長鏈非編碼RNA HOTAIR通過調控PIK3R3促進肝癌HepG2細胞的轉移和侵襲*
張婧婧, 吳 偉, 王 鵬, 楊景瑞, 段巨洪, 于海川△
(新鄉醫學院 1醫學檢驗學院和分子診斷與醫學檢驗技術河南省協同創新中心, 2第三附屬醫院,河南 新鄉 453003)
?
長鏈非編碼RNA HOTAIR通過調控PIK3R3促進肝癌HepG2細胞的轉移和侵襲*
張婧婧1, 吳 偉2, 王 鵬2, 楊景瑞1, 段巨洪1, 于海川1△
(新鄉醫學院1醫學檢驗學院和分子診斷與醫學檢驗技術河南省協同創新中心,2第三附屬醫院,河南 新鄉 453003)
目的: 探討長鏈非編碼RNA HOX轉錄反義RNA (HOTAIR)對肝癌HepG2細胞轉移和侵襲的影響。方法: 運用免疫組化技術檢測磷脂酰肌醇3-激酶調節亞基3(phosphoinositide-3-kinase regulatory subunit 3,PIK3R3)在正常肝臟組織和肝癌組織的表達;運用qPCR和Western blot檢測慢病毒LV3-shHOTAIR和LV3-shPIK3R3對HOTAIR和PIK3R3基因的沉默效率;Transwell侵襲實驗檢測HOTAIR和PIK3R3的表達對肝癌細胞HepG2侵襲能力的影響;劃痕實驗檢測HOTAIR和PIK3R3的表達對肝癌細胞HepG2遷移能力的影響;qPCR檢測沉默HOTAIR和PIK3R3后miR-214的表達;qPCR檢測轉染miR-214 mimics和miR-214 inhibitor后HOTAIR和PIK3R3的表達;雙螢光素酶報告基因系統檢測miR-214對HOTAIR和PIK3R3轉錄活性的影響。結果: 和正常肝組織比較,PIK3R3在肝癌組織中的表達明顯增加;沉默HOTAIR和PIK3R3基因后,肝癌細胞株HepG2的侵襲和轉移能力明顯降低;沉默HOTAIR和PIK3R3基因后,miR-214表達上調;轉染miR-214 mimics后,HOTAIR和PIK3R3的表達降低;轉染miR-214 inhibitor后,HOTAIR和PIK3R3的表達上調;雙螢光素酶報告基因系統檢測結果顯示miR-214可以直接調控HOTAIR和PIK3R3的轉錄活性。結論: HOTAIR可以通過miR-214調控PIK3R3的表達,從而促進肝癌細胞的侵襲和遷移能力
HOX轉錄反義RNA; 肝癌; 磷脂酰肌醇3-激酶調節亞基3
肝細胞癌是我國常見的一種惡性腫瘤,其發病率較高,侵襲性極強,進展非常迅速,預后極差。對患者生命造成嚴重威脅[1-2]。生長較為迅速,早期就出現淋巴管、血管以及遠處器官轉移等惡性臨床病理特點。與肝癌患者預后較差有關。探索肝癌發生、發展的生物學機制,并闡述肝癌發生和進展的分子生物學機制,可以為肝癌的診治提供新的重要途徑[3]。
近年來,盡管手術方式的改進、化療和放療效果的不斷提高,但是肝癌患者尤其是晚期肝癌患者的5年生存率仍然很低。腫瘤轉移是肝癌患者死亡的最主要的原因[4-5]。腫瘤轉移第一步是降低腫瘤細胞黏附能力,增強細胞運動性,然后浸潤周圍臨近組織和遠處轉移[6]。目前腫瘤轉移的分子生物學機制尚不明確,這對腫瘤治療造成很大的障礙。
長鏈非編碼RNA(long noncoding RNA,lncRNA)是一類長度大于200個核苷酸、不編碼蛋白的RNA分子。其可在表觀遺傳水平、轉錄和轉錄后水平調控基因的表達, 從而廣泛參與機體的生理與病理過程。 越來越多的證據表明lncRNA參與肝癌的發生與轉移過程。 研究表明在肝細胞癌中有一部分異常表達的lncRNA與腫瘤的發生、轉移、進展或診斷有關[7-8]。 HOTAIR是一種反義lncRNA,同時也是第一個被發現具有反式轉錄作用的lncRNA。Bayram等[9]研究發現,人體11 種成纖維細胞中轉錄自HOX基因的非編碼RNA 中有231 個lncRNA,同時在位于第12號染色體的HOXC基因座發現了一種特殊的lncRNA,其作用方式和其它lncRNA不同,主要是通過反式作用沉默染色質,并且編碼該lncRNA的DNA 僅一條鏈可以被轉錄,同時與HOX基因序列也不在同一條DNA 鏈上,因此將其命名為HOX轉錄反義RNA(HOX transcript antisense RNA,HOTAIR)。先前的研究發現,HOTAIR可以調控卵巢癌、乳腺癌、結腸癌等腫瘤的惡性生物學行為[9-10]。有文獻報道HOTAIR在肝癌中可以調控肝癌細胞的惡性生物學行為,但是在肝癌中其機制尚不明確。因此我們擬研究HOTAIR在肝癌細胞中的作用及其機制。
材 料 和 方 法
1 細胞株與主要試劑
人肝癌細胞株HepG2購自武漢大學中國典型培養物保藏中心。細胞在含10%胎牛血清的RPMI-1640,37 ℃、5% CO2條件下培養。胎牛血清和RPMI-1640培養基均購自Gibco;PIK3R3兔單克隆抗體均購自Abcam;Transwell小室購自Millipore;Matrigel購自BD;miR-214 mimics、miR-214 inhibitors、磷脂酰肌醇3-激酶調節亞基3(phosphoinositide-3-kinase regulatory subunit 3,PIK3R3)和HOTAIR沉默及對照慢病毒購自上海吉瑪制藥技術有限公司;PIK3R3和HOTAIR沉默慢病毒載體分別命名為LV3-shPIK3R3和LV3-shHOTAIR,其中LV3-NC為對照。miR-214、PIK3R3和HOTAIR的qPCR引物以及RNA提取試劑盒、逆轉錄試劑盒、PCR試劑盒均購自廣州復能基因有限公司。
2 實驗方法
2.1 免疫組化實驗 30例肝癌組織從手術中獲得;癌旁組織為距離腫瘤組織旁2 cm的組織。石蠟包埋組織,切片厚度4 μm。免疫組化實驗步驟按免疫組化SP試劑盒(北京博奧森生物技術有限公司)說明書操作,經組織脫蠟、水化,在枸櫞酸鹽緩沖液(pH 6.0)中使用微波爐抗原修復30 min,冷卻至室溫;然后用PBS洗3 min 3次,3% H2O237 ℃孵育15 min,PBS洗3 min 3次,5%驢血清(Abcam)37 ℃水浴30 min,兔抗人PIK3R3多克隆抗體(1∶100)37 ℃水浴2 h,PBS洗3 min 3次,加辣根過氧化物酶標記的驢抗兔IgG(1∶200,北京博奧森生物技術有限公司)孵育,PBS洗3 min 3次,辣根酶標記鏈酶卵白素工作液(北京博奧森生物技術有限公司)37 ℃水浴20 min,PBS洗3 min 3次,二氨基聯苯胺(DAB)顯色。陰性對照運用PBS代替 I 抗。所有切片的閱片經3名病理科醫生獨立完成,每個人選擇22個具有代表性的高倍視野(×400),同時計數每個標本陽性細胞數的比例,陽性結果的判定按照De Falco 等[11]描述的方法判定并評分:陽性細胞數不到1%為0分;陽性細胞數占1%到20%為1分;陽性細胞數占21%到40%為2分;陽性細胞數占41%到60%為3分;陽性細胞數超過61%為4分。
2.2 qPCR 檢測miR-214、PIK3R3和HOTAIR的表達 用TRIzol (Gibco)抽提組織總RNA,逆轉錄,qPCR擴增miR-214、PIK3R3和HOTAIR,PIK3R3和HOTAIR用GAPDH作為內參照,miR-214使用U6作為內參照。反應條件為95 ℃ 10 min;95 ℃ 10 s,60 ℃ 20 s,72 ℃ 10 s,重復35個循環。每個樣品設3個平行復孔,取平均值。反應結束后,由軟件自動得出螢光反應曲線以及每個標本反應體系的擴增效率及Ct值,實驗重復3次。
2.3 PIK3R3蛋白的檢測 裂解細胞以每孔20 μg上樣,經聚丙烯酰胺凝膠電泳后移至PVDF膜,然后加入山羊抗人PIK3R3多克隆抗體(稀釋度1∶5 000)孵育2 h后洗膜,再加入辣根過氧化物酶標記驢抗羊IgG,采用化學熒光發光法(ECL)顯影。運用凝膠成像系統成像,計算PIK3R3相對表達量。
2.4 Transwell侵襲實驗檢測肝癌細胞的遷移侵襲能力 所有試劑及器材均于冰上預冷,將Transwell小室置于24孔板內,小室內膜均勻涂抹Matrigel膠 50 μL(0.2 g/L),37 ℃孵育15 min,使膠凝固;消化、離心、計數細胞后,按照2.5×107/L用無血清培養基稀釋細胞,制成細胞懸液;按照每孔200 μL,將細胞懸液加入Transwell上室,同時在Transwell下室加入10% 胎牛血清+培養基500 μL,放入37 ℃孵箱培養;甲醛固定,結晶紫染色10 min,然后用棉簽輕輕擦拭內膜上的細胞。顯微鏡下計數4個高倍視野下穿過濾膜的細胞數。所有實驗重復3次。
2.5 劃痕實驗檢測肝癌細胞的遷移能力 將HepG2細胞接種于6孔板,待細胞融合度生長在90%時,用200 μL消毒槍頭從上而下劃線,并在顯微鏡下觀察,測量劃痕的初始距離(0 h);在24 h、48 h和72 h后,分別測量劃痕的距離,并拍照,計算細胞的遷移率。所有實驗重復3次。各組細胞任意3個部位的劃痕的寬度為D,則遷移率=[D(t=24 h、48 h或72 h)-D(t=0 h)]/D(t=0 h)。
2.6 雙螢光素酶報告基因檢測 于24孔板接種穩定轉染的細胞,細胞濃度為2×104。放置于細胞培養箱培養過夜。觀察細胞狀態,細胞融合約70%時轉染質粒。轉染試劑采用EndofectinTM-Plus。根據說明書步驟操作。將質粒DNA(3.2 μg)與phRL-TK(0.8 μg)按1∶4混合后,加入12 μL EndoFectinTM試劑。一邊輕輕渦旋裝有質粒溶液的試管,一邊將DMEM培養基稀釋的EndoFectinTM試劑滴加至試管中。充分混勻后,室溫靜置 10 min以形成 DNA-EndofectinTM復合物。將復合物加入24孔板中,輕輕搖細胞培養板,使均勻混合。24 h后,傾倒細胞培養液,每孔加入200 μL報告基因細胞裂解液。于室溫下裂解10 min,待細胞充分裂解后,12 000 r/min離心5 min,移液器吸取上清用于后續測定。融解海腎螢光素酶檢測緩沖液,待達到室溫。將海腎螢光素酶檢測底物(100×)放置于冰盒上備用。按照檢測每個孔的樣品需要100 μL檢測工作液的量,配置適量海腎螢光素酶檢測工作液。運用多功能酶標儀檢測。每個組3個復孔。并記錄數據。
3 統計學分析
采用SPSS 20.0軟件,計量數據以均數±標準差(mean±SD)表示,2組間均數比較采用t檢驗;3組
之間比較采用單因素方差分析,事后兩兩比較采用SNK-q檢驗。以P<0.05為差異有統計學意義。
結 果
1 PIK3R3在肝癌組織中表達增加
免疫組化結果顯示,PIK3R3定位在細胞膜和細胞漿。PIK3R3在肝癌組織中呈強陽性表達,在癌旁組織中呈弱陽性表達。肝癌組織中PIK3R3的表達明顯高于癌旁組織,差異有統計學顯著性(P<0.05),見圖1。
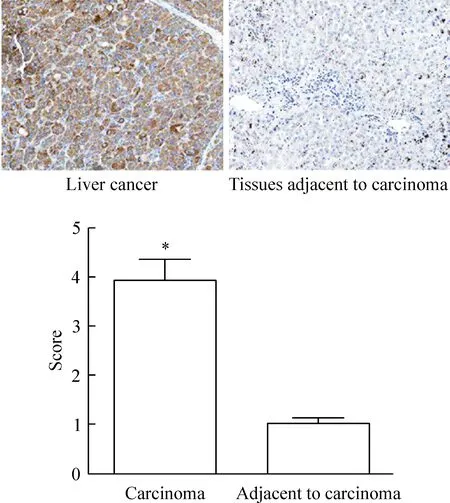
Figure 1.The expression of PIK3R3 in the liver cancer detected by immunohistochemical staining (×20). Mean±SD.n=3.*P<0.05vsadjacent to carcinoma.
圖1 免疫組化檢測PIK3R3在肝癌組織和癌旁組織的表達情況
2 HOTAIR通過miR-214調控 PIK3R3的表達
通過生物信息學預測(TargetScan)表明,HOTAIR和PIK3R3互為內源競爭性RNA。HOTAIR和PIK3R3可能通過miR-214相互調控。我們的結果發現,和對照組(LV3-NC)比較,肝癌細胞株HepG2感染慢病毒LV3-shHOTAIR后,PIK3R3的mRNA和蛋白水平明顯下調(P<0.05);和對照組(LV3-NC)比較,肝癌細胞株HepG2感染慢病毒LV3-shPIK3R3后,HOTAIR的mRNA水平明顯下調(P<0.05),提示HOTAIR和 PIK3R3可以相互調控。分別沉默HOTAIR和PIK3R3后,miR-214表達上調(P<0.05),提示HOTAIR可能通過miR-214調控PIK3R3的表達,見圖2。

Figure 2.HOTAIR regulated PIK3R3 by miR-214. A: silencing ofHOTAIRregulated the mRNA and protein expression of PIK3R3; B: silencing ofPIK3R3 regulated the mRNA expression of HOTAIR; C: silencing ofHOTAIRandPIK3R3 regulated the expression of miR-214. Mean±SD.n=3.*P<0.05vsLV3-NC group.
圖2 HOTAIR通過miR-214調控 PIK3R3的表達
3 HOTAIR和PIK3R3是miR-214的直接靶點
為了進一步證實HOTAIR和PIK3R3互為內源競爭性RNA,運用miR-214 mimics和miR-214 inhibitor轉染HepG2細胞。結果顯示,和對照組比較,轉染miR-214 mimics后,HOTAIR和 PIK3R3的表達降低;轉染miR-214 inhibitors后,HOTAIR和 PIK3R3的表達上調。同時雙螢光素酶報告系統檢測發現,HOTAIR和PIK3R3是miR-214的直接靶點,可能通過miR-214相互調控,見圖3。
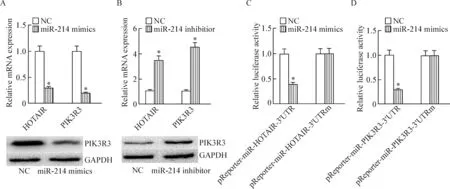
Figure 3.HOTAIR and PIK3R3 were direct targets of miR-214. A: the expression of HOTAIR and PIK3R3 was decreased when transfected with miR-214 mimics; B: the expression of HOTAIR and PIK3R3 was increased when transfected with miR-214 inhibitor; C: transfection of miR-214 mimics suppressed luciferase activity of miR-214 and HOTAIR binding sites; D: transfection of miR-214 mimics suppressed luciferase activity of miR-214 and PIK3R3 binding sites. Mean±SD.n=3.*P<0.05vsNC group.
圖3 HOTAIR和PIK3R3是miR-214的直接靶點
4 沉默HOTAIR和PIK3R3后,肝癌細胞株HepG2侵襲能力降低
Transwell結果顯示,感染LV3-shHOTAIR或者LV3-shPIK3R3后,肝癌細胞HepG2通過Matrigel基質膠的細胞數量分別為45.5±6.8和 50.7±4.7,明顯少于感染LV3-NC的HepG2細胞,差異有統計學顯著性(P<0.05),說明沉默HOTAIR和PIK3R3可以抑制肝癌細胞HepG2的侵襲能力,見圖4。
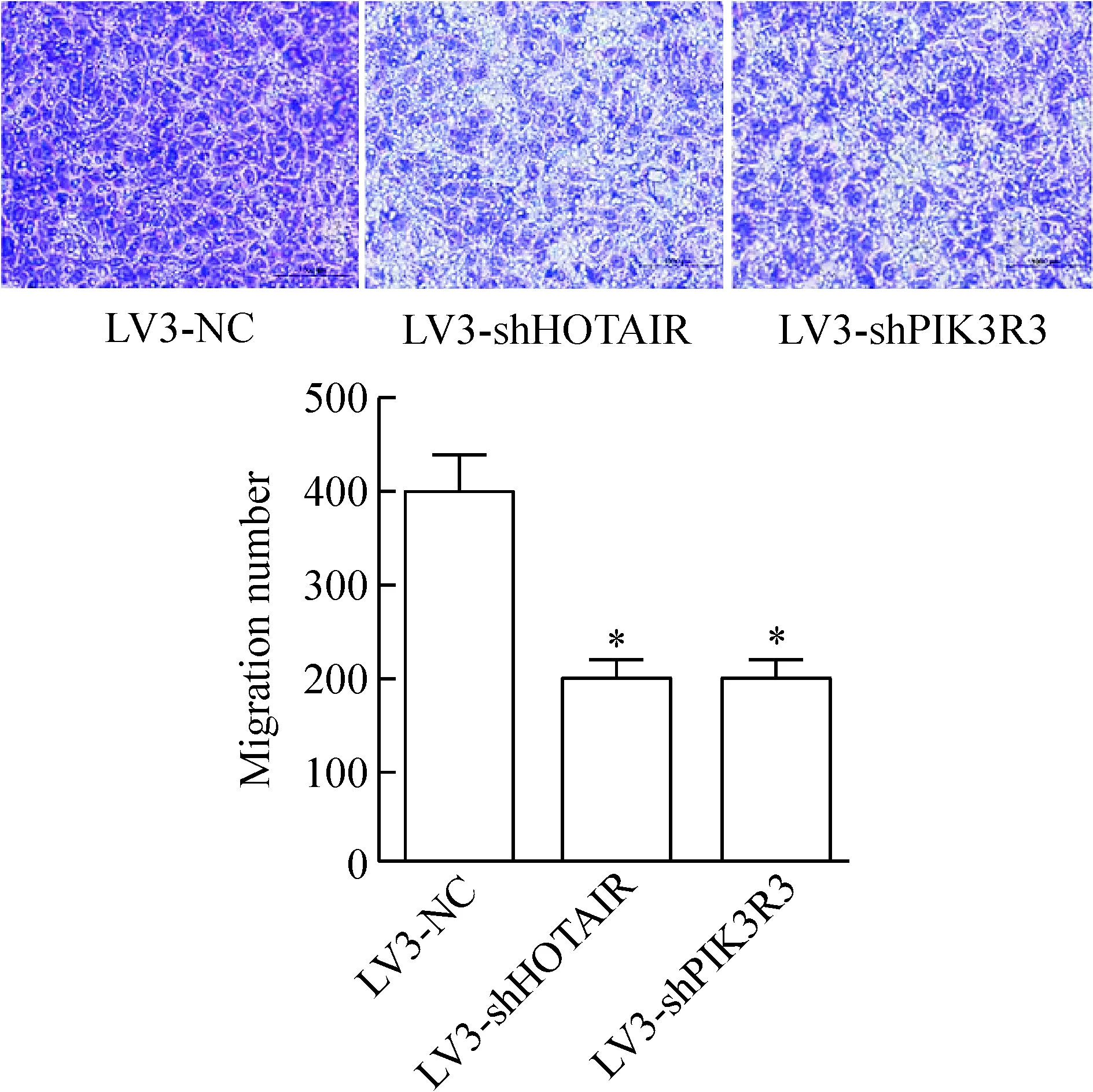
Figure 4.The effects ofHOTAIRandPIK3R3 silencing on the invasion ability of HepG2 cells detected by Transwell Matrigel invasion assay. Mean±SD.n=3.*P<0.05vsLV3-NC group.
圖4 Transwell侵襲實驗檢測沉默HOTAIR和PIK3R3對肝癌細胞HepG2侵襲能力的影響
5 沉默HOTAIR和PIK3R3后,肝癌細胞株HepG2的遷移能力降低
劃痕實驗結果表明,與LV3-NC轉染細胞組比較,在48 h時,LV3-shHOTAIR或LV3-shPIK3R3感染細胞組的遷移率明顯降低,差異有統計學顯著性,表明沉默HOTAIR和PIK3R3后,肝癌細胞株HepG2遷移能力降低,見圖5。
討 論
HOTAIR由HOXC基因的反義鏈轉錄產生,可以通過招募PCR2蛋白復合體,從而促使組蛋白H3K27的甲基化修飾,進而下調抑癌基因的表達,最后促進腫瘤的生長和轉移[12-14]。Yang等[15]運用檢測50 位肝癌患者肝癌組織和癌旁組織中HOTAIR 的表達發現,肝癌組織中HOTAIR 表達水平明顯高于癌旁組織,同時他們研究發現,肝癌復發患者中有65.7% 的患者HOTAIR 存在高表達狀態,但是未現復發患者HOTAIR低表達。因此推測HOTAIR可以作為肝癌患者預后的一個獨立標志。Ishibashi等[16]研究發現,在64位肝癌患者肝癌組織檢測發現, 13例患者存在HOTAIR的高表達,和不表達HOTAIR的患者比, HOTAIR高表達患者的腫瘤體積更大,預后顯著更差,因此提出HOTAIR可以作為判斷肝癌患者預后的一個獨立因子。Li 等[17]等研究發現,HOTAIR通過下調SETD2調控肝癌干細胞的干性,從而促進肝癌的惡性生物學行為。我們的研究發現,沉默HOTAIR后肝癌HepG2細胞侵襲和轉移能力降低。這說明HOTAIR在肝癌的發生和發展中發揮重要的作用。

Figure 5. The effects ofHOTAIRandPIK3R3 silencing on the migration ability of HepG2 cells detected by wound healing assay. Mean±SD.n=3.*P<0.05vsLV3-NC group.
圖5 劃痕實驗檢測沉默HOTAIR和PIK3R3對肝癌細胞HepG2轉移能力的影響
目前關于lncRNA的研究包括生物信息學預測結合實驗驗證以及生物芯片篩選結合實驗驗證[18-20]。lncRNA的作用機制非常復雜,涉及調控蛋白的穩定性或者定位[20-22]和DNA以及RNA相互作用調控基因表達[23-25]、lncRNA的“海綿作用”調控靶基因轉錄、通過影響轉錄因子和啟動子的結合抑制附近基因的轉錄、形成miRNA或者Piwi調控基因的表達[26]、調控mRNA前體的可變剪接、作為內源性競爭性RNA發揮調控作用、表觀遺傳學調控基因的調控[27]、與miRNA產生調控網絡的相互作用等多個方面。本研究通過生物信息學分析我們的研究證實,HOTAIR通過miR-214調控PIK3R3的表達,從而促進肝癌的發生和發展。
磷脂酰肌醇3-激酶是細胞內脂質底物的轉換器,可以被蛋白酪氨酸激酶受體富集和激活,進而產生第二信使參與多條信號通路,調控細胞的增殖和轉移[28]。PIK3R3在結腸癌中可以促進其生長和轉移,抑癌因子miR-152可以直接靶向PIK3R3抑制結腸癌的惡性生物學行為[29]。有研究發現,PIK3R3可以誘導EMT從而促進結腸癌的轉移能力[30]。在肝癌當中,miR-132和miR-511也可以通過靶向PIK3R3抑制肝癌細胞HepG2的增殖、侵襲和轉移能力[31-32]。在三陰性乳腺癌中,PIK3R3的高表達可以促進其轉移能力[33]。本研究中,我們發現,PIK3R3在肝癌組織中表達增加,同時沉默PIK3R3后,肝癌HepG2細胞轉移和侵襲能力降低,這和前面的報道一致。在本研究中,我們發現PIK3R3在肝癌組織中表達明顯增加,同時體外實驗發現沉默PIK3R3后肝癌細胞轉移和侵襲能力降低。這說明PIK3R3在體內和體外均可以調控肝癌細胞的生長。
Chen 等[34]研究發現,在肝癌中,miR-214可以通過靶向成纖維細胞生長因子受體1(fibroblast growth factor receptor 1,FGFR1),從而抑制肝癌細胞的轉移。另一項研究表明,順鉑通過促進microRNA-214表達抑制肝癌細胞HepG2及Hep3b的增殖[35]。在肝癌細胞Hep3b中,過表達miR-214后,肝癌細胞增殖能力明顯被抑制。這說明miR-214參與了肝癌的發生和進展。我們的研究發現,miR-214可以直接靶向調控HOTAIR和PIK3R3。這說明HOTAIR和PIK3R3促進腫瘤的作用,并且也間接說明HOTAIR和PIK3R3之間的相互調控是通過miR-214來實現的。
綜上所述,本研究表明HOTAIR在肝癌細胞的侵襲遷移中起重要作用,并對其相關機制做進一步探討,表明HOTAIR可能通過miR-214調控PIK3R3的表達,從而調控肝癌侵襲遷移的作用。這提示HOTAIR可能參與了肝癌的發展和轉移過程,有可能成為預測疾病發展、治療效果及預測預后的生物標志物。本研究主要從體外實驗研究,后續將深入研究HOTAIR對肝癌在體內的作用。
[1] Nielsen SR, Quaranta V, Linford A, et al. Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis[J]. Nat Cell Biol, 2016, 18(5):549-560.
[2] Zhang Y, Xi Y, Fang J, et al. Identification and characterization of monoclonal antibodies against GP73 for use as a potential biomarker in liver cancer screening and diagnosis[J]. J Immunoassay Immunochem, 2016, 37(4):390-406.
[3] Wang Y, He H, Chang J, et al. Mueller matrix microscope: a quantitative tool to facilitate detections and fibrosis scorings of liver cirrhosis and cancer tissues[J]. J Biomed Opt, 2016, 21(8):71112.
[4] Hong SH, Eun JW, Choi SK, et al. Epigenetic reader BRD4 inhibition as a therapeutic strategy to suppress E2F2-cell cycle regulation circuit in liver cancer[J]. Oncotarget, 2016, 7(22):32628-32640.
[5] Silberhumer GR, Paty PB, Denton B, et al. Long-term oncologic outcomes for simultaneous resection of synchronous metastatic liver and primary colorectal cancer[J]. Surgery, 2016, 160(1):67-73.
[6] Araújo OC, Rosa AS, Fernandes A, et al. RASSF1A and DOK1 promoter methylation levels in hepatocellular carcinoma, cirrhotic and non-cirrhotic liver, and correlation with liver cancer in brazilian patients[J]. PLoS One, 2016,11(4):e0153796.
[7] Ma Y, Huang D, Yang F, et al. Long noncoding RNA highly upregulated in liver cancer regulates the tumor necrosis factor-α-induced apoptosis in human vascular endothelial cells[J].DNA Cell Biol, 2016, 35(6):296-300.
[8] Dickson I. Hepatocellular carcinoma: a role for lncRNA in liver cancer[J]. Nat Rev Gastroenterol Hepatol, 2016,13(3):122-123.
[9] Bayram S, Sümbül AT, DadaE. A functionalHOTAIRrs12826786 C>T polymorphism is associated with breast cancer susceptibility and poor clinicopathological characteristics in a Turkish population: a hospital-based case-control study[J]. Tumour Biol, 2016, 37(4):5577-5584.
[11]De Falco M, Fedele V, Cobellis L, et al. Pattern of expression of cyclin D1/CDK4 complex in human placenta during gestation[J]. Cell Tissue Res, 2004, 317(2):187-194.
[12]Liu Y, Wang B, Liu X, et al. Epigenetic silencing of p21 by long non-coding RNA HOTAIR is involved in the cell cycle disorder induced by cigarette smoke extract[J]. Toxicol Lett, 2016, 240(1):60-67.
[13]Betancur JG, Tomari Y. Cryptic RNA-binding by PRC2 components EZH2 and SUZ12[J]. RNA Biol, 2015,12(9):959-965.
[14]Sharma S, Mandal P, Sadhukhan T, et al. Bridging links between long noncoding RNA HOTAIR and HPV oncoprotein E7 in cervical cancer pathogenesis[J]. Sci Rep, 2015, 5:11724.
[15]Yang Z, Zhou L, Wu LM, et al. Overexpression of long non-coding RNA HOTAIR predicts tumor recurrence in hepatocellular carcinoma patients following liver transplantation[J]. Ann Surg Oncol, 2011,18(5):1243-1250.
[16]Ishibashi M, Kogo R, Shibata K, et al. Clinical significance of the expression of long non-coding RNA HOTAIR in primary hepatocellular carcinoma[J]. Oncol Rep, 2013, 29(3):946-950.
[17]Li H, An J, Wu M, et al. LncRNA HOTAIR promotes human liver cancer stem cell malignant growth through downregulation of SETD2[J]. Oncotarget, 2015, 6(29): 27847-27864.
[18]Zhou M, Wang X, Shi H, et al. Characterization of long non-coding RNA-associated ceRNA network to reveal potential prognostic lncRNA biomarkers in human ovarian cancer[J]. Oncotarget, 2016, 7(11):12598-12611.
[19]Guo Q, Cheng Y, Liang T, et al. Comprehensive analysis of lncRNA-mRNA co-expression patterns identifies immune-associated lncRNA biomarkers in ovarian cancer malignant progression[J]. Sci Rep, 2015, 5:17683.
[20]Liu E, Liu Z, Zhou Y. Carboplatin-docetaxel-induced activity against ovarian cancer is dependent on up-regulated lncRNA PVT1[J]. Int J Clin Exp Pathol, 2015, 8(4):3803-3810.
[21]Tian T, Li C, Xiao J, et al. Quantitative assessment of the polymorphisms in theHOTAIRlncRNA and cancer risk: A meta-analysis of 8 case-control studies[J]. PLoS One, 2016,11(3):e0152296.
[22]Gao JZ, Li J, DU JL, et al. Long non-coding RNA HOTAIR is a marker for hepatocellular carcinoma progression and tumor recurrence[J]. Oncol Lett, 2016, 11(3):1791-1798.
[23]Liu S, Cui B, Dai ZX, et al. Long non-coding RNA HOTAIR promotes Parkinson’s disease induced by MPTP through up-regulating the expression of LRRK2[J]. Curr Neurovasc Res, 2016,13(2):115-120.
[24]Zhou Q, Chen F, Fei Z, et al. Genetic variants of lncRNA HOTAIR contribute to the risk of osteosarcoma[J]. Oncotarget, 2016,DOI: 10.18632/oncotarget.7957.
[25]Liu FT, Qui C, Zhang Y, et al. The association of HOTAIR expression with clinicopathological features and prognosis in gastric cancer patients[J]. Panminerva Med, 2016, 58(2):167-174.
[26]Zhang L, Liu Z, Li X, et al. Low long non-coding RNA HOTAIR expression is associated with down-regulation of Nrf2 in the spermatozoa of patients with asthenozoospermia or oligoasthenozoospermia[J]. Int J Clin Exp Pathol, 2015, 8(11):14198-14205.
[27]Berrondo C, Flax J, Kucherov V, et al. Expression of the long non-coding RNA HOTAIR correlates with disease progression in bladder cancer and is contained in bladder cancer patient urinary exosomes[J]. PLoS One, 2016, 11(1):e0147236.
[28]Wang G, Yang X, Li C, et al. PIK3R3 induces epithelial-to-mesenchymal transition and promotes metastasis in colorectal cancer[J]. Mol Cancer Ther, 2014, 13(7): 1837-1847.
[29]Li B, Xie Z, Li B. miR-152 functions as a tumor suppressor in colorectal cancer by targeting PIK3R3[J]. Tumour Biol, 2016, 37(8):10075-10084.
[30]Wang G, Yang X, Li C, et al. PIK3R3 induces epithelial-to-mesenchymal transition and promotes metastasis in colorectal cancer[J]. Mol Cancer Ther, 2014, 13(7):1837-1847.
[31]Liu K, Li X, Cao Y, et al. MiR-132 inhibits cell proliferation, invasion and migration of hepatocellular carcinoma by targeting PIK3R3[J]. Int J Oncol, 2015, 47(4):1585-1593.
[32]Cao G, Dong W, Meng X, et al. MiR-511 inhibits growth and metastasis of human hepatocellular carcinoma cells by targeting PIK3R3[J]. Tumour Biol, 2015, 36(6):4453-4459.
[33]Klahan S, Wu MS, Hsi E, et al. Computational analysis of mRNA expression profiles identifies the ITG family and PIK3R3 as crucial genes for regulating triple negative breast cancer cell migration[J]. Biomed Res Int, 2014, 2014:536591.
[34]Chen DL, Wang ZQ, Zeng ZL, et al. Identification of microRNA-214 as a negative regulator of colorectal cancer liver metastasis by way of regulation of fibroblast growth factor receptor 1 expression[J]. Hepatology, 2014, 60(2):598-609.
[35]劉子文, 杜永星, 由 磊, 等. 順鉑通過促進microRNA-214表達抑制肝癌細胞增殖[J].基礎醫學與臨床,2014, 34(9):1199-1203.
(責任編輯: 盧 萍, 羅 森)
Long noncoding RNA HOTAIR promotes liver cancer HepG2 cell migration and invasion by regulating PIK3R3
ZHANG Jing-jing1, WU Wei2, WANG Peng2, YANG Jing-rui1, DUAN Ju-hong1, YU Hai-chuan1
(1CollaborativeInnovationCenterofHenanProvince,InstituteofMedicalLaboratoryScienceandMolecularDiagnosticsandMedicalLaboratoryTechnology,2TheThirdAffiliatedHospital,XinxiangMedicalUniversity,Xinxiang453003,China.E-mail:fjingsc@163.com)
AIM: To investigate the effect of HOX transcript antisense RNA (HOTAIR) on the migration and invasion abilities of liver carcinoma HepG2 cells.METHODS: The expression of phosphoinositide-3-kinase regulatory subunit 3 (PIK3R3) in the liver cancer and normal liver tissues was detected by immunohistochemistry. The efficiency of gene silencing ofHOTAIRorPIK3R3 by LV3-shHOTAIR or LV3-shPIK3R3 was determined by qPCR and Western blot. The migration and invasion abilities of HepG2 cells after silencing ofHOTAIRandPIK3R3 were measured by wound healing assay and Transwell Matrigel invasion assay. The expression of miR-214 after silencing ofHOTAIRandPIK3R3 was analyzed by qPCR. The expression of HOTAIR and PIK3R3 in the HepG2 cells was also evaluated by qPCR after transfected with miR-214 mimics or miR-214 inhibitor. Dual-luciferase reporter assay system was used to determine the regulatory effect of miR-214 on HOTAIR andPIK3R3 expression.RESULTS: PIK3R3 expression increased significantly in the liver cancer tissues compared with normal liver tissues. The abilities of invasion and metastasis of hepatocellular carcinoma were reduced after silencing ofHOTAIRandPIK3R3. miR-214 expression was increased when silencing ofHOTAIRandPIK3R3 was performed. HOTAIR and PIK3R3 expression was reduced after transfection with miR-214 mimics. HOTAIR and PIK3R3 expression was increased after transfection with miR-214 inhibitor. The results of dual-luciferase reporter assay test showed that miR-214 directly regulatedHOTAIRandPIK3R3 transcription activity. CONCLUSION: HOTAIR regulates the expression of PIK3R3 through miR-214, thus promoting the migration and invasion abilities in the liver cancer cells.
HOX transcript antisense RNA; Liver cancer; Phosphoinositide-3-kinase regulatory subunit 3
1000- 4718(2016)10- 1775- 07
2016- 05- 18
2016- 06- 20
國家自然科學基金資助項目(No.31301135)
△通訊作者 Tel: 13938715600; E-mail: fjingsc@163.com
R730.23
A
10.3969/j.issn.1000- 4718.2016.10.008
雜志網址: http://www.cjpp.net

