PAX2穩(wěn)轉(zhuǎn)細胞系構(gòu)建及對細胞遷移和侵襲能力的作用研究
·論著·
PAX2穩(wěn)轉(zhuǎn)細胞系構(gòu)建及對細胞遷移和侵襲能力的作用研究
李里,宮亮,吳玉斌
作者單位:121000遼寧省錦州市,遼寧醫(yī)學(xué)院附屬第一醫(yī)院風(fēng)濕免疫科(李里),耳鼻喉科(宮亮);中國醫(yī)科大學(xué)附屬盛京醫(yī)院小兒腎臟風(fēng)濕免疫科(吳玉斌)
通信作者:吳玉斌,110004 遼寧省沈陽市,中國醫(yī)科大學(xué)附屬盛京醫(yī)院小兒腎臟風(fēng)濕免疫科;E-mail:772652284@qq.com
【摘要】目的 探討PAX2穩(wěn)轉(zhuǎn)細胞系構(gòu)建及生物學(xué)作用。方法選擇處于對數(shù)生長期大鼠腎小管上皮細胞,實驗分為穩(wěn)轉(zhuǎn)組、空載組、對照組。穩(wěn)轉(zhuǎn)組采用陽離子脂質(zhì)體轉(zhuǎn)導(dǎo)將pEGFP-PAX2轉(zhuǎn)入大鼠腎小管上皮細胞,空載組采用同樣方法配置空載質(zhì)粒轉(zhuǎn)染溶液,對照組不添加質(zhì)粒轉(zhuǎn)染溶液。經(jīng)G418篩選,建立穩(wěn)定轉(zhuǎn)染細胞系。通過細胞劃痕實驗及Transwell實驗檢測PAX2穩(wěn)轉(zhuǎn)細胞系的細胞遷移率及細胞侵襲作用。結(jié)果應(yīng)用G418篩選14 d,穩(wěn)轉(zhuǎn)組大鼠腎小管上皮細胞有明顯克隆長出,建立PAX2基因穩(wěn)定轉(zhuǎn)染腎小管上皮細胞系。穩(wěn)轉(zhuǎn)組在熒光顯微鏡下可見綠色熒光,基因融合表達獲得成功。穩(wěn)轉(zhuǎn)組細胞PAX2條帶密度與β-action吸光度比值為(1.00±0.04),空載組為(0.83±0.03),對照組為(0.85±0.04),3組吸光度比值比較,差異有統(tǒng)計學(xué)意義(F=398.7,P<0.05);其中穩(wěn)轉(zhuǎn)組吸光度比值高于空載組和對照組,差異均有統(tǒng)計學(xué)意義(P<0.05)。細胞劃痕后18 h,對照組、空載組和穩(wěn)轉(zhuǎn)組細胞遷移率分別為(39.34±5.34)%、(40.56±7.54)%和(83.72±7.12)%,3組細胞遷移率比較,差異有統(tǒng)計學(xué)意義(F=431.8,P<0.05);其中穩(wěn)轉(zhuǎn)組細胞遷移率高于對照組和空載組,差異均有統(tǒng)計學(xué)意義(P<0.05)。各組細胞培養(yǎng)48 h后,對照組、空載組和穩(wěn)轉(zhuǎn)組遷移細胞分別為(23.33±3.63)、(22.00±8.14)、(45.67±7.14),3組遷移細胞數(shù)比較,差異有統(tǒng)計學(xué)意義(F=248.5,P<0.05);其中穩(wěn)轉(zhuǎn)組遷移細胞數(shù)高于對照組和空載組,差異均有統(tǒng)計學(xué)意義(P<0.05)。結(jié)論pEGFP-PAX2質(zhì)粒成功轉(zhuǎn)染大鼠腎小管上皮細胞,并成功篩選出高效穩(wěn)定表達PAX2的穩(wěn)轉(zhuǎn)細胞系。PAX2穩(wěn)轉(zhuǎn)增加了腎小管上皮細胞的遷移及侵襲能力。
【關(guān)鍵詞】PAX2基因;細胞侵襲;細胞運動;腎小管;上皮細胞;大鼠
基金項目:遼寧省博士科研啟動基金計劃資助項目(20141136)
【中圖分類號】R 692.6
收稿日期:(2015-07-02;修回日期:2015-09-12)
李里,宮亮,吳玉斌.PAX2穩(wěn)轉(zhuǎn)細胞系構(gòu)建及對細胞遷移和侵襲能力的作用研究[J].中國全科醫(yī)學(xué),2015,18(35):4325-4329.[www.chinagp.net]
Li L,Gong L,Wu YB.Study on the construction of PAX2 stably transfected cell lines and their effects on cell migration and invasion ability[J].Chinese General Practice,2015,18(35):4325-4329.
Study on the Construction of PAX2 Stably Transfected Cell Lines and Their Effects on Cell Migration and Invasion AbilityLILi,GONGLiang,WUYu-bin.DepartmentofRheumatologyandImmunology,theFirstAffiliatedHospitalofLiaoningMedicalUniversity,Jinzhou121000,China
Abstract【】ObjectiveTo investigate the construction and biological effects of PAX2 stably transfected cell lines.MethodsRat renal tubular epithelial cells at exponential phase were selected,and the cells were divided into stably transfected group,empty-loading group and control group.For stably transfected group,cationic liposome transduction was conducted to transfer pEGFP-PAX2 into rat renal tubular epithelial cells;for empty-loading group,cationic liposome transduction was also conducted with empty-loading plasmid transfection solution;for control group,no plasmid transfection solution was added.By G418 selection,stably transfected cell lines were constructed.Cell wound scratch assay and Tanswell test were conducted to measure cell migration rate and cell invasion of PAX2 stably transfected cell lines.ResultsG418 selection was undertaken for 14 days,the cells of stably transfected group had obvious cloning growth,and PAX2 stably transfected cell lines were constructed.Green fluorescence was observed under fluorescence microscope in stably transfected group,and successful gene fusion expression was made.The ratio of the PAX2 strip density to the absorbancy of β-action was (1.00±0.04) for stably transfected group,(0.83±0.03)for empty-loading group and (0.85±0.04)for control group.The three groups were significantly different in optical density ratio(F=398.7,P<0.05);the stably transfected group was higher than empty-loading group in optical density ratio(P<0.05).At 18 hours after cell scratch was made,the cell migration rates of control group,empty-loading group and stably transfected group were(39.34±5.34)%,(40.56±7.54)% and(83.72±7.12)%,with significant differences among the three groups(F=431.8,P<0.05)and the stably transfected group higher than control group and empty-loading group(P<0.05).At 48 hours of culture,the numbers of migrated cells of control group,empty-loading group and stably transfected group were(23.33±3.63),(22.00±8.14)and(45.67±7.14),with significant differences among the three groups(F=248.5,P<0.05)and stably transfected group higher than control group and empty-loading group(P<0.05).ConclusionpEGFP-PAX2 plasmid is successfully transfected into rat renal tubular epithelial cells,and stably transfected cell lines with highly effective and stable expression of PAX2 are successfully constructed.The stable transfection of PAX2 increases migration and invasion of renal tubular epithelial cells.
【Key words】PAX2 gene;Cell invasion;Cell movement;Kidney tubules;Epithelial cells;Rats
PAX2(Paired Box2)基因是胚胎發(fā)育基因,在胚胎發(fā)育過程中發(fā)揮重要的調(diào)控作用。尤其在腎臟,其是腎臟發(fā)育過程中前腎、中腎和后腎重要的調(diào)控基因。PAX2基因主要通過誘導(dǎo)腎小管上皮細胞轉(zhuǎn)分化發(fā)揮調(diào)控作用[1-3]。有學(xué)者[4-7]發(fā)現(xiàn)在病變大鼠體內(nèi)出現(xiàn)PAX2基因重新表達,并可能調(diào)控轉(zhuǎn)分化作用。在體外研究中發(fā)現(xiàn)PAX2基因轉(zhuǎn)染腎小管上皮細胞后出現(xiàn)纖維表型標(biāo)志物的增加以及上皮細胞表型標(biāo)志物的減少[8]。判定細胞出現(xiàn)上皮細胞轉(zhuǎn)分化的標(biāo)志除了出現(xiàn)表型的轉(zhuǎn)變外還表現(xiàn)為細胞侵襲及遷移能力的增加。本研究將首先構(gòu)建PAX2穩(wěn)轉(zhuǎn)細胞系,進一步探討PAX2穩(wěn)轉(zhuǎn)細胞系細胞侵襲及遷移能力,為臨床疾病治療提供實驗依據(jù)。
1材料與方法
1.1材料大鼠腎小管上皮細胞株(NRK52E),購自上海中科院;pEGFP-PAX2質(zhì)粒(含Neo抗性基因),購自Clontech 公司;Transwell細胞小室,購自Corning公司。
1.2方法
1.2.1pEGFP-PAX2穩(wěn)定轉(zhuǎn)染腎小管上皮細胞系的構(gòu)建及篩選選擇處于對數(shù)生長期大鼠腎小管上皮細胞,其生長狀態(tài)良好。分為穩(wěn)轉(zhuǎn)組、對照組、空載組。將pEGFP-grp78質(zhì)粒4 μg加入無血清培養(yǎng)液245 μl,再加入Lipofectamine 2000,將上述液體混合,在室溫靜置30 min。同樣方法配置空載質(zhì)粒轉(zhuǎn)染溶液。對照組不加人質(zhì)粒轉(zhuǎn)染溶液。在無血清無抗生素的培養(yǎng)液中將腎小管上皮細胞洗滌2次,再將上述轉(zhuǎn)染液與細胞加入培養(yǎng)孔中,在5% CO237 ℃培養(yǎng)箱中培養(yǎng)6 h后,將轉(zhuǎn)染液棄除,再加入胎牛血清繼續(xù)培養(yǎng)。細胞傳代,再用G418的選擇培養(yǎng)基篩選,篩選14 d后pEGFP-PAX2重組質(zhì)粒轉(zhuǎn)染組有克隆形成,建立穩(wěn)定轉(zhuǎn)染細胞系。
1.2.2穩(wěn)定轉(zhuǎn)染細胞系鑒定在熒光顯微鏡下觀察各組細胞,藍色激光激發(fā),隨機選一視野,發(fā)出綠色熒光的細胞并攝像。PAX2 mRNA的表達檢測:在24孔板中加入PBS漂洗2次,取1×106細胞加Trizol 1.0 ml,按試劑盒操作說明用三氯甲烷分離、采用異丙醇RNA沉淀、將RNA再溶解,提取細胞總RNA。按照試劑盒說明書配制20 μl RT反應(yīng)體系;PCR反應(yīng):引物設(shè)計:上游:5′-CAACGGTGAGAAGAGGAAACGAG-3′,下游:5′-TAATGCTGCTGGGTGAAGGTGTC-3′,β-actin作為內(nèi)參照,按照試劑盒說明配制50 μl PCR反應(yīng)體系,結(jié)束后進行瓊脂凝膠電泳檢測并攝像;經(jīng)圖像分析儀測定吸光度值,即檢測基因產(chǎn)物吸光度值與內(nèi)參照吸光度的比值為A值。以對照組和空載組的腎小管上皮細胞作為對照。
1.2.3細胞劃痕試驗細胞鋪板:前1天將培養(yǎng)瓶中細胞用0.25%胰蛋白酶消化,接種1×105細胞至6孔培養(yǎng)板中,當(dāng)細胞融合至80%~90%時開始進行實驗。棄去培養(yǎng)基,應(yīng)用200 μl pipette tip使培養(yǎng)板出現(xiàn)劃痕,應(yīng)用培養(yǎng)基清洗細胞表面2次,再加入培養(yǎng)基中,在鏡下觀察10、18 h,并于100倍鏡下拍照記錄。并計算各組遷移率。遷移率用來描述細胞遷移修復(fù)速度,具體計算方法:遷移率=(0 h細胞劃痕寬度-10 h細胞劃痕寬度或18 h細胞劃痕寬度)/0 h細胞劃痕寬度×100%。
1.2.4Transwell實驗將50 μl的Matrigel(1 μg/μl)平鋪在Transwell的8 μm孔徑的聚碳酸酯微孔濾膜上,37 ℃放置1 h,室溫干燥過夜。將600 μl含20%胎牛血清的NIH3T3細胞的上清加入Transwell下室,將200 μl對照組、空載組和穩(wěn)轉(zhuǎn)組細胞懸液加入上室,每種細胞設(shè)3個復(fù)孔,培養(yǎng)48 h后取出小室,將膜上層未穿越細胞拭去,用多聚甲醇固定30 min,蘇木精染色15 min,PBS洗去多余蘇木精,鏡下計數(shù)穿到膜背細胞數(shù)。計數(shù)3個不同視野,取平均值(×200)。
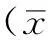
2結(jié)果
2.1穩(wěn)定轉(zhuǎn)染細胞系的構(gòu)建及篩選應(yīng)用G418篩選14 d,穩(wěn)轉(zhuǎn)組大鼠腎小管上皮細胞有明顯克隆長出,建立了PAX2基因穩(wěn)定轉(zhuǎn)染腎小管上皮細胞系(見圖1)。
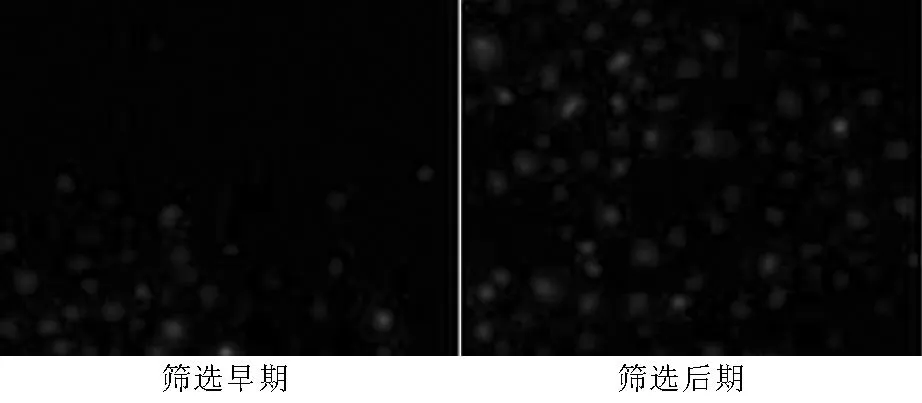
圖1 PAX2基因轉(zhuǎn)染腎小管上皮細胞的G418篩選(×200)
Figure 1G418 selection of renal tubular epithelial cells after PAX2 gene transfection
2.2穩(wěn)定轉(zhuǎn)染細胞系鑒定
2.2.1各組細胞綠色熒光表達穩(wěn)轉(zhuǎn)組及空載組在熒光顯微鏡下均可見綠色熒光,對照組未見綠色熒光(見圖2),基因融合表達獲得成功。
2.2.2各組細胞PAX2 mRNA表達穩(wěn)轉(zhuǎn)組細胞PAX2條帶密度與β-action吸光度比值為(1.00±0.04),空載組為(0.83±0.03),對照組為(0.85±0.04)。3組吸光度比值比較,差異有統(tǒng)計學(xué)意義(F=398.7,P<0.05);其中穩(wěn)轉(zhuǎn)組吸光度比值高于空載組和對照組,差異均有統(tǒng)計學(xué)意義(P<0.05,見圖3)。
2.3PAX2穩(wěn)定轉(zhuǎn)染后腎小管上皮細胞運動遷移改變細胞劃痕后10 h,對照組、空載組和穩(wěn)轉(zhuǎn)組細胞遷移率分別為(23.00±3.10)%、(24.33±4.02)%和(43.12±7.13)%。3組細胞遷移率比較,差異有統(tǒng)計學(xué)意義(F=501.6,P<0.05)。細胞劃痕后18 h,對照組、空載組和穩(wěn)轉(zhuǎn)組細胞遷移率分別為(39.34±5.34)%、(40.56±7.54)%和(83.72±7.12)%。3組細胞遷移率比較,差異有統(tǒng)計學(xué)意義(F=431.8,P<0.05);其中穩(wěn)轉(zhuǎn)組細胞遷移率高于對照組和空載組,差異均有統(tǒng)計學(xué)意義(P<0.05,見圖4)。
2.4PAX2穩(wěn)定轉(zhuǎn)染對腎小管上皮細胞侵襲能力的影響各組細胞培養(yǎng)48 h后,計數(shù)膜下表面的細胞數(shù)。對照組、空載組和穩(wěn)轉(zhuǎn)組遷移細胞分別為(23.33±3.63)、(22.00±8.14)、(45.67±7.14),3組遷移細胞數(shù)比較,差異有統(tǒng)計學(xué)意義(F=248.5,P<0.05);其中穩(wěn)轉(zhuǎn)組遷移細胞數(shù)高于對照組和空載組,差異均有統(tǒng)計學(xué)意義(P<0.05,見圖5)。
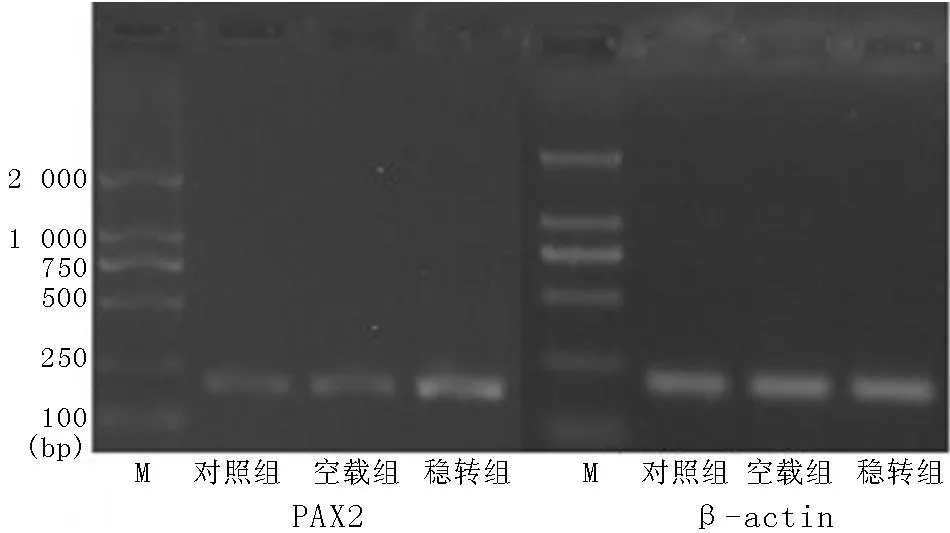
圖3 3組細胞PAX2 mRNA表達的RT-PCR電泳圖
Figure 3RT-PCR eelectrophoretogram of PAX2 mRNA expression of the three groups
3討論
PAX2基因為胚胎發(fā)育基因,既往研究表明PAX2在病理腎臟出現(xiàn)重新表達,認(rèn)為其可能通過調(diào)控腎小管上皮細胞轉(zhuǎn)分化調(diào)控疾病發(fā)生,但具體機制不清楚。腎小管上皮細胞轉(zhuǎn)分化發(fā)生中重要的一步即細胞出現(xiàn)了遷移及侵襲能力的增強[9],故本研究通過建立PAX2穩(wěn)轉(zhuǎn)細胞系,探討PAX2轉(zhuǎn)染是否導(dǎo)致細胞的侵襲及遷移能力增強,為進一步探討PAX2基因調(diào)控腎小管上皮細胞轉(zhuǎn)分化提供依據(jù)。
圖2 熒光顯微鏡觀察pEGFP-PAX2在腎小管上皮細胞中的表達(×200)

圖4 3組0 h、10 h、18 h細胞遷移率比較
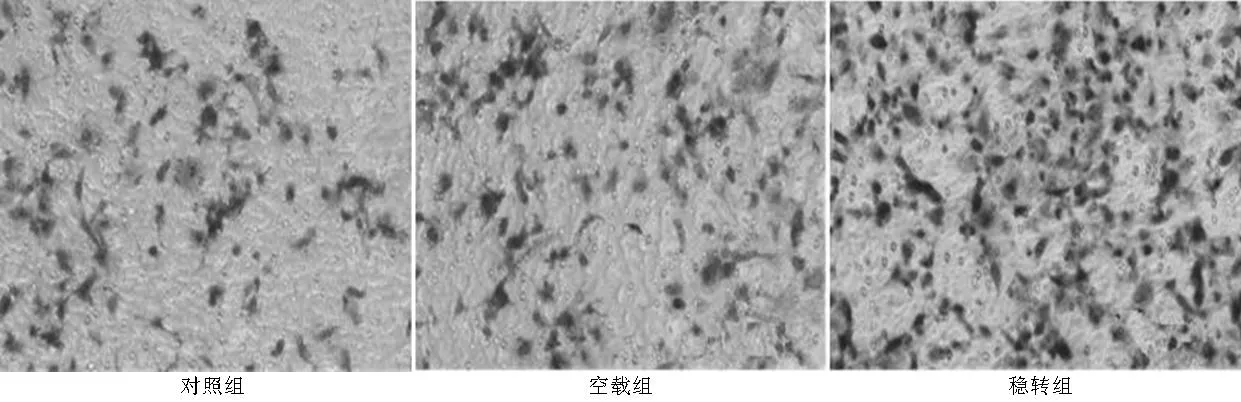
圖5 3組細胞侵襲能力比較(×200)
本實驗應(yīng)用脂質(zhì)體介導(dǎo)轉(zhuǎn)染法將PAX2質(zhì)粒轉(zhuǎn)染入大鼠腎小管上皮細胞,G418是目前廣泛應(yīng)用的穩(wěn)定轉(zhuǎn)染篩選試劑[10],本實驗應(yīng)用該試劑進行了大鼠腎小管上皮細胞篩選,獲得了穩(wěn)定轉(zhuǎn)染PAX2基因的腎小管上皮細胞系。通過熒光顯微鏡觀察及RT-PCR鑒定,穩(wěn)定轉(zhuǎn)染PAX2組熒光表達明顯增加,PAX2 mRNA表達也明顯增加,其表達較對照組及空載組差異有統(tǒng)計學(xué)意義,證明穩(wěn)定轉(zhuǎn)染細胞系構(gòu)建成功。同時也表明脂質(zhì)體介導(dǎo)轉(zhuǎn)染法可以成功進行基因的穩(wěn)定表達轉(zhuǎn)染。
細胞遷移實驗是通過細胞受傷后的自動修復(fù)能力來評估,如單層細胞在受傷24 h內(nèi),其只靠細胞移動來愈合修復(fù),并不會出現(xiàn)反應(yīng)性修復(fù)增生[11],故本實驗應(yīng)用細胞劃痕實驗評估細胞遷移運動能力。結(jié)果表明穩(wěn)轉(zhuǎn)組細胞遷移速度較空載組及對照組明顯增快,表明過表達PAX2可明顯增加腎小管上皮細胞遷移能力。本試驗通過目前較為常用的Transwell實驗探討細胞侵襲能力,細胞在含有Matrigel的聚碳酸酯微孔中穿過,是在模擬體內(nèi)細胞穿過細胞外基質(zhì)的過程,可以反映細胞的侵襲能力[12],本研究結(jié)果表明穩(wěn)轉(zhuǎn)組穿透的細胞數(shù)較空載組及對照組明顯增加,說明PAX2可明顯增加細胞侵襲能力。
本文價值:
本研究首次通過構(gòu)建PAX2穩(wěn)轉(zhuǎn)細胞系探討其生物學(xué)作用,發(fā)現(xiàn)PAX2轉(zhuǎn)染可以增加腎小管上皮細胞的遷移及侵襲能力,這可能是PAX2基因誘導(dǎo)腎小管上皮細胞轉(zhuǎn)分化的重要機制,為臨床疾病的治療提供實驗依據(jù)。
參考文獻
[1]Huang B,Pi L,Chen C,et al.WT1 and Pax2 re-expression is required for epithelial-mesenchymal transition in 5/6 nephrectomized rats and cultured kidney tubular epithelial cells[J].Cells Tissues Organs,2012,195(4):296-312.
[2]Zhou TB,Qin YH,Lei FY,et al.Association of PAX2 with cell apoptosis in unilateral ureteral obstruction rats[J].Ren Fail,2012,34(2):194-202.
[3]Li L,Wu Y,Zhang W.PAX2 re-expression in renal tubular epithelial cells and correlation with renal interstitial fibrosis of rats with obstructive nephropathy[J].Ren Fail,2010,32(5):603-611.
[4]Cohen T,Loutochin O,Amin M,et al.PAX2 is reactivated in urinary tract obstruction and partially protects collecting duct cells from programmed cell death[J].Am J Physiol Renal Physiol,2007,292(4):F1267-1273.
[5]Yi ZW,Zhu CP,Dang XQ,et al.PAX2 expression of pathological kidney in children with nephrotic syndrome and acute glomerulonephritis[J].Journal of Clinical Research,2005,22(11):1526-1530.(in Chinese)
易著文,朱翠平,黨西強,等.腎病綜合征和急性腎炎患兒腎組織PAX2的表達及臨床意義[J].醫(yī)學(xué)臨床研究,2005,22(11):1526-1530.
[6]Geng WM,Yi ZW,He XJ,et al.Function of PAX2 in tubular epithelium transdifferentiation[J].Journal of Clinical Pediatrics,2007,25(4):284-287.(in Chinese)
耿文茂,易著文,何小解,等.PAX2在腎小管上皮細胞轉(zhuǎn)分化中的作用[J].臨床兒科雜志,2007,25(4):284-287.
[7]Pi L,Jiang T,Ouyang J,et al.Role of PAX2 gene in renal tubular epithelial cell transdifferentiation[J].Central China Medical Journal,2007,31(1):7-10.(in Chinese)
皮蕾,姜儻,歐陽涓,等.PAX2基因在腎小管上皮細胞轉(zhuǎn)分化中的作用[J].華中醫(yī)學(xué)雜志,2007,31(1):7-10.
[8]Li L,Wu Y,Yang Y.Paired box 2 induces epithelial-mesenchymal transition in normal renal tubular epithelial cells of rats[J].Mol Med Rep,2013,7(5):1549-1554.
[9]Rastaldi MP.Epithelial-mesenchymal transition and its implications for the development of renal tubulointerstitial fibrosis[J].J Nephrol,2006,19(4):407-412.
[10]Liu Y.Epithelial to mesenchymal transition in renal fibrogenesis:pathologic significance,molecular mechanism,and therapeutic intervention[J].J Am Soc Nephrol,2004,15(1):1-12.
[11]Rastaldi MP,F(xiàn)errario F,Giardino L,et al.Epithelial-mesenchymal transition of tubular epithelial cells in human renal biopsies[J].Kidney Int,2002,62(1):137-146.
[12]Yang J,Liu Y.Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis[J].Am J Pathol,2011,159(4):1465-1475.
(本文編輯:賈萌萌)

