區(qū)域動(dòng)脈灌注5-FU改善大鼠重癥急性胰腺炎相關(guān)的肺損傷
李強(qiáng),金約朋,白永愉,黃新策,周蒙滔
?
·論著 基礎(chǔ)研究·
區(qū)域動(dòng)脈灌注5-FU改善大鼠重癥急性胰腺炎相關(guān)的肺損傷
李強(qiáng)1,金約朋2,白永愉1,黃新策1,周蒙滔2
(1.溫州醫(yī)科大學(xué) 第一臨床醫(yī)學(xué)院,浙江 溫州 325035;2.溫州醫(yī)科大學(xué)附屬第一醫(yī)院 肝膽外科,浙江溫州 325000)
[摘 要]目的 研究區(qū)域動(dòng)脈灌注(regional arterial infusion,RAI)5-氟尿嘧啶(5-fluorouracil,5-FU)對(duì)大鼠重癥急性胰腺炎(severe acute pancreatitis,SAP)相關(guān)的急性肺損傷(acute lung injury,ALI)的作用及其可能的機(jī)制。方法 36只健康成年雄性SD大鼠隨機(jī)分成3組:對(duì)照組(C組)、胰腺炎組(SAP組)、區(qū)域動(dòng)脈灌注5-FU治療組(5-FU組)。逆行胰膽管注射5%牛磺膽酸鈉(1 mL/kg)建立SAP模型。5-FU組在誘導(dǎo)SAP模型后立即行區(qū)域動(dòng)脈灌注5-FU(40 mg/kg)治療,C組和SAP組區(qū)域動(dòng)脈灌注等量的生理鹽水。建模成功后分別于12、24 h取標(biāo)本并處死大鼠,胰腺和肺臟組織送病理學(xué)檢查,檢測(cè)肺組織濕干比,肺組織髓過(guò)氧化物酶(myeloperoxidase,MPO)活性,血淀粉酶和血中TNF-α、IL-1β、IL-6的含量。結(jié)果 與C組比較,SAP組與5-FU組血淀粉酶、血中促炎細(xì)胞因子(TNF-α、IL-1β、IL-6)、肺組織濕干比、肺組織MPO均顯著升高(P<0.05);與SAP組比較,5-FU組上述指標(biāo)均顯著下降(P<0.05)。光鏡下可見(jiàn)SAP組出現(xiàn)明顯的肺損傷,5-FU區(qū)域灌注治療后肺損傷減輕,病理學(xué)評(píng)分下降。結(jié)論 區(qū)域動(dòng)脈灌注5-FU對(duì)大鼠重癥急性胰腺炎相關(guān)的急性肺損傷有改善作用,其作用機(jī)制可能與抑制促炎細(xì)胞因子的過(guò)度表達(dá)有關(guān)。
[關(guān)鍵詞]重癥急性胰腺炎;急性肺損傷;5-氟尿嘧啶;區(qū)域動(dòng)脈灌注;細(xì)胞因子;大鼠
重癥急性胰腺炎(SAP)并發(fā)癥率和死亡率高,急性肺損傷(ALI)是其中比較常見(jiàn)的并發(fā)癥,與炎癥介質(zhì)的過(guò)度激活有密切的關(guān)系。臨床上,5-FU治療SAP取得了值得肯定的療效,但其對(duì)SAP相關(guān)的ALI未見(jiàn)文獻(xiàn)報(bào)道。本實(shí)驗(yàn)通過(guò)建立大鼠SAP相關(guān)的ALI模型,觀察5-FU對(duì)SAP大鼠急性肺損傷的治療效果,并探討其可能的機(jī)制。
1 材料和方法
1.1 材料及試劑
清潔級(jí)健康成年雄性SD大鼠,體重250~300 g,購(gòu)于溫州醫(yī)科大學(xué)實(shí)驗(yàn)動(dòng)物中心。5-FU注射液(天津金耀氨基酸有限公司);牛磺膽酸鈉(美國(guó)Sigma公司);亞甲蘭染色液,HE染色劑和水合氯醛(北京索萊寶科技有限公司);PE10導(dǎo)管(寧波安萊軟件與器械有限公司);大鼠TNF-α、IL-1β、IL-6 ELISA試劑盒(美國(guó)eBioscience公司);MPO試劑盒(南京建成生物工程所)。
1.2 動(dòng)物分組與模型
雄性SD大鼠36只,術(shù)前禁食12 h,不禁水。隨機(jī)分成三組(n=12),每組又分成12 h和24 h兩個(gè)亞組(n=6)。(1)C組:常規(guī)10%水合氯醛溶液(3 mL/kg),腹部皮下注射麻醉后開(kāi)腹,僅翻動(dòng)胰腺后關(guān)腹。(2)SAP組:依照Aho法[1]建立大鼠SAP模型。應(yīng)用微泵逆行胰管內(nèi)注射5%牛磺膽酸鈉以0.1 mL/min的速度,劑量1 mL/kg。(3)5-FU組:同上SAP制模后,胃左動(dòng)脈插管亞甲蘭染色確定導(dǎo)管位置[2]后行5-FU區(qū)域動(dòng)脈灌注,5-FU動(dòng)脈灌注劑量40 mg/kg,灌注速度6.0 mL/h。C組和SAP組給予動(dòng)脈灌注等量的生理鹽水。大鼠清醒后自由飲水,禁食。
1.3 標(biāo)本采集及指標(biāo)檢測(cè)
各組建模后12和24 h,分別再次麻醉大鼠,抽血、取標(biāo)本,檢測(cè)如下指標(biāo):(1)采用全自動(dòng)生化儀(日本日立7600)檢測(cè)血清淀粉酶;(2)采用ELISA法檢測(cè)血清中的細(xì)胞因子TNF-α、IL-1β、IL-6水平;(3)采集右肺上葉用生理鹽水洗凈后在濾紙上吸干水分并稱重,記錄肺濕重。后置于80 ℃電熱恒溫干燥箱中烘烤72 h,再次稱重,記錄肺干重。肺組織濕干比計(jì)算公式:[(肺濕重-肺干重)/肺干重×100%]。(4)采集左肺制成肺組織勻漿,離心取上清液用于檢測(cè)MPO活性。(5)取部分胰腺、肺組織置于4%多聚甲醛溶液中固定用于HE染色,觀察肺、胰腺組織改變。胰腺組織按照Schmidt標(biāo)準(zhǔn)[3],即根據(jù)胰腺間質(zhì)水腫、腺泡細(xì)胞壞死、出血和脂肪壞死、炎癥細(xì)胞浸潤(rùn)進(jìn)行評(píng)分,每項(xiàng)按照嚴(yán)重程度分別評(píng)分為0~4分。肺組織按照Osman標(biāo)準(zhǔn)[4],根據(jù)肺水腫和炎癥細(xì)胞的浸潤(rùn)程度進(jìn)行評(píng)分,每項(xiàng)按照嚴(yán)重程度分別評(píng)分為0~3分。由有經(jīng)驗(yàn)的病理科醫(yī)師雙盲進(jìn)行病理評(píng)分。
1.4 統(tǒng)計(jì)學(xué)分析
2 結(jié)果
2.1 胰腺組織光鏡觀察
C組大鼠胰腺鏡下結(jié)構(gòu)基本正常(圖1,C組)。SAP組胰腺腺體結(jié)構(gòu)紊亂,腺泡細(xì)胞腫脹,葉間隙增寬,可見(jiàn)灶性壞死,壞死區(qū)內(nèi)腺泡結(jié)構(gòu)消失,部分腺泡細(xì)胞空泡化,伴有炎癥細(xì)胞浸潤(rùn),24 h組腺泡結(jié)構(gòu)破壞更加嚴(yán)重,炎癥細(xì)胞浸潤(rùn)更加明顯(圖1,SAP組)。5-FU組與SAP組比較,胰腺間質(zhì)水腫、胰腺腺泡細(xì)壞死、炎癥細(xì)胞浸潤(rùn)均明顯減輕(圖1,5-FU組)。
2.2 肺組織光鏡觀察
C組大鼠肺組織鏡下結(jié)構(gòu)基本正常(圖2,C組)。SAP組肺組織鏡下可見(jiàn)肺泡和間質(zhì)水腫、出血,肺泡間隔增寬或塌陷,大量炎性細(xì)胞浸潤(rùn)及紅細(xì)胞滲出,肺組織結(jié)構(gòu)紊亂,24 h組以上改變更加明顯(圖2,SAP組)。5-FU組與SAP組比較肺間質(zhì)水腫、紅細(xì)胞滲出及炎細(xì)胞浸潤(rùn)均減輕(圖2,5-FU組)。

圖1 大鼠胰腺組織HE染色(×400)
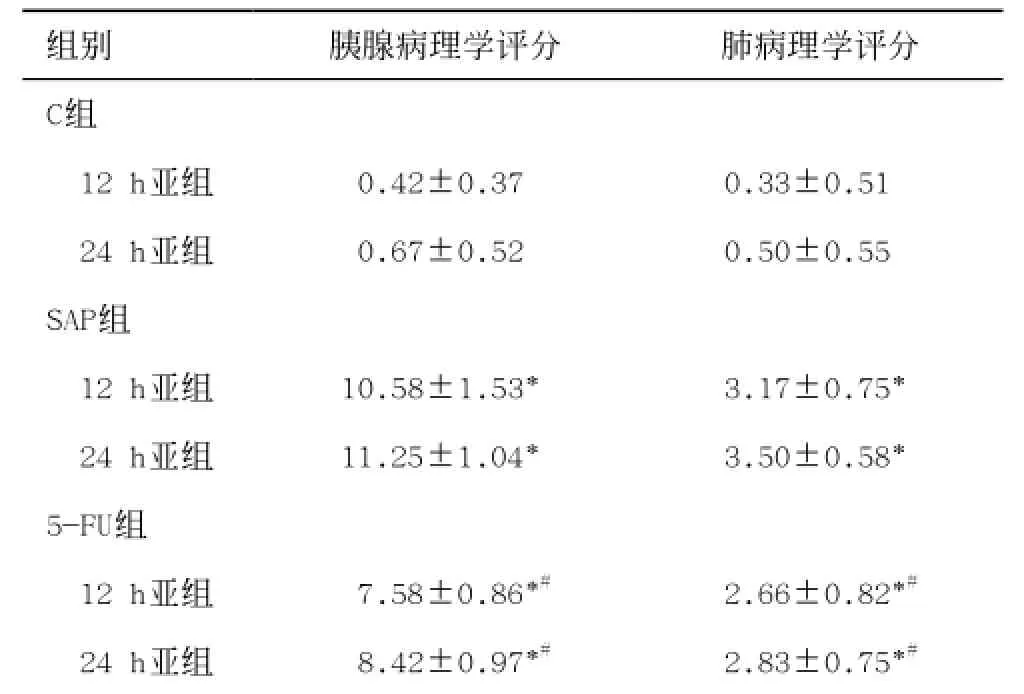
表1 各組胰腺和肺病理學(xué)評(píng)分( ±s)表示
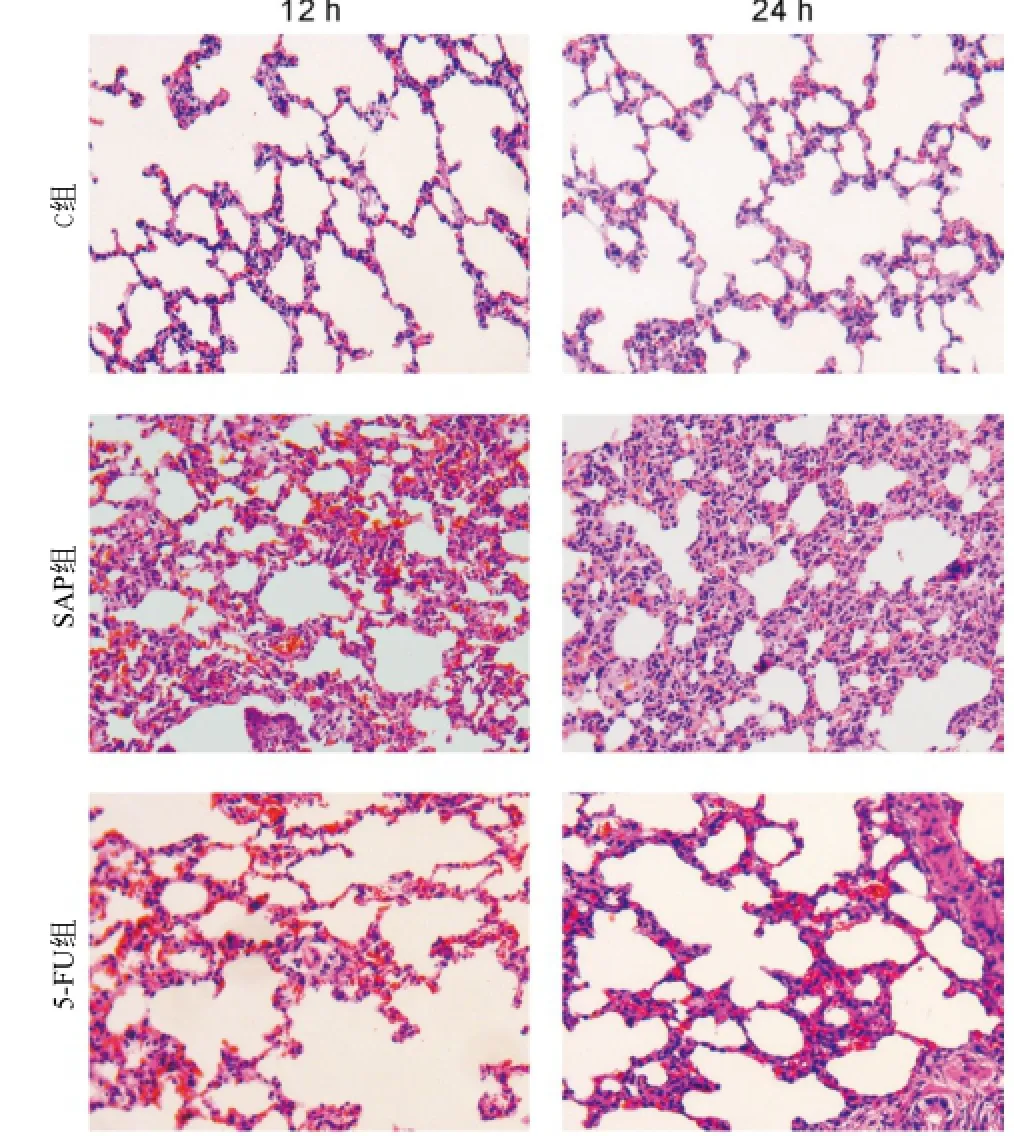
圖2 大鼠肺組織HE染色(×400)
2.3 胰腺和肺組織的病理學(xué)評(píng)分
SAP組胰腺和肺的病理學(xué)評(píng)分最高,胰腺和肺的病理?yè)p害嚴(yán)重。區(qū)域動(dòng)脈灌注5-FU后,胰腺和肺的病理?yè)p害程度減輕、病理學(xué)評(píng)分減少(P<0.05)。具體見(jiàn)表1。
2.4 各組血淀粉酶、血清中促炎細(xì)胞因子(TNF-α、IL-1β、IL-6)含量、肺組織濕干比、肺組織MPO活性改變
血淀粉酶SAP組與5-FU組在12 h和24 h均較對(duì)照組明顯升高(P<0.05)。區(qū)域動(dòng)脈灌注5-FU治療后血清中促炎細(xì)胞因子(TNF-α、IL-1β、IL-6)、肺組織濕干比。中性粒細(xì)胞浸潤(rùn)指標(biāo)MPO較SAP組均明顯下降,具有統(tǒng)計(jì)學(xué)意義(P<0.05)。具體見(jiàn)表2、表3。

表2 12 h各組血淀粉酶、TNF-α、IL-1β、IL-6的含量、肺組織濕干比、肺組織MPO活性改變

表3 24 h各組血淀粉酶、TNF-α、IL-1β、IL-6的含量、肺組織濕干比、肺組織MPO活性改變
3 討論
重癥急性胰腺炎(SAP)發(fā)生時(shí),胰腺壞死物質(zhì)吸收入血,刺激炎癥細(xì)胞分泌的炎癥介質(zhì)如TNF-α、IL-1β、IL-6等細(xì)胞因子觸發(fā)炎癥級(jí)聯(lián)反應(yīng),導(dǎo)致全身炎癥反應(yīng)綜合征(SIRS),嚴(yán)重的可導(dǎo)致多臟器功能衰竭(MODS),是SAP致死的主要原因[5]。其中肺損傷是SAP中最易發(fā)生也是最危險(xiǎn)的的胰外器官損傷[6],目前認(rèn)為與炎癥細(xì)胞的浸潤(rùn)和高濃度的炎癥介質(zhì)導(dǎo)致的肺血管損傷有密切的關(guān)系。本實(shí)驗(yàn)通過(guò)逆行胰膽管注射牛磺膽酸鈉成功建立SAP相關(guān)的急性肺損傷模型,具體改變?nèi)缦拢阂认俳M織病理呈典型的SAP改變,同時(shí)伴有血淀粉酶升高,肺組織病理可見(jiàn)肺間質(zhì)水腫、肺泡間隔增寬,炎性細(xì)胞浸潤(rùn)及紅細(xì)胞滲出。
RAI通過(guò)動(dòng)脈導(dǎo)管灌注藥物,使其直接到達(dá)病變部位,有效提高藥物在胰腺的濃度,并延長(zhǎng)藥物在胰腺的接觸時(shí)間,而外周血漿藥物濃度增加并不明顯,從而提高療效而減少藥物的毒副作用[7-9]。
自從上世紀(jì)70年代,5-FU就被用于治療急性胰腺炎[10-11]。長(zhǎng)期以來(lái),5-FU被認(rèn)為可干擾DNA和RNA的合成,抑制胰腺腺泡細(xì)胞合成和胰淀粉酶和胰蛋白酶的分泌[11]。關(guān)于5-FU治療急性胰腺炎的機(jī)制的探討,涉及多方面:一項(xiàng)研究表明5-FU確能誘導(dǎo)LPS激活的大鼠巨噬細(xì)胞凋亡[12];也有研究表明區(qū)域動(dòng)脈灌注5-FU對(duì)急性壞死性大鼠胰腺微循環(huán)缺血起到改善作用[13];近年來(lái)有研究認(rèn)為5-FU可阻斷某些炎癥介質(zhì)的作用[14-15],5-FU可抑制過(guò)高的促炎細(xì)胞因子和抗炎細(xì)胞因子的水平,使炎癥反應(yīng)維持在一個(gè)較低的水平,既防止急性胰腺炎早期的免疫過(guò)激狀態(tài),又有效地避免急性胰腺炎中后期的免疫抑制[16]。同時(shí)我們前期的研究也顯示,5-FU聯(lián)合奧曲肽對(duì)治療犬急性胰腺炎起到良好的治療效果[17]。
已有研究證實(shí)急性胰腺炎發(fā)病初期的胰腺損傷啟動(dòng)機(jī)體過(guò)度炎癥反應(yīng),釋放TNF-α、IL-1β、IL-6等促炎細(xì)胞因子,誘發(fā)免疫細(xì)胞的過(guò)激狀態(tài),是誘導(dǎo)ALI發(fā)生的主要原因之一[18-21]。本研究結(jié)果顯示,5-FU區(qū)域動(dòng)脈灌注組與SAP組比較,大鼠血中TNF-α、IL-1β、IL-6的含量降低顯著,胰腺和肺病理?yè)p害減輕顯著,說(shuō)明通過(guò)區(qū)域動(dòng)脈灌注5-FU治療SAP相關(guān)的ALI效果明顯。本實(shí)驗(yàn)評(píng)價(jià)肺損傷的指標(biāo)除肺病理學(xué)檢查外還包括:肺濕干比,肺組織MPO。其中肺濕干比作為肺部滲出指標(biāo),反映了肺水腫的程度。誘導(dǎo)SAP后肺濕干比上升,在區(qū)域動(dòng)脈灌注5-FU治療后出現(xiàn)下降,提示5-FU減輕了肺水腫。在ALI中,中性粒細(xì)胞的積聚起到了重要的作用,大量的中性粒細(xì)胞激活后會(huì)導(dǎo)致炎癥級(jí)聯(lián)反應(yīng),嚴(yán)重的會(huì)出現(xiàn)呼吸窘迫綜合征。肺組織MPO反映了肺組織內(nèi)中性粒細(xì)胞的數(shù)量,5-FU治療后MPO的減少提示中性粒細(xì)胞在肺組織內(nèi)積聚減少。
綜上所述,本實(shí)驗(yàn)結(jié)果表明區(qū)域動(dòng)脈灌注5-FU對(duì)大鼠SAP相關(guān)的ALI起到治療作用,降低了血淀粉酶和血中促炎細(xì)胞因子(TNF-α、IL-1β、IL-6)濃度,減少了中性粒細(xì)胞在肺組織內(nèi)的過(guò)度積聚,減輕肺損傷,其主要機(jī)制可能與抑制促炎細(xì)胞因子的過(guò)度表達(dá)有關(guān)。
參考文獻(xiàn):
[1] AHO H J, KOSKENSALO S M, NEVALAINEN T J. Experimental pancreatitis in the rat. Sodium taurocholate-induced acute haemorrhagic pancreatitis [J]. Scand J Gastroenterol,1980, 15(4): 411-416.
[2] 謝陽(yáng)云, 余夙慧, 陳必成, 等. 逆行胃左動(dòng)脈行腹腔干灌注治療大鼠胰腺炎的模型制作 [J]. 肝膽胰外科雜志,2013, 25 (1): 47-50.
[3] SCHMIDT J, LEWANDROWSI K, WARSHAW A L, et al. Morphometric characteristics and homogeneity of a new model of acute pancreatitis in the rat [J]. Int J Pancreatol, 1992, 12 (1): 41-51.
[4] OSMAN M O, KRISTENSEN J U, JACOBSEN N O, et al. A monoclonal anti-interleukin 8 antibody (ws-4) inhibits cytokine response and acute lung injury in experimental severe acute necrotising pancreatitis in rabbits [J]. Gut, 1998, 43(2): 232-239.
[5] DIMAGNO M J, DIMAGNO E P. New advances in acute pancreatitis [J]. Curr Opin Gastroenterol, 2007, 23(5): 494-501.
[6] STEER M L. Relationship between pancreatitis and lung diseases [J]. Resp Physiol, 2001, 128(1): 13-16.
[7] 陸永良, 姚行, 李鴻偉, 等. 區(qū)域動(dòng)脈灌注5-氟尿嘧啶對(duì)急性壞死性胰腺炎大鼠胰腺血流的改善作用 [J]. 中華實(shí)驗(yàn)外科雜志2001, 18(1): 56-57.
[8] ZHOU M T, CHEN B C, SUN H W, et al. Continuous regional arterial infusion with fluorouracil and octreotide attenuates severe acute pancreatitis in a canine model [J]. Plos One, 2012,7(5).
[9] XIE Y, ZHOU Z, LI M, et al. Pharmacokinetic comparison between regional arterial infusion and intravenous injection with 5-Fluorouracil in experimental severe acute pancreatitis [J]. Latin Am J Pharm, 2013, 32: 1-7.
[10] JOHNSON R, BARONE R, DAS GUPTA T K, et al. Treatment of experimental acute pancreatitis with 5-fluorouracil (5fu) [J]. Rev Surg, 1973, 30(1): 64-66.
[11] KINAMI Y, MIYAZAKI I, KAWAMURA M, et al. Clinical effects of anticancer drugs to pancreatic diseases as protein synthesis inhibitors [J]. Gastroenterol Jpn, 1976, 11(2): 123-132.
[12] WANG W, ZHOU X, YANG F, et al. The effects of 5-fluorouracil on severe acute pancreatitis-inducing apoptosis of macrophages [J]. Pancreas, 2014, 43(4): 660-663.
[13] 余昶, 周為中, 張啟瑜, 等. 重癥急性胰腺炎行區(qū)域性動(dòng)脈灌注治療的研究 [J]. 現(xiàn)代中西醫(yī)結(jié)合雜志, 16(17): 2341-2342.
[14] 高友兵, 汪訓(xùn)實(shí), 王嶸, 等. 5-氟尿嘧啶區(qū)域動(dòng)脈灌注對(duì)SAP大鼠炎性因子的影響 [J]. 醫(yī)學(xué)臨床研究, 2006, 23(10): 1562-1563.
[15] 馮新富, 蘆靈軍, 陳曉理, 等. 5-氟尿嘧啶對(duì)大鼠急性胰腺炎炎癥相關(guān)細(xì)胞因子的調(diào)節(jié)作用 [J]. 中國(guó)普通外科雜志2003, 12(10): 747-750.
[16] CHEN X L, CIREN S Z, ZHANG H, et al. Effect of 5-fu on modulation of disarrangement of immune-associated cytokines in experimental acute pancreatitis [J]. World J Gastroenterol, 2009, 15(16): 2032-2037.
[17] 周蒙滔, 張啟瑜, 施紅旗, 等. 早期區(qū)域動(dòng)脈灌注氟尿嘧啶、奧曲肽治療急性壞死性胰腺炎的機(jī)制探討 [J]. 肝膽胰外科雜志, 2001, 13(3): 149-151.
[18] ZHANG H, NEUHOFER P, SONG L, et al. IL-6 trans-signaling promotes pancreatitis-associated lung injury and lethality [J]. J Clin Invest, 2013, 123(3): 1019-1031.
[19] DE-MADARIA E, MARTINEZ J, SEMPERE L, et al. Cytokine genotypes in acute pancreatitis association with etiology,severity, and cytokine levels in blood [J]. Pancreas, 2008, 37 (3): 295-301.
[20] STIMAC D, FISIC E, MILIC S, et al. Prognostic values of il-6, il-8, and il-10 in acute pancreatitis [J]. J Clin Gastroenterol,2006, 40(3): 209-212.
[21] MAYER J, RAU B, GANSAUGE F, et al. Inflammatory mediators in human acute pancreatitis: clinical and pathophysiological implications [J]. Gut, 2000, 47(4): 546-552.
(本文編輯:魯翠濤)
·論著 基礎(chǔ)研究·
[中圖分類號(hào)]R576
[文獻(xiàn)標(biāo)識(shí)碼]A
Doi:10.11952/j.issn.1007-1954.2016.04.007
[收稿日期]2015-11-18
[基金項(xiàng)目]浙江省自然科學(xué)基金項(xiàng)目(LY12H03007);國(guó)家自然科學(xué)基金項(xiàng)目(81370563);浙江省醫(yī)藥衛(wèi)生重大科技計(jì)劃項(xiàng)目(WKJ2012-2-033);省科技廳公益性應(yīng)用項(xiàng)目(2012C23108);溫州市科技局項(xiàng)目(H20110016)。
[第一作者簡(jiǎn)介]李強(qiáng)(1990-),男,安徽阜陽(yáng)人,在讀碩士。
[通訊作者簡(jiǎn)介]周蒙滔,主任醫(yī)師,博士生導(dǎo)師,E-mail:zmt0417@hotmail.com。and the 5-FU group increased significantly (P<0.05). However, compared with the SAP group at 12 and 24 h,all those parameters were attenuated in the 5-FU group (P<0.05). Acute lung injury was observed in SAP group under light microscope. The RAI with 5-FU attenuated the SAP associated acute lung injury and decreased the pathological scores of lungs. Conclusion 5-FU has beneficial effects on SAP associated acute lung injury in rats,which mechanism possibly involves the alleviation the overexpression of proinflammatory cytokines.
Regional arterial infusion with 5-fluorouracil attenuates acute pancreatitis associated lung in-jury in rats model
LI Qiang1, JIN Yu
e-peng2, BAI Yong-yu1, HUANG Xin-che1, ZHOU Meng-tao2. 1The First Clinical Medical College, Wenzhou Medical University, Wenzhou, Zhejiang 325035, China;2Department of Hepatobiliary Surgery, the First Affiliated Hospital of Wenzhou Medical University , Wenzhou, Zhejiang 325000,China
Abstractobjective To assess the effects of 5-fluorouracil (5-FU) administered with regional arterial infusion (RAI) on severe acute pancreatitis (SAP) associated acute lung injury (ALI) in rats and to explore the underlying mechanisms. Methods Thirty-six male Sprague-Dawley (SD) rats were randomly divided into 3 groups: control group (C group), severe acute pancreatitis group (SAP group), and 5-FU administered with regional arterial infusion group (5-FU group). SAP model was induced by 5% sodium taurocholate (1 mL/kg) injected into the pancreatic duct. 5-FU (40 mg/kg) was immediately administered with RAI to the 5-FU group following the establishment of the SAP model. Saline was administered the same way to other two groups. The pathological changes in the pancreas and lungs were observed and scored. Serum amylase activity, lung wet/dry weight ratio,lung myeloperoxidase (MPO) activity, and the level of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β)and interleukin-6 (IL-6) in the serum were measured at 12 h and 24 h following the induction of SAP. Results Compared with the control group, the serum amylase activity, the levels of TNF-α, IL-1β and IL-6 in the serum,lung wet/dry weight ratio, lung MPO activity, as well as the pathological scores of lungs for both the SAP group
Key wordssevere acute pancreatitis; acute lung injury; 5-fluorouracil; regional arterial infusion; cytokines;rats

