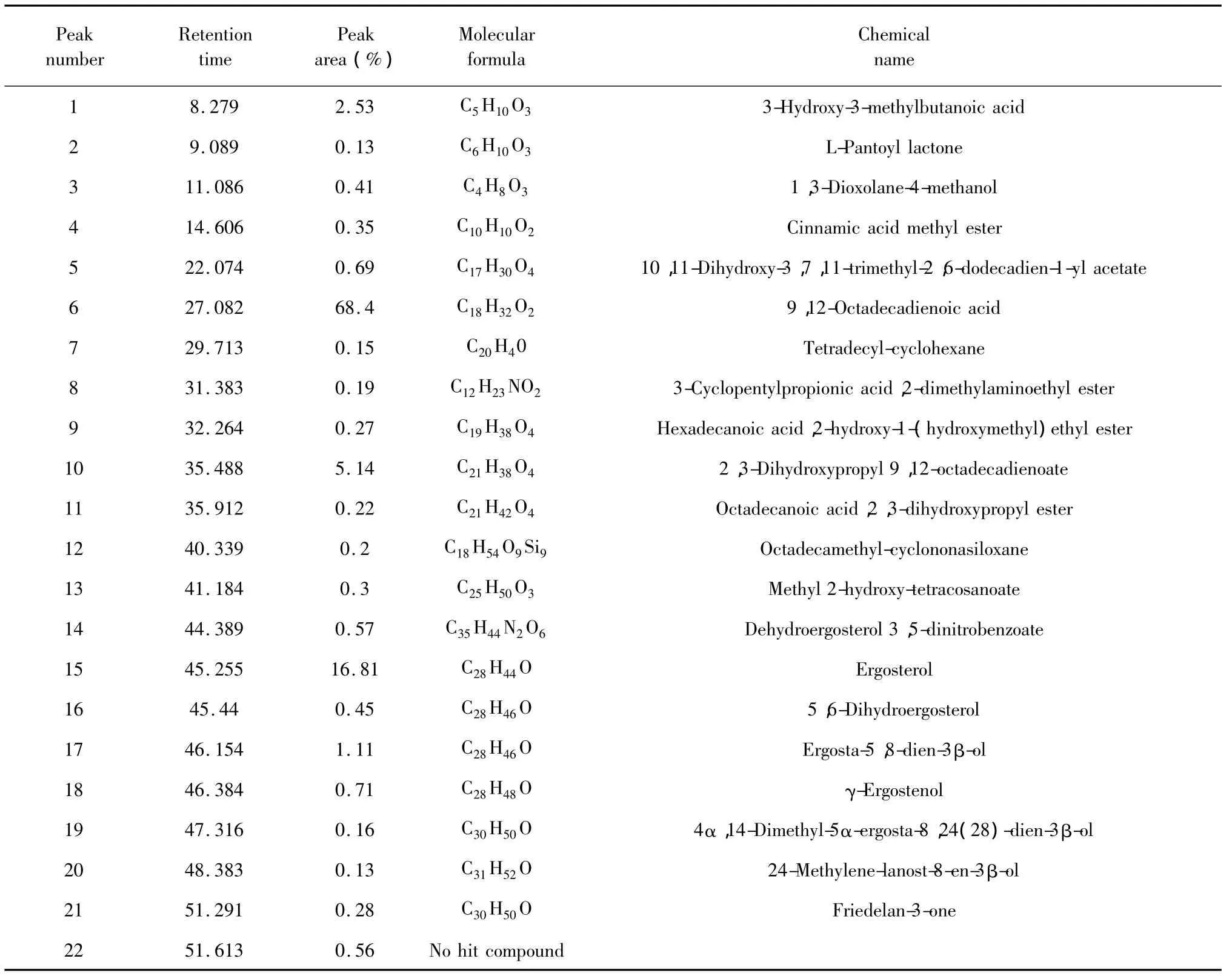
Table 4 Results of chem ical com positions analysis
Conclusion
This study indicated that a quadratic polynomialmodel could be successfully employed to optimize the extraction conditions of T.matsutake essential oil by supercritical carbon dioxide extraction.From the response surface plots,three factors(temperature,pressure and the concentration of themodifier)were found to significantly influence the yield of the essential oil,both independently and interactively.Under the optimal conditions,the experimental values agreed with the predicted values.Thus,thismethodology was a successmodel to examine the non-linear nature of independent variables and their effect in short-term experiments.Furthermore,twenty two compounds were identified and quantified by GC-MS.The results showed that 9,12-octadecadienoic acid,ergosterol and 2,3-dihydroxypropyl 9,12-octadecadienoate were the major constituents in the T.matsutake extract.Many studies showed that 9,12-octadecadienoic acid and ergosterol can inhibitmelanin synthesis.Further research was currently ongoing to elucidate the relationships between the chemical composition and its activity.
1 Mau JL,Lin HC,Song SF.Antioxidant properties of several specialtymushrooms.Food Res Int,2002,35:519-526.
2 Hoshi H,Yagi Y,Iijima H,et al.Isolation and characterization of a novel immunomodulatory R-glucan-protein complex from the mycelium of Tricholoma matsutake in basidiomycetes.J Agric Food Chem,2005,53:8948-8956.
3 Ebina T,Kubota T,Ogamo N,et al.Antitumor effect of a peptide-glucan preparation extracted from amycelium of Tricholomamatsutake(S.Ito and Imai)Sing.Biotherapy,2002,16:255-259.
4 Rizvi S.Supercritical fluid processing of food and biomaterials.Blackie Acade Professional,1994:44-50.
5 Casas L,Mantell C,Rodroguez M,et al.Supercritical fluid extraction of bioactive compounds from sunflower leaves with carbon dioxide and water on a pilot plant scale,J.Supercritical Fluids,2008,45:37-42.
6 Shucheng L,Feng Y,Chaohua Z,et al.Optimization of process parameters for supercritical carbon dioxide extraction of Passiflora seed oil by response surfacemethodology,J.Supercritical Fluids,2009,48:9-14.
7 Liu J,Han B,Li G,et al.Solubility of the non-ionic surfactant tetraethylene glycol n-laurel ether in supercritical CO2 with n-pentanol,Fluid Phase Equilibrium,2001,15:247-254.
8 Mani S,Jaya S,Vadivambal R.,etal.Optimization of solvent extraction ofmoringa(Moringa oleifera)seed kernel oil using response surfacemethodology.Food Bioprod Proc,2007,85:328-335.
9 Li G,Song C,You J,et al.Optimization of red pepper seed oil extraction using supercritical CO2and analysis of the composition by reversed phase HPLC-FLD-MS/MS.Int J Food Sci Technol,2011,46:44-51.
10 Huang W,Xue A,Niu H,et al.Optimized ultrasonic-assisted extrction of flavonoids from Folium Eucommiae and evaluation of antioxidantactivity inmulti-test systems in vitro.Food Chem,2009,114:1147-1154.
11 Pourmortazavi S,Hajimirsadeghi S.Supercritical fluid extraction in plant essential and volatile oil analysis.JChromatogr A,2007,1162:2-24.
12 Gomes P,Mata V,Rodrigues A,etal.Production of rose geranium oil using supercritical fluid extraction.J Supercrit Fluids,2007,41:50-60.
13 Pinho P,Ribeiro B,Goncalves R,et al.Correlation between the pattern volatiles and the overall aroma of wild edible mushrooms.JAgric Food Chem,2008,56:1704-1712.
14 Cho I,Kim S,Choi H,et al.Characterization of aroma-active compounds in raw and cooked pine-mushrooms(Tricholoma matsutake Sing.).JAgric Food Chem,2006,54:6332-6335.
15 Cho I,ChoiH,Kim Y,etal.Difference in the volatile composition of pine-mushrooms(Tricholoma matsutake Sing.)according to their grades.J Agric Food Chem,2006,54:4820-4825.
16 Cho I,Lee S,Kim S,et al.Differentiation of aroma characteristics of pine-mushrooms(Tricholoma matsutake Sing.)of different grades using gas chromatography-olfactometry and sensory analysis.JAgric Food Chem,2007,55:2323-2328.

