雷公藤紅素衍生物合成與活性測定
單偉光,施 航,占扎君
(浙江工業大學 藥學院,浙江 杭州 310014)
雷公藤紅素衍生物合成與活性測定
單偉光,施航,占扎君
(浙江工業大學 藥學院,浙江 杭州 310014)
摘要:雷公藤紅素是一種從中藥雷公藤中提取的一種具有很強抗癌活性的三萜化合物,為找到具有更好抗癌活性和成藥性的雷公藤紅素衍生物,在雷公藤紅素C-29羧基設計并合成10個新型的衍生物,并通過MMT法,測定了衍生物對A549和HepG2腫瘤細胞的抗癌活性,實驗結果顯示10個衍生物都具有很好的抗癌活性,化合物C4和C8抗癌活性最強,并且具有良好的水溶性,是較好的體內實驗備選化合物.
關鍵詞:雷公藤紅素;抗癌;毒性;MTT法
Synthesis and anti-cancer evaluation of novel celastrol analogues
SHAN Weiguang, SHI Hang, ZHAN Zhajun
(College of Pharmaceutical Science, Zhejiang University of Technology, Hangzhou 3100, China)
Abstract:Celastrol, a natural quinone methide triterpenoid originally isolated from root bark of the traditional Chinese herb “Thunder of God Vine”, showed remarkable anticancer activity. In order to find the celasrol analogues of better anticancer activity and druggability, Ten novel celastrol analogues were designed and synthesized. Their cytotoxicity were evaluated against 2 human cancer cell lines(A549 and HepG2) by MTT method. Ten celastrol analogues all showed better inhibitions than positive drug. Among all of the derivatives, C4 and C6 were the most suitable candidate compounds for in vivo experiment in the future because of their high activity and water solubility.
Key words:celastrol; anticancer; cytotoxicity; MTT method
雷公藤紅素(celastrol)是一種五環的木栓烷型三萜化合物,又名南蛇藤堿,是第一個從雷公藤根部提取出來的三萜類化合物[1-2].三萜類化合物雷公藤紅素具有獨特的化學結構基團,能夠和半胱氨酸殘基中的巰基發生邁克爾加成,生成共軛加成產物[3],該化合物在體內通過發生加成反應,影響蛋白或酶的活性、調節多種細胞信號途徑等,進而產生藥理作用.據報道,雷公藤紅素具有多種顯著的生物活性,例如,抗腫瘤、抗免疫與抗炎、抗病毒以及抗神經衰退性疾病等[4-6].雷公藤紅素的抗腫瘤活性在很久之前已經被研究證實,但是很長一段時間機制不明確,直到2006年Yang等[7]首次實驗證明雷公藤紅素可以誘導癌細胞凋亡,使得腫瘤細胞壞死,從而引發了雷公藤紅素抗癌機制的研究熱浪;之后相繼有研究表明雷公藤紅素對多種腫瘤細胞的治療都有很好的療效,例如前列腺癌細胞、神經膠質瘤細胞、口腔鱗狀癌細胞、黑素瘤、乳腺癌、白血病和肺癌等等[8-10].
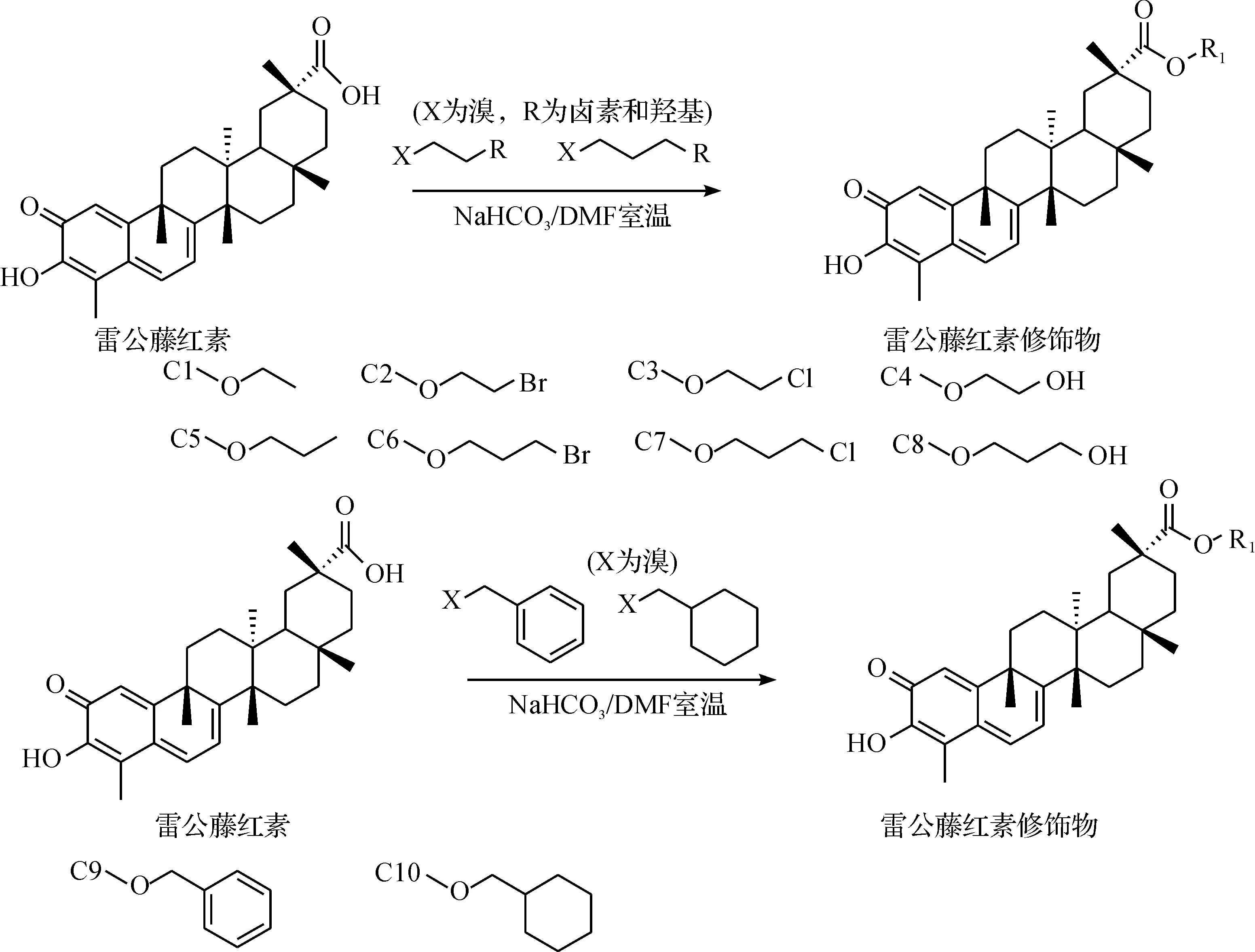
國內外開展了不少雷公藤紅素修飾物的研究[11-13],主要是針對C-3羥基和C-29羧基成一些酯基和一些酰胺鍵,本次研究的創新處在于在C-29位的常規修飾基團中加入一些鹵元素、苯環、六圓環及羥基,試圖改變整個分子的結構以及電子云密度,從而產生不同的抗癌活性.
1合成研究
1.1實驗材料與儀器
實驗材料:雷公藤紅素(課題組自提分離);鹵代烷試劑(AR,Aladdin Chemistry Co.Ltd.);碳酸氫鈉(CR,天津永大化學試劑有限公司);無水硫酸鈉(CR,上海四赫維化工有限公司);硅膠(青島海洋化工廠);溶劑均為國產分析純.
實驗儀器:磁力攪拌器(杭州大衛科教儀器);真空干燥箱(上海精宏實驗設備有限公司);真空油泵(臺州博奧真空設備有限公司);恒溫鼓風干燥箱(上海精宏實驗設備有限公司);數控超聲波清洗器(昆山市禾創超聲儀器有限公司);旋轉蒸發儀(BUCHI);循環水式多用真空泵(杭州大衛科教儀器);紫外燈(上海顧村電光儀器廠);電子天平(max 220 g,d=0.1 mg,德國塞多利斯);冰箱(青島海爾股份有限公司).
1.2衍生物的合成
如圖1所示,取紅素(50 mg, 0.11 mmol)溶于N,N-二甲基甲酰胺(DMF,4 mL)中,加入碳酸氫鈉少量,再加入相應的二鹵代烷、鹵代環烷烴(0.3 mmol),室溫下攪拌數小時.TLC法檢測反應程度,觀察雷公藤紅素點消失后,反應終止.加入去離子水(15 mL),乙酸乙酯萃取3 次,合并有機層.有機層再用飽和食鹽水洗3次,無水硫酸鈉干燥,旋轉蒸發儀濃縮得棕色油狀物.粗產物經快速柱層析(油醚:丙酮)法分離純化,產物經減壓蒸除溶劑、真空干燥得深棕色固體C1~C10.所得產物經氫譜、碳譜和質譜等方法確定其結構.雷公藤紅素衍生物的合成路線為
1.3衍生物的圖譜歸屬
C1:1H-NMR(CDCl3,500 MHz):0.55(3H,s,CH3),1.12(3H,s,CH3),1.21(3H,s,CH3),1.25(3H,t,J=7.0 Hz,H-2′),1.27(3H,s,CH3),1.45(3H,s,CH3),2.25(3H,s,CH3),3.98(2H,m,H-1′),6.35(1H,d,J=7.0 Hz7.0,H-7),6.55(1H,s,H-1),7.03(1H,d,J=7.0 Hz,H-6).13C-NMR(CDCl3,125 MHz):10.2,14.0,18.4,21.6,28.7,29.3,29.8,30.6,30.7,31.6,32.8,33.6,34.8,36.4,38.2,39.5,40.2,43.0,44.3,45.1,60.3,117.3,118.2,119.5,127.5,134.3,146.1,164.9,170.4,178.2,178.4.ESI-MS:m/z479.3[M+H]+.
C2:1H-NMR(CDCl3,500 MHz):0.55(3H,s,CH3),1.09(3H,s,CH3),1.21(3H,s,CH3),1.27(3H,s,CH3),1.45(3H,s,CH3),2.19(3H,s,CH3),3.45(2H,t,J=6.5 Hz,H-2′),4.10(1H,m,H-1′a),4.27(1H,m,H-1′b),6.31(1H,d,J=7.0 Hz,H-7),6.49(1H,s,H-1),7.09(1H,d,J=7.0 Hz,H-6).13C-NMR(CDCl3,125 MHz):10.2,18.4,21.5,28.4,28.8,29.6,29.8,30.6,30.7,31.5,32.7,33.5,34.6,36.3,38.1,39.4,40.4,42.8,44.2,44.9,63.9,117.0,118.0,119.5,127.3,134.0,146.1,164.6,169.8,177.7,178.2.ESI-MS:m/z559.3[M+H]+.
C3:1H-NMR(CDCl3,500 MHz):0.55(3H,s,CH3),1.09(3H,s,CH3),1.21(3H,s,CH3),1.27(3H,s,CH3),1.45(3H,s,CH3),2.19(3H,s,CH3),3.64(2H,t,J=6.5 Hz,H-2′),4.08(1H,m,H-1′a),4.23(1H,m,H-1′b),6.34(1H,d,J=7.0 Hz,H-7),6.53(1H,s,H-1),7.01(1H,d,J=7.0 Hz,H-6).13C-NMR(CDCl3,125 MHz):10.2,18.6,21.6,28.6,29.6,29.7,29.8,30.8,31.5,32.7,33.6,34.7,36.3,38.2,39.5,40.5,41.6,42.9,44.3,45.0,64.2,117.1,118.1,119.5,127.4,134.0,146.1,164.7,169.8,177.9,178.4.ESI-MS:m/z513.3[M+H]+.
C4:1H-NMR(CDCl3,500 MHz):0.55(3H,s,CH3),1.12(3H,s,CH3),1.21(3H,s,CH3),1.26(3H,s,CH3),1.44(3H,s,CH3),2.20(3H,s,CH3),3.78(2H,m,H-2′),4.01(1H,m,H-1′a),4.09(1H,m,H-1′b),6.35(1H,d,J=7.0 Hz,H-7),6.53(1H,s,H-1),7.02(1H,d,J=7.0 Hz,H-6).13C-NMR(CDCl3,125 MHz):10.2,18.5,21.6,28.7,29.3,29.8,30.6,30.7,31.6,32.8,33.6,34.8,36.4,38.2,39.5,40.2,43.0,44.3,45.1,60.9,66.1,117.2,118.2,119.6,127.5,134.1,146.1,164.8,170.0,178.4,178.5.ESI-MS:m/z485.3[M+H]+.
C5:1H-NMR(CDCl3,500 MHz):0.55(3H,s,CH3),0.97(3H,m,H-3′),1.12(3H,s,CH3),1.19(3H,s,CH3),1.27(3H,s,CH3),1.45(3H,s,CH3),2.24(3H,s,CH3),3.82(1H,m,H-1′a),3.93(1H,m,H-1′b),6.35(1H,d,J=7.0 Hz,H-7),6.54(1H,s,H-1),7.02(1H,d,J=7.0 Hz,H-6).13C-NMR(CDCl3,125 MHz):10.2,14.0,18.4,21.6,21.7,28.7,29.3,29.7,30.6,30.7,31.6,32.8,33.5,34.8,36.4,38.2,39.4,40.4,43.0,44.3,45.1,66.3,117.3,118.2,119.5,127.4,134.0,146.0,164.7,170.0,178.3,178.4.ESI-MS:m/z493.1[M+H]+.
C6:1H-NMR(CDCl3,500 MHz):0.54(3H,s,CH3),1.09(3H,s,CH3),1.21(3H,s,CH3),1.27(3H,s,CH3),1.45(3H,s,CH3),2.19(3H,s,CH3),3.44(2H,t,J=6.5 Hz H-3′),3.98(1H,m,H-1′a),4.10(1H,m,H-1′b),6.33(1H,d,J=7.0 Hz,H-7),6.52(1H,s,H-1),7.00(1H,d,J=7.0 Hz,H-6).13C-NMR(CDCl3,125 MHz):10.2,18.6,21.6,28.6,29.2,29.3,29.6,29.8,30.6,30.7,31.5,32.7,33.5,34.6,36.3,38.1,39.4,40.4,42.8,44.3,44.9,62.0,117.0,118.1,119.5,127.4,134.0,146.0,164.6,169.7,178.3,178.2.ESI-MS:m/z573.3[M+H]+.
C7:1H-NMR(CDCl3,500 MHz):0.55(3H,s,CH3),1.09(3H,s,CH3),1.21(3H,s,CH3),1.27(3H,s,CH3),1.45(3H,s,CH3),2.19(3H,s,CH3),3.56(2H,t,J=6.5 Hz H-3′),3.95(1H,m,H-1′a),4.09(1H,m,H-1′b),6.31(1H,d,J=7.0 Hz,H-7),6.49(1H,s,H-1),7.01(1H,d,J=7.0 Hz,H-6).13C-NMR(CDCl3,125 MHz):10.1,18.6,21.5,28.5,29.6,29.7,29.8,30.4,30.7,31.5,32.6,33.4,34.7,36.3,38.1,39.3,40.5,41.0,42.8,44.2,44.9,61.0,117.1,118.1,119.5,127.3,133.9,146.o,164.6,169.8,177.9,178.2.ESI-MS:m/z527.3[M+H]+.
C8:1H-NMR(CDCl3,500 MHz):0.55(3H,s,CH3),1.10(3H,s,CH3),1.18(3H,s,CH3),1.27(3H,s,CH3),1.45(3H,s,CH3),2.21(3H,s,CH3),3.68(2H,m,H-3′),4.01(1H,m,H-1′a),4.13(1H,m,H-1′b),6.35(1H,d,J=7.0 Hz,H-7),6.54(1H,s,H-1),7.02(1H,d,J=7.0 Hz,H-6).13C-NMR(CDCl3,125 MHz):10.2,18.5,21.6,28.7,29.3,29.7,29.8,30.6,30.7,31.6,32.8,33.6,34.8,36.4,38.2,39.5,40.5,43.0,44.3,45.1,59.4,61.5,117.2,118.2,119.6,127.5,134.1,146.1,164.8,170.0,178.4,178.5.ESI-MS:m/z499.3[M+H]+.
C9:1H-NMR(CDCl3,500 MHz):0.50(3H,s,CH3),1.09(3H,s,CH3),1.20(3H,s,CH3),1.26(3H,s,CH3),1.43(3H,s,CH3),2.21(3H,s,CH3),4.94(1H,d,J=7.5 Hz,H-1′a),5.02(1H,d,J=7.5 Hz,H-1′b),6.33(1H,d,J=7.0 Hz,H-7),6.49(1H,s,H-1),7.01(1H,d,J=7.0 Hz,H-6),7.27-7.35(5H,m,H-Ar).13C-NMR(CDCl3,125 MHz):10.2,18.4,21.6,28.6,29.3,29.8,30.6,30.8,31.6,32.8,33.6,34.8,36.4,38.2,39.5,40.4,42.9,44.2,45.0,66.3,117.3,118.1,119.6,127.4,127.5,128.2,128.3,128,6 134,1 134.1,135,7,146.0,164.7,170.1,177.9,178.3.ESI-MS:m/z541.3[M+H]+.
C10:1H-NMR(CDCl3,500 MHz):0.53(3H,s,CH3),1.13(3H,s,CH3),1.21(3H,s,CH3),1.27(3H,s,CH3),1.46(3H,s,CH3),2.22(3H,s,CH3),3.64(H,m,H-1′a),3.80(H,m,H-1′b),6.36(1H,d,J=7.0 Hz,H-7),6.54(1H,s,H-1),7.03(1H,d,J=7.0 Hz,H-6).13C-NMR(CDCl3,125 MHz):10.2,18.4,21.6,25.7,25.8,28.7,29.7,29.7,29.8,29.9,30.6,30.8,31.6,32.8,33.5,34.9,36.5,38.2,39.5,40.5,43.0,44.4,45.1,69.7,117.0,118.1,119.5,127.5,134.0,146.1,164.7,169.9,178.3,178.4.ESI-MS:m/z547.3[M+H]+.
2衍生物抗腫瘤活性研究
2.1實驗材料
實驗細胞(人肺癌細胞株A549,人肝癌細胞株HepG2[14];MEM培養基(南京凱基生物);DMEM培養基(南京凱基生物);RPMI-1640培養基(南京凱基生物);胎牛血清(Hyclone);胰酶(吉諾生物醫藥);細胞凍存液(南京凱基生物);PBS(吉諾生物醫藥);MTT(Sigma);DMSO(CR,上海凌峰化學有限公司).
2.2實驗儀器
超凈臺(SW-CJ-1F,上海博迅實業醫療設備廠);CO2培養箱(Thermo scientific);倒置相差顯微鏡(Olympus, CKX41);酶標儀(TECAN Sunrise);高壓蒸汽滅菌鍋(上海博迅實業醫療設備廠);96孔培養板(Corning Incorporated);高速離心機(LG10-2.4A).
2.3實驗操作
采用MTT法[15-16],在37 ℃,體積比為5%的CO2培養箱內,取對數生長期的各細胞(3×104個/mL),接種于96孔培養板(180 μL/孔)中貼壁生長.24 h后,加入不同濃度藥物及陽性對照物,以含細胞培養基為空白對照孔,每個濃度設5個平行孔.置于37 ℃,體積比為5% CO2培養箱中培養.第48 h時加入5 mg/mL MTT溶液(20 μL/孔),混勻后,繼續孵育4 h.第52 h時,停止培養,小心倒去96孔板內培養基,加入DMSO(150 uL/孔),振蕩溶解,10 min內酶標儀于570 nm波長處測定OD值.最后根據IC50計算公式和軟件計算出各化合物IC50值.
2.4IC50實驗結果和討論
選取雷公藤紅素以及10個衍生物,以卡鉑作為陽性藥,測出IC50值,如表1所示.合成所得的化合物C1~C10活性都要高于陽性對照藥卡鉑.C9和C10這兩個衍生物,芳烴衍生物C9活性要好于環烷烴衍生物C10.在直鏈烴衍生物中,羥基衍生物C4和C8活性最強.
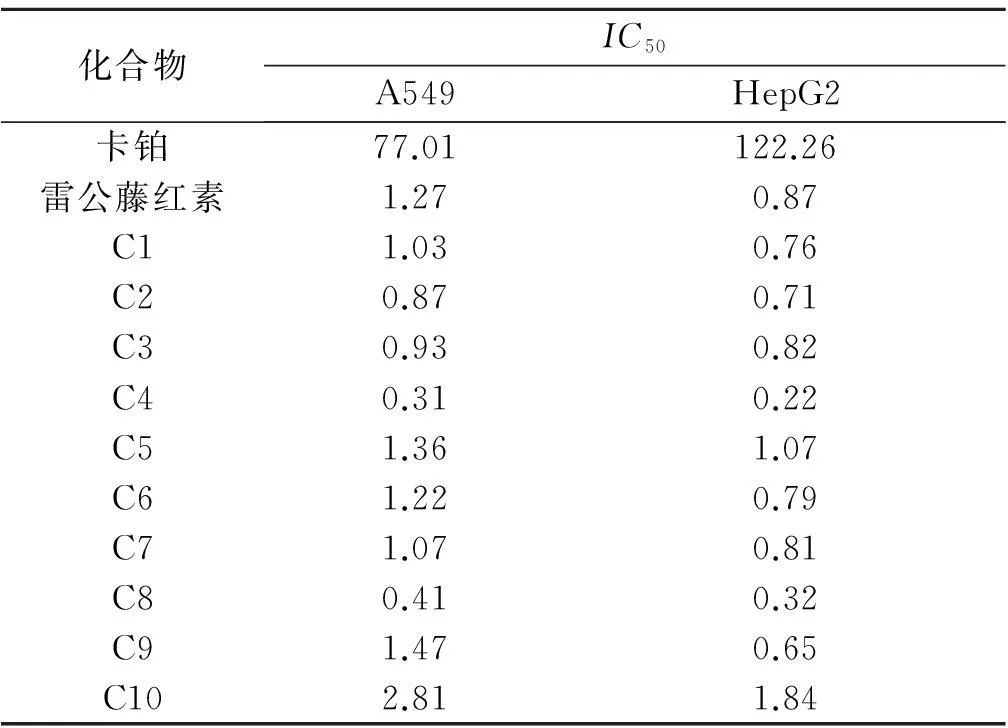
表1 雷公藤紅素衍生物的IC50
3結論
本實驗對具有較強抗癌活性的木栓烷型三萜化合物雷公藤紅素C-29-羧基進行修飾,得到了10個新型的衍生物,并對衍生物進行抗癌活性測定,采用人肺癌細胞株A549和人肝癌細胞株HepG2,運用MTT法算出了IC50.衍生物C1~C4以及C6~C8都表現出了比雷公藤紅素強的抗癌活性,引入了鹵素、苯環、羥基都明顯提升了化合物抗癌活性,而引入六圓環化合物活性有明顯下降.衍生物中化合物C4和C8抗癌活性最強,且C4和C8是衍生物中唯一兩個極性大于紅素的衍生物,可以作為未來體內實驗的候選藥物.
參考文獻:
[1]劉為萍,劉素香,唐慧珠,等.雷公藤研究新進展[J].中草藥,2010,41(7):1215-1218.
[2]韋登明,黃光照.雷公藤及其單體的藥理和毒理病理學研究進展[J].中藥材,2003,26(12):894-897.
[3]SREERAMULU S, GANDE S L, GOBEL M, et al. Molecular mechanism of inhibition of the human protein complex Hsp90-Cdc37 a kinome chaperone-cochaper one by triterpene celastrol[J]. Angewandte Chemie International Edition,2009,48:5853-5855.
[4]胡凱,葛衛紅.雷公藤紅素藥理活性研究進展[J].亞太傳統醫藥,2012,8(11):179-181.
[5]CHAPELSKY S, BATTY S, FROST M, et al. Inhibition of anthrax lethal toxin-induced cytolysis of RAW264.7 cells by celastrol[J]. Plos One,2008(1):1-6.
[6]錢利武,周國勤,陳師農.雷公藤紅素抗精神衰退性疾病研究概況[J].中國現代應用藥學,2011,7(28):629-633.
[7]YANG H J, CHEN D, CUI C, et al. Celastrol a triterpene extracted from the chinese “Thunder of God Vine” is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice[J]. Cancer Research,2006,66(9):4758-4765.
[8]WESTERHEIDE S D. Celastrols as inducers, of the heat shock response and cytoprotection[J]. Journal of Biological Chemistry,2004,279(53):56053-56060.
[9]GE P, JI X, DING Y, et al. Celastrol causes apoptosis and cell cycle arrest in rat glioma cells[J]. Neurological Research,2010,32(1):94-100.
[10]PENG B, XU L, CAO F, et al. HSP90 inhibitor celastrol arrests, human monocytic leukemia cell U937 at G0/G1 in thiol-containing agents reversible way[J]. Molecular Cancer,2010,9(1):79.
[11]SUN H, XU L, YU P, et al. Synthesis and preliminary evaluation of neuroprotection of celastrol analogues in PC12 cells[J]. Bioorganic & Medicinal Chemistry Letters,2010,20(13):3844-3847.
[12]王家強,劉軍峰,劉珂.雷公藤紅素衍生物的合成與評價[J].中草藥,2009,40:201.
[13]ABBAS S, BHOUMIK A, DAHL R, et al.Preclinical studies of celastrol and acetyl isogambogic acid in melanoma[J]. Clinical Cancer Research,2007,13(22):6769-6778.
[14]宋必衛,駱錢芬.BAPTA-AM對OGD-再灌致HepG-2細胞損傷的保護作用研究[J].浙江工業大學學報,2013,41(2):133-137.
[15]竺佳,朱泱平,徐佳,等.七葉皂苷鈉對P388小鼠白血病細胞的體內外增殖抑制作用[J].浙江工業大學學報,2009,37(2):123-125.
[16]唐嵐,趙亞,單海峰,等.甜瓜蒂中葫蘆素類成分分離及體外抗癌活性研究[J].浙江工業大學學報,2012,40(4):388-391.
(責任編輯:陳石平)
文章編號:1006-4303(2015)06-0607-04
中圖分類號:O626.4
文獻標志碼:A
作者簡介:單偉光(1961—),男,浙江寧波人,教授,博士生導師,主要從事天然產物化學以及藥品質量控制標準的研究,E-mail:swg@zjut.edu.cn.
基金項目:國家自然科學基金資助項目(20702049)
收稿日期:2015-04-15