Impact of Pitx3 gene knockdown on glial cell linederived neurotrophic factor transcriptional activity in dopaminergic neurons
Jing Chen, Xiao-yu Kang, Chuan-xi Tang, Dian-shuai Gao,
1 Experiment Teaching Center of Morphology, Xuzhou Medical University, Xuzhou, Jiangsu Province, China
2 Teaching and Research Section of Neurobiology and Anatomy, Xuzhou Medical University, Xuzhou, Jiangsu Province, China
Impact of Pitx3 gene knockdown on glial cell linederived neurotrophic factor transcriptional activity in dopaminergic neurons
Jing Chen1, Xiao-yu Kang2, Chuan-xi Tang2, Dian-shuai Gao2,*
1 Experiment Teaching Center of Morphology, Xuzhou Medical University, Xuzhou, Jiangsu Province, China
2 Teaching and Research Section of Neurobiology and Anatomy, Xuzhou Medical University, Xuzhou, Jiangsu Province, China
How to cite this article:Chen J, Kang XY, Tang CX, Gao DS (2017) Impact of Pitx3 gene knockdown on glial cell line-derived neurotrophic factor transcriptional activity in dopaminergic neurons. Neural Regen Res 12(8):1347-1351.
Graphical Abstract
Pitx3 is strongly associated with the phenotype, dif f erentiation, and survival of dopaminergic neurons.e relationship between Pitx3 and glial cell line-derived neurotrophic factor (GDNF) in dopaminergic neurons remains poorly understood.e present investigation sought to construct and screen a lentivirus expression plasmid carrying a rat Pitx3 short hairpin (sh)RNA and to assess the impact ofPitx3gene knockdown on GDNF transcriptional activity in MES23.5 dopaminergic neurons.ree pairs of interference sequences were designed and separately ligated into GV102 expression vectors.ese recombinant plasmids were transfected into MES23.5 cells and western blot assays were performed to detect Pitx3 protein expression. Finally, the most ef f ective Pitx3 shRNA and a dual-luciferase reporter gene plasmid carrying the GDNF promoter region (GDNF-luciferase) were cotransfected into MES23.5 cells. Sequencing showed that the synthesized sequences were identical to the three Pitx3 interference sequences. Inverted fl uorescence microscopy revealed that the lentivirus expression plasmids carrying Pitx3-shRNA had 40–50% transfection ef fi ciency. Western blot assay conf i rmed that the corresponding Pitx3 of the third knockdown sequence had the lowest expression level. Dual-luciferase reporter gene results showed that the GDNF transcriptional activity in dopaminergic cells cotransfected with both plasmids was decreased compared with those transfected with GDNF-luciferase alone. Together, the results showed that the designed Pitx3-shRNA interference sequence decreased Pitx3 protein expression, which decreased GDNF transcriptional activity.
nerve regeneration; neurodegeneration; Parkinson’s disease; glial cell line-derived neurotrophic factor; Pitx3; MES23.5 cells; short hairpin RNA; gene knockdown; plasmid; dual-luciferase reporter gene; neural regeneration
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative condition aer Alzheimer’s disease. Its major pathological feature is the chronic and progressive loss of dopaminergic (DA) neurons in the substantia nigra of the midbrain, which leads to clinical symptoms including high muscular tension, gait abnormality, resting tremor, and dyskinesia (Spillantini et al., 1997; Damier et al., 1999).
Glial cell line-derived neurotrophic factor (GDNF), a member of the transforming growth factor-β superfamily, was originally cloned and purified as a protective factor for damaged DA neurons (Lin et al., 1993; He and Yan, 2015). In the nigrostriatal system, GDNF provides specific trophic support and exerts survival-promoting andpost-damage repair ef f ects on DA neurons (Roussa et al., 2004; Yin et al., 2015). GDNF increases the expression of the transcription factors Nurr1 and Pitx3 to sustain DA neuron survival (Lei et al., 2011). After GDNF and GDNF-family receptor alpha-1 (GFRα1) form a complex, the recruited RET receptor protein can also increase the expression of transcription factors such as Pitx3 and Nurr1 (Olanow et al., 2015).
Transcription factors, such as Pitx3, Nurr1, and Msx1, regulate gene expression in DA neurons (Smidt et al., 2000, 2004; Kim et al., 2003; Andersson et al., 2006). Among these transcription factors, expression of Pitx3 is highest in DA neurons in the embryonic midbrain (Semina et al., 1997; Smidt et al., 1997; Messmer et al., 2007). Pitx3 knockout mice have decreased numbers of DA neurons in the substantia nigra (Maxwell et al., 2005), indicating that Pitx3 is a specif i c factor that regulates DA neuron development and is crucial for establishment and maintenance of the nigrostriatal pathway (Smidt et al., 2004). Interference of Pitx3 expression leads to decreased DA levels and DA neuron loss in the substantia nigra (Le et al., 2015), while Pitx3 overexpression promotes the dif f erentiation of DA neuron precursors from stem cells (Chung et al., 2005; Martinat et al., 2006) and signif i cantly increases GDNF levels in SY5Y cells (Peng et al., 2007).
In this study, we employed genetic engineering to construct a lentiviral plasmid for interference with Pitx3 expression to explore how Pitx3 regulates GDNF expression.
Materials and Methods
Plasmids, bacterial strains, cell strains, and reagents
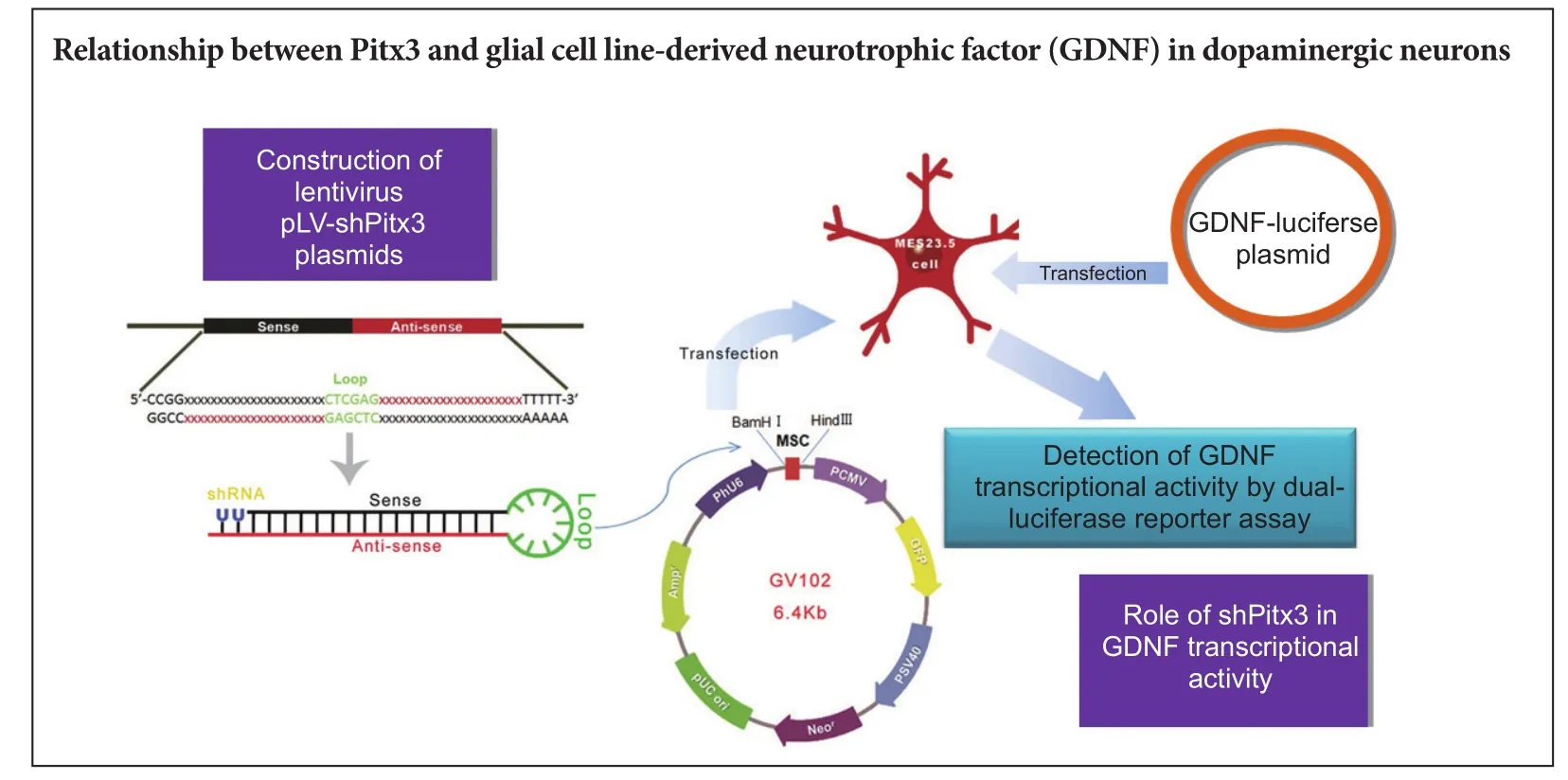
Construction of lentiviral vector pLV-shPitx3 carrying interference sequences
Screening Pitx3-short hairpin (sh)RNA interference plasmids
High-glucose Dulbecco’s modified Eagle’s medium (Gibco, Grand Island, NY, USA) with 10% fetal bovine serum (Gibco) was used to culture MES23.5 cells (Shanghai Cell Institute, Chinese Academy of Sciences, China) in a 6-well plate. LipofectamineTM2000 (Gibco) was used to transiently transfect the empty vector and the 3568-1 (1,072 bp), 3569-1 (1,133 bp), and 3570-1 (1,196 bp) shRNA plasmids into MES23.5 cells, which subsequently became the four experimental groups (Control, Sh-3568, Sh-3569 and Sh-3570;n≥ 3). All four plasmids contained green fluorescent protein and fluorescence peaked 48 hours after transient transfection. Cell samples were collected, and western blot assays were performed to determine the comparative Pitx3 protein expression level. Based on comparison with the control group, the plasmid with the best interference ef f ect was used in subsequent experiments.
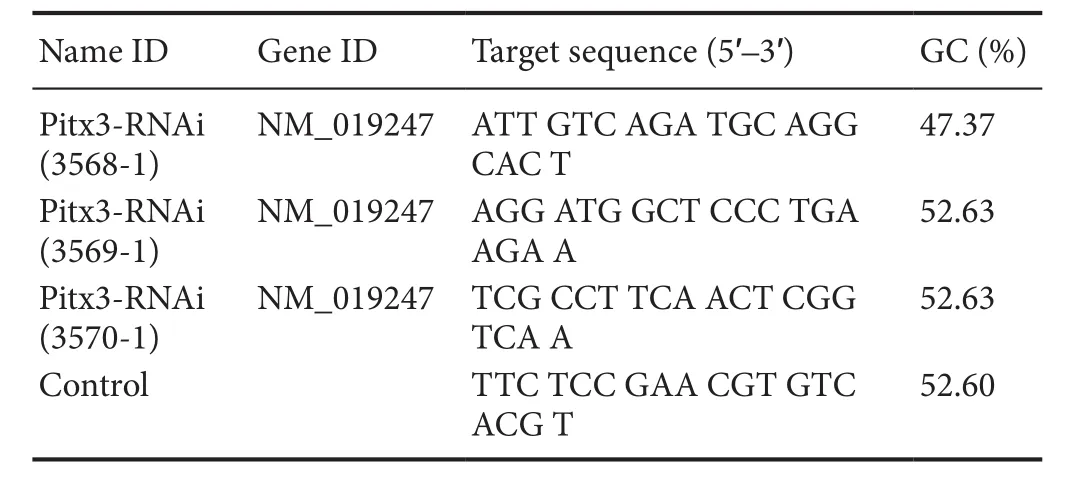
Table 1 Interference and control target sequence
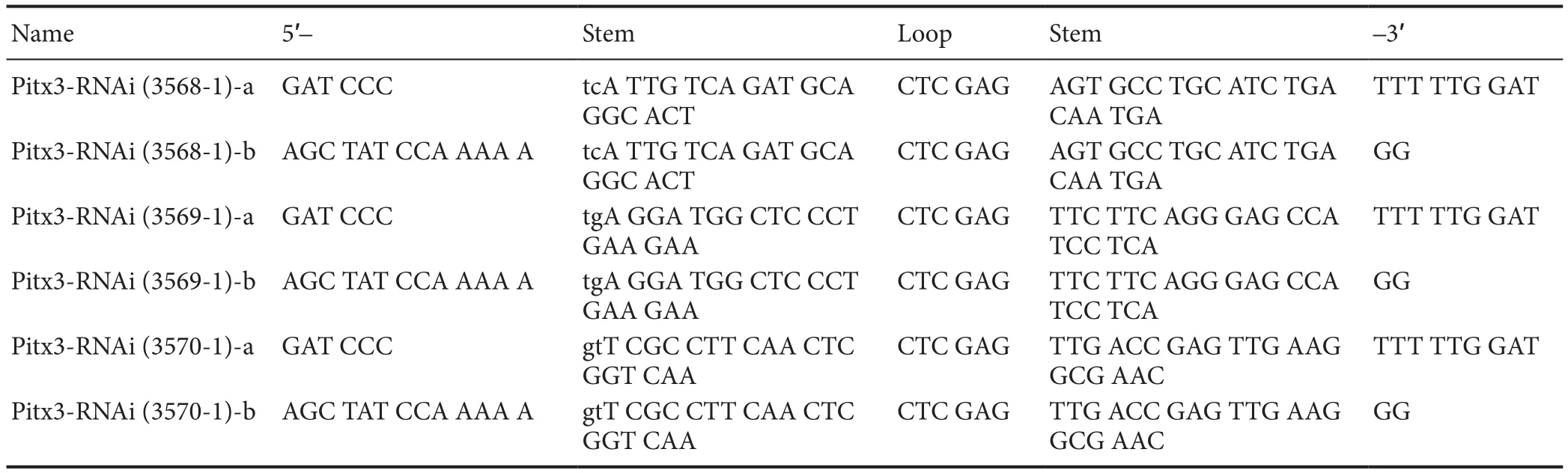
Table 2 shRNA sequences ofPitx3

Figure 1 Vector map and sequencing results of the interference plasmid.
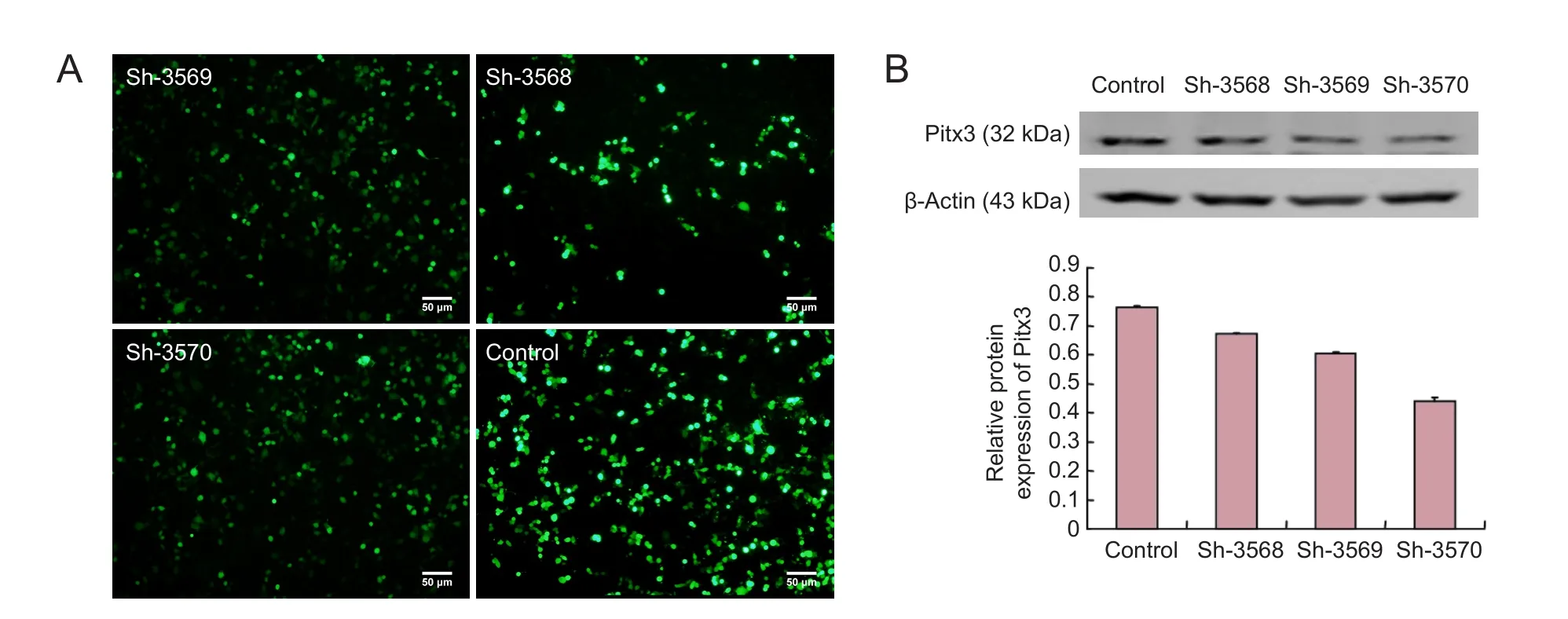
Figure 2 Screening interference plasmids.
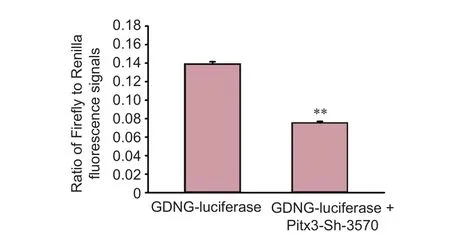
Figure 3 Ef f ects ofPitx3gene interference on glial cell line-derived neurotrophic factor (GDNF) transcriptional activity.
Western blot assay
The cells were collected and lysed with a protein extraction kit (Beyotime Company, Jiangsu Province, China). The bicinchoninic acid assay was used to measure protein concentrations before the samples were denatured at 100°C for 10 minutes, followed by centrifugation. The prepared samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and membrane transfer. Aer blocking, membranes were incubated with the primary antibodies anti-Pitx3 (rabbit polyclonal antibody; Sigma, St. Louis, MO, USA) and anti-β-actin (mouse monoclonal antibody; Santa Cruz Biotechnology, Santa Cruz, CA, USA), then secondary antibodies (IRDye 680RD goat anti-rabbit and IRDye 800CW goat anti-mouse; LI-COR Biosciences, USA). Protein bands were visualized using an Odyssey scanner (LI-COR Biosciences, Lincoln, NE, USA). ImageJ software (Wayne Rasband, National Institutes of Health, USA) was used for analysis. Each blot was representative of at least three experiments.
Detection of GDNF with the luciferase reporter gene
The upstream 1,449 bp sequence of the GDNF gene promoter region was designed (synthesized and validated byShanghai Jikai Genchem Co., Ltd.) and ligated into a GV306 dual-luciferase reporter gene vector. Cultured MES23.5 DA neurons were seeded into a 12-well plate and when they reached 80% conf l uency, they were cotransfected with Pitx3-shRNA and GDNF-luciferase plasmids or with the GDNF-luciferase plasmid alone. A dual-luciferase reporter assay system kit (Promega, Madison, WI, USA) was used to detect GDNF transcriptional activity, which was measured as the ratio of Firef l y to Renilla fl uorescence signals.
Statistical analysis
All analyses were performed with SPSS 13.0 soware (SPSS, Chicago, IL, USA). Histograms were generated with Sigma-Plot 13.0 soware (Systat Inc., San Jose, CA, USA). Data are presented as the mean ± SD. Independent samplet-test, oneway analysis of variance and the least signif i cant dif f erence test were used for statistical analysis, andP< 0.05 was considered statistically signif i cant.
Results
Construction of lentivirus pLV-shPitx3 plasmids
Plasmid sh-3570 had the best knockdown ef f ect
Using LipofectamineTM2000, the three expression plasmids were transiently transfected into MES23.5 cells. Aer 48 hours, fluorescence was detectable, indicating that the transfection was successful. Western blot assay was used to determine the knockdown ef fi ciency of the plasmids, which showed that sh-3570 exerted the strongest knockdown ef f ect (Figure 2).
Detecting the impact of Pitx3 knockdown on GDNF transcriptional activity
After three stable passages, MES 23.5 DA neurons were transfected with GDNF-luciferase plasmid with or without Pitx3-shRNA. After 48 hours, the cells were collected and subjected to luciferase measurement.e results showed decreased transcriptional activity of GDNF in the cells cotransfected with Pitx3-sh-3570 andGDNF-luciferase plasmids compared with those transfected with the GDNF-luciferase plasmid alone (Figure 3;P< 0.01).
Discussion
PD is characterized by the loss of DA neurons. Neurotrophic factors can exert protective effects on neurons (Kuhlmann et al., 2006; Wang et al., 2014; Duan et al., 2016); therefore, application of neurotrophic factors in PD can slow chronic degeneration and enhance the functional activity of residual DA neurons. GDNF is an important factor for sustaining central nervous system development (Carnicella et al., 2009) and is also a critical neurotrophic factor for treating PD (Pascual et al., 2008; Xing et al., 2016). Studies have shown that GDNF can slow DA neuron degeneration, enhance the functional activity of residual DA neurons, and promote differentiation of new DA neurons (Sun et al., 2004; Yang et al., 2011; Wang et al., 2016).
In this study, three Pitx3 interference sequences were designed and synthesized. The recombinant plasmids containing interference sequences of Pitx3 were transfected into MES23.5 cells and western blot assays showed that sh-3570 exerted the strongest knockdown effect on Pitx3. Through luciferase reporter gene assays, we further explored whether Pitx3 exerts an effect on GDNF expression. The reporter gene is part of a reporter system that uses fluorescein as a substrate to detect firefly luciferase activity, allowing it to detect an interaction between a transcription factor and the promoter region of a target gene. We designed a luciferase reporter gene plasmid carrying the GDNF promoter region and a plasmid carrying a sequence to decreasePitx3gene expression and transfected them into MES23.5 cells. The results showed that GDNF transcriptional activity was significantly lower in cells with Pitx3 knockdown, indicating that this intervention decreased GDNF transcription.ese data imply that Pitx3 may participate in PD protection by regulating GDNF transcription, in agreement with previous studies. Moreover, in light of the complex relationship between GDNF and Nurr1 in DA neurons (Galleguillos et al., 2010; Decressac et al., 2013; Volakakis et al., 2015), the mechanism by which Pitx3 participates in GDNF protection of DA neurons may be very complex.is will be a subject of our further research.
In summary, we successfully constructed a plasmid carrying a sequence to knockdown Pitx3 expression and a luciferase reporter plasmid expressing a GDNF promoter. We transfected them into MES23.5DA neurons and demonstrated that interfering with Pitx3 expression decreased GDNF transcription. Our results lay a foundation to further explore mechanisms to increase GDNF levels to protect DA neurons in PD. It is of importance to identify changes in the GDNF promoter region that alter GDNF expression, and other possible factors, by interfering withPitx3gene expression.
Author contributions:JC designed this study, performed experiments, collected data, analyzed data, interpreted the data and wrote the paper. XYK performed experiments, collected and analyzed data. CXT collected,analyzed and interpreted the data. DSG designed this study, obtained funding, administrated and supported technology. All authors approved the fi nal version of the paper.
Conf l icts of interest:None declared.
Data sharing statement:
Plagiarism check:Checked twice by ienticate.
Peer review:Externally peer reviewed.
Open access statement:
Andersson E, Tryggvason U, Deng Q, Friling S, Alekseenko Z, Robert B, Perlmann T, Ericson J (2006) Identif i cation of intrinsic determinants of midbrain dopamine neurons. Cell 124:393-405.
Carnicella S, Ron D (2009) GDNF--a potential target to treat addiction. Pharmacoler 122:9-18.
Chung S, Hedlund E, Hwang M, Kim DW, Shin BS, Hwang DY, Kang UJ, Isacson O, Kim KS (2005)e homeodomain transcription factor Pitx3 facilitates dif f erentiation of mouse embryonic stem cells into AHD2-expressing dopaminergic neurons. Mol Cell Neurosci 28:241-252.
Damier P, Hirsch EC, Agid Y, Graybiel AM (1999)e substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain 122:1437-1448.
Decressac M, Volakakis N, Bj?rklund A, Perlmann T (2013) NURR1 in Parkinson disease-from pathogenesis to therapeutic potential. Nat Rev Neurol 9:629-636.
Duan K, Wang X, Yang Z, Wang B, Wang M, Zhang H, Deng X (2016) Therapeutic effect of GDNF gene-modified mesencephalic neural stem cell transplantation in a rat model of Parkinson disease. Nan Fang Yi Ke Da Xue Xue Bao 36:32-38.
Galleguillos D, Fuentealba JA, Gómez LM, Saver M, Gómez A, Nash K, Burger C, Gysling K, Andrés ME (2010) Nurr1 regulates RET expression in dopamine neurons of adult rat midbrain. J Neurochem 114:1158-1167.
He J, Yan B (2015) Dif f erentiation of neural stem cells induced by glial cell line-derived neurotrophic factor. Zhongguo Zuzhi Gongcheng Yanjiu 19:8167-8171.
Kim H, Quan X, Seong Y, Kim J (2014) Impaired motor coordination in Pitx3 overexpression mice. Biochem Biophys Res Commun 446:1211-1218.
Kim KS, Kim CH, Hwang DY, Seo H, Chung S, Hong SJ, Lim JK, Anderson T, Isacson O (2003) Orphan nuclear receptor Nurr1 directly transactivates the promoter activity of the tyrosine hydroxylase gene in a cell-specif i c manner. J Neurochem 85:622-634.
Kuhlmann T, Remington L, Cognet I, Bourbonniere L, Zehntner S, Guilhot F, Herman A, Guay-Giroux A, Antel JP, Owens T, Gauchat JF (2006) Continued administration of ciliary neurotrophic factor protects mice from inf l ammatory pathology in experimental autoimmune encephalomyelitis. Am J Pathol 169:584-598.
Le W, Zhang L, Xie W, Li S, Dani JA (2015) Pitx3 def i ciency produces decreased dopamine signaling and induces motor def i cits in Pitx3(-/-) mice. Neurobiol Aging 36:3314-3320.
Lei Z, Jiang Y, Li T, Zhu J, Zeng S (2011) Signaling of glial cell line-derived neurotrophic factor and its receptor GFRα1 induce Nurr1 and Pitx3 to promote survival of graed midbrain-derived neural stem cells in a rat model of Parkinson disease. J Neuropathol Exp Neurol 70:736-747.
Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F (1993) GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 260:1130-1132.
Martinat C, Bacci JJ, Leete T, Kim J, Vanti WB, Newman AH, Cha JH, Gether U, Wang H, Abeliovich A (2006) Cooperative transcription activation by Nurr1 and Pitx3 induces embryonic stem cell maturation to the midbrain dopamine neuron phenotype. Proc Natl Acad Sci U S A 103:2874-2879.
Maxwell SL, Ho HY, Kuehner E, Zhao S, Li M (2005) Pitx3 regulates tyrosine hydroxylase expression in the substantia nigra and identif i es a subgroup of mesencephalic dopaminergic progenitor neurons during mouse development. Dev Biol 282:467-479.
Messmer K, Remington MP, Skidmore F, Fishman PS (2007) Induction of tyrosine hydroxylase expression by the transcription factor Pitx3. Int J Dev Neurosci 25:29-37.
Olanow CW, Bartus RT, Volpicelli-Daley LA, Kordower JH (2015) Trophic factors for Parkinson’s disease: To live or let die. Mov Disord 30:1715-1724.
Pascual A, Hidalgo-Figueroa M, Piruat JI, Pintado CO, Gómez-Díaz R, López-Barneo J (2008) Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci 11:755-761.
Peng C, Fan S, Li X, Fan X, Ming M, Sun Z, Le W (2007) Overexpression of pitx3 upregulates expression of BDNF and GDNF in SHSY5Y cells and primary ventral mesencephalic cultures. FEBS Lett 581:1357-1361.
Roussa E, Krieglstein K (2004) GDNF promotes neuronal dif f erentiation and dopaminergic development of mouse mesencephalic neurospheres. Neurosci Lett 361:52-55.
Semina EV, Reiter RS, Murray JC (1997) Isolation of a new homeobox gene belonging to the Pitx/Rieg family: expression during lens development and mapping to the aphakia region on mouse chromosome 19. Hum Mol Genet 6:2109-2116.
Smidt MP, Asbreuk CH, Cox JJ, Chen H, Johnson RL, Burbach JP (2000) A second independent pathway for development of mesencephalic dopaminergic neurons requires Lmx1b. Nat Neurosci (3:337-341.
Smidt MP, Smits SM, Burbach JP (2004) Homeobox gene Pitx3 and its role in the development of dopamine neurons of the substantia nigra. Cell Tissue Res 318:35-43.
Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha-synuclein in Lewy bodies. Nature 388:839-840.
Sun ZH, Lai YL, Li P, Zuo HC, Xie ZP (2004) GDNF augments Survival and diferentiation of TH -positive neurons in neural progenitor cells. Cell Biol Int 28:323-325.
Van den Munckhof P, Luk KC, Ste-Marie L, Montgomery J, Blanchet PJ, Sadikot AF, Drouin J (2003) Pitx3 is required for motor activity and for survival of a subset of midbrain dopaminergic neurons. Development 130:2535-2542.
Volakakis N, Tiklova K, Decressac M, Papathanou M, Mattsson B, Gillberg L, Nobre A, Bj?rklund A, Perlmann T (2015) Nurr1 and retinoid x receptor ligands stimulate ret signaling in dopamine neurons and can alleviate α-synuclein disrupted gene expression. J Neurosci 35:14370-14385.
Wang K, Demir IE, D’Haese JG, Tierunk E, Kujundzic K, Schorn S, Xing B, Kehl T, Friess H, Ceyhan GO (2014)e neurotrophic factor neurturin contributes toward an aggressive cancer cell phenotype, neuropathic pain and neuronal plasticity in pancreatic cancer. Carcinogenesis 35:103-113.
Wang T, Chen J, Tang CX, Zhou XY, Gao DS (2016) Inverse expression levels of ephrina3 and ephrina5 contribute to dopaminergic dif f erentiation of human SH-SY5Y cells. J Mol Neurosci 59:483-492.
Xing HX, Jiang JK, Qin LY, Wang YM (2016) Minocycline af f ects the expression of glial cell derived neurotrophic factor family in a rat model of Parkinson’s disease. Zhongguo Zuzhi Gongcheng Yanjiu 20:4020-4028.
Yang C, Zhou L, Gao X, Chen B, Tu J, Sun H, Liu X, He J, Liu J, Yuan Q (2011) Neuroprotective effects of bone marrow stem cells overexpressing glial cell line-derived neurotrophic factor on rats with intracerebral hemorrhage and neurons exposed to hypoxia/reoxygenation. Neurosurgery 68:691-704.
Yin XF, Xu HM, Jiang YX, Zhi YL, Liu YX, Xiang HW, Liu K, Ding XD, Sun P (2015) Lentivirus-mediated Persephin over-expression in Parkinson’s disease rats. Neural Regen Res 10:1814-1818.
Copyedited by Turnley A, Maxwell R, Wang J, Li CH, Qiu Y, Song LP, Zhao M
*< class="emphasis_italic">Correspondence to: Dian-shuai Gao, Ph.D., gds@xzhmc.edu.cn.
Dian-shuai Gao, Ph.D., gds@xzhmc.edu.cn.
orcid: 0000-0001-8567-0238 (Dian-shuai Gao)
10.4103/1673-5374.213557
Accepted: 2017-06-20
- 中國神經再生研究(英文版)的其它文章
- A progressive compression model of thoracic spinal cord injury in mice: function assessment and pathological changes in spinal cord
- A novel triple immunoenzyme staining enables simultaneous identif i cation of all muscle fi ber types on a single skeletal muscle cryosection from normal, denervated or reinnervated rats
- Optical coherence tomography and T cell gene expression analysis in patients with benign multiple sclerosis
- Dried Rehmannia root protects against glutamateinduced cytotoxity to PC12 cells through energy metabolism-related pathways
- Dexmedetomidine mitigates isof l urane-induced neurodegeneration in fetal rats during the second trimester of pregnancy
- Cold water swimming pretreatment reduces cognitive def i cits in a rat model of traumatic brain injury