M- (Sm, Pr, Ga)摻雜TiO2帶隙及電子結構的第一原理研究
房玉真, 孔祥晉, 劉軍海, 崔守鑫, 王東亭
(1.聊城大學 化學化工學院,聊城 252059; 2.聊城大學 物理科學與信息工程學院,聊城 252059)
1 Introduction
Titanium dioxide (TiO2) has drawn intense interest as promising material for photochemical applications[1-3]. It has excellent characteristics, such as long term stability and non-toxicity. Whereas factors that limit the usefulness of titania are its wide band-gaps (Eg= 3.20 eV for the anatase phase and 3.00 eV for the rutile phase), which can only be excited by UV light irradiation, i.e. only about 5% of the solar spectrum can be absorbed by TiO2[4-6]. Therefore, reducing the band gap of TiO2to make it photosensitive to the visible-light region has become one of the most important goals in photo-catalyst studies. In recent years, doping with non-metals and metal ions into the semiconductor matrix of TiO2have been used widely[7-9]. Doping with non-metal dopants (C, S, N, Br, Cl, etc.) can shift the top of the valence band to higher energies to reduce their forbidden band gaps[10-12]. On the other hand, a series of metal ions such as W6+, V5+, Ce4+, Zr4+, Fe3+, Cu2+, La3+, Pd2+, Cr3+, Ag+and Nd3+have been investigated[13-15], which promote the separation of photo-generated electrons and holes, and reduce electron-hole recombination. Some of them are manifested in experimental studies as significantly enhanced photo-catalytic activity in the visible region[16-19]. Although the optical properties of several doped TiO2systems have been studied, a number of questions arise simultaneously, including dopant metal ions type, the doping amounts and the dopant states on the doped TiO2. Lanthanides with special electronic layer structure (4fn6s2or 4fn- 15d6s2), rich level, and easy to produce more electronic configuration, doping TiO2lattice by lanthanide elements can increase the separation efficiency of photo-generated electron hole, and to improve the photo-catalytic activity[20]. According to the published literature, Ga doping will induce oxygen vacancies and create defect levels near conduction band in TiO2, which act as electron traps and enhance the separation of photo generated electron-hole pairs[21, 22]. It’s quite difficult to obtain the configuration, energy, electronic structure and the electronic distribution of different types of dopants in TiO2by experimental means. Meanwhile, a detailed study is necessary to understand the fundamental mechanism of chemical bonds in doped TiO2. As well as the first-principles electronic structure theory has played a crucial role in understanding various physical and chemical properties of TiO2[23, 24].
Based on the above reasons,we choose Sm, Pr and Ga as the dopant metal ions type in this work, and a systematic analysis of the dopant characteristics of elements (Sm/Pr/Ga) in anatase TiO2were investigated by the first principles. The optimized lattice parameters and electronic structures, as well as the effects of different kinds of dopants on electronic structures and the chemical bonds were compared. The understanding of the chemical bond between the dopant and O (or Ti) will be critical to improve the optical performance of TiO2-based photo-catalysts.
2 Computational methods
To examine the impacts of doping with Sm, Pr, Ga (M) element on the photo-catalytic activity of TiO2, a super-cell with M-Ti15O32was built where the M element substitutes for the Ti atom. All the calculations were performed using the CASTEP code based on first-principles density functional theory. The exchange and correlation interactions were modeled using the generalized gradient approximation and the Perdew-Burke-Ernzerhof (PBE) functional. The cutoff kinetic energy of the electron wave function was 380 eV, and the k-point sampling set 7×3×3 division of the reciprocal unit cell based on the Monkhorst-Pack scheme was found to be converged. In the geometrical optimization, all forces on atoms were converged to less than 0.3 eV/? , the maximum ionic displacement was within 0.001 ? and the total stress tensor was reduced to the order of 0.5 GPa. In this work, the band gap calculated by GGA is lower than the experimental value, which has also been accepted internationally for the result of the function itself, and it can not be used to make accurate calculation of the absolute energy. But as an effective approximation method, the relative value is still very accurate, and it can be used to analyze the band structure and electronic properties.
3 Results and discussion
3.1 Optimized structures and electronic properties
In this work, the pure,and the M- (Sm, Pr, Ga) doped anatase TiO2were studied. The calculated models are shown in Fig. 1, the green atom represents the substituted Ti atom, and their optimized lattice parameters and volumes are listed in Table 1.
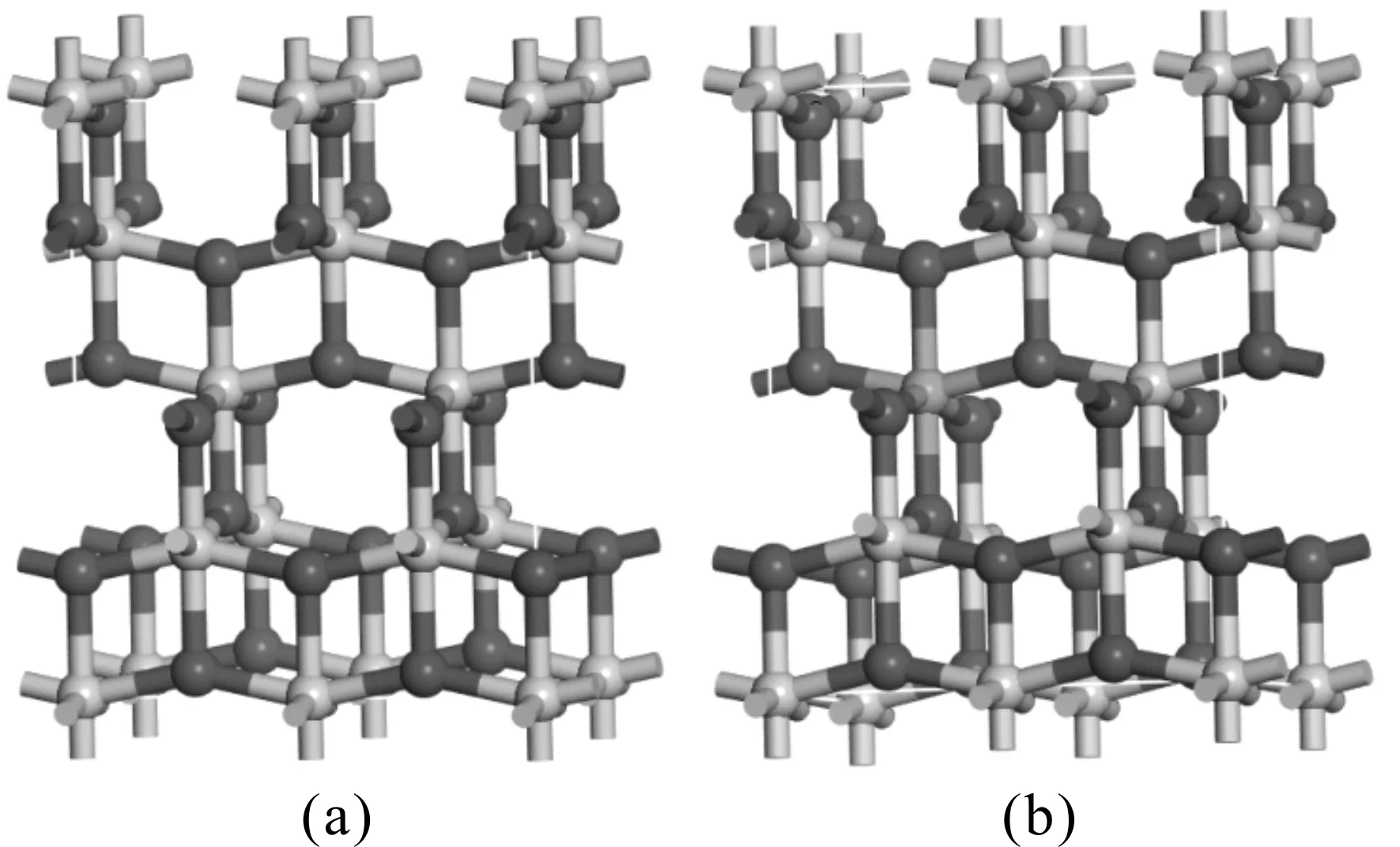
Fig. 1 The calculated models. (a) pure anatase TiO2; (b) M-doped anatase TiO2
For the pure anatase phase of TiO2, our calculated lattice parameters area=7.5648,b=3.7824,c=9.5102 ?, which are in excellent agreement with the previously reported experimental values[25], and all of lattice parameters in the doped systems are larger than that of pure TiO2except for Ga-doped TiO2. This can be understood by the fact that the radii of impurity atoms Sm (1.80 ?) and Pr (1.83 ?) are larger than that of Ti (1.45 ? ) and the radii of impurity atoms Ga (1.40 ?) are shorter. Although doping with foreign elements results in the variation of lattice constants, the deformations of a and c are less 4%, considering the larger radius of dopant atoms, the calculated models of the pure and M-doped anatase TiO2are structurally stable.
Table 1 The lattice parameters and volumes of the doped anatase TiO2supercells
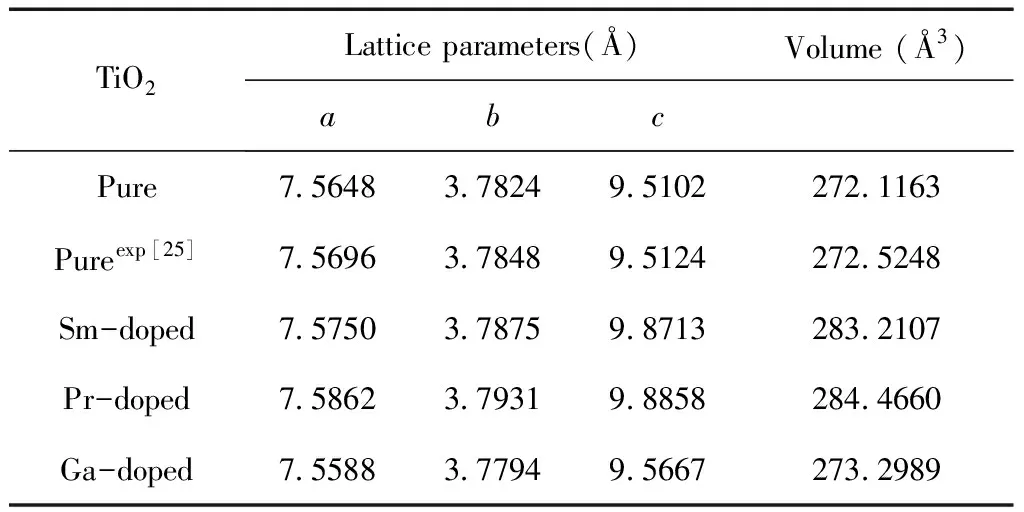
TiO2Lattice parameters(?)Volume (?3)abcPure7.56483.78249.5102272.1163Pureexp[25]7.56963.78489.5124272.5248Sm-doped7.57503.78759.8713283.2107Pr-doped7.58623.79319.8858284.4660Ga-doped7.55883.77949.5667273.2989
To further understand the chemical bond characteristic, we calculated theMulliken populations. The results for M-doped anatase TiO2are summarized in Table 2. For the pure anatase TiO2structure, the average Ti-O bond length is 1.947 ?, there is also a tiny change compared with those of the Sm-, Pr-and Ga-doped structures, and their average Ti-O bond lengths are 1.972 ?, 1.957 ? and 1.961 ?, respectively. In the case of doped TiO2systems, the lengths of all these Ti-O bonds have an increase due to the dopant atoms locating on the geometry optimization, which may relax oxygen atom away from the surface of titanium atom and into the dopants atoms. The longer distance of Ti-O bond length results in a weaker interaction between titanium and oxygen atom and hence the covalent bond is weakened and the ionic bond enhanced, and the Ti-O bond in Sm-doped case has ion bond characteristics. The average Sm-O, Pr-O, and Ga-O bond lengths are 2.216 ?, 2.409 ? and 1.992 ? respectively, considering the different radius of metal atoms, the Sm-O, Pr-O and Ga-O bonds have higher ion bond characteristics than Ti-O bond.
The bond strength is also judged by the population value, as a general rule, the larger population is, the stronger covalent bond is, and vice versa.The average Mulliken populations of Ti-O are 0.407, 0.419, 0.419 and 0.422 for the pure, Sm-, Pr- and Ga-doped structures, respectively, their corresponding values of Sm-O, Pr-O and Ga-O are 0.228, 0.264 and 0.292. As a result, the covalence of Ti-O bond enhanced after doping metal elements, and M-O bond has ion bond characteristics, which have been proved by the bond length discussed above.
Table 2 Average Mulliken populations (MP), bond length (BL) and net charge (NC) in the pure, and the M-doped (M= Sm, Pr, or Ga) anatase TiO2.
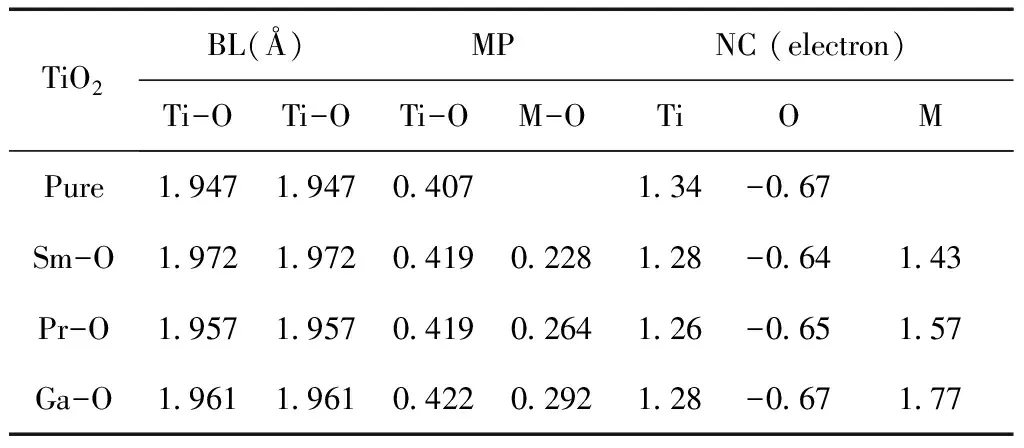
TiO2BL(?)MPNC (electron)Ti-OTi-OTi-OM-OTiOMPure1.9471.9470.4071.34-0.67Sm-O1.9721.9720.4190.2281.28-0.641.43Pr-O1.9571.9570.4190.2641.26-0.651.57Ga-O1.9611.9610.4220.2921.28-0.671.77
The atomic charge are also shown in Table 2, the net charges on Ti, Sm, Pr, and Ga are 1.34, 1.43, 1.57 and 1.77 e, respectively. Their corresponding values of O atom are -0.67, -0.64, -0.65 and -0.67 e in the pure Sm-, Pr- and Ga-doped structures. The most negatively charged atoms are the oxygen sites, which is slightly lower in pure anatase TiO2than those in Sm-doped and Pr-doped structures. The charge density near the dopants M is shown in Fig. 2 intuitively (only showing one plane[110]). From Fig. 2, we can see that the covalent bond strengths between the metal atom and adjacent O atoms increase in order of Ti-O, Pr-O, Sm-O and Ga-O. There is a covalent bonding behavior in the former two cases, but ionic bonding characteristic in the latter two case. These imply that more electron transfer from the Ga or Sm atom to an adjacent O atom rather than the sharing of electrons between Ti or Pr and O atoms.
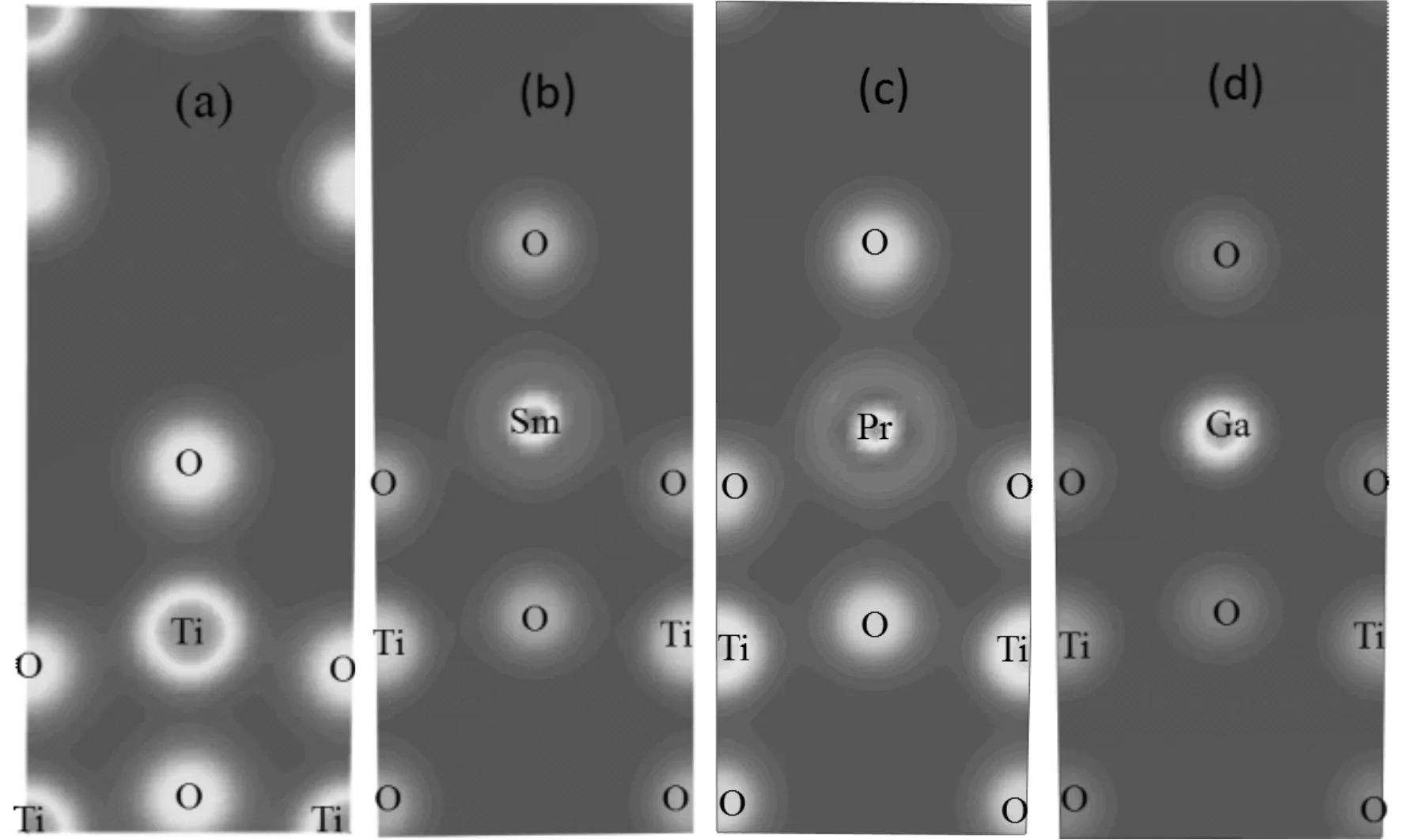
Fig. 2 The charge density of the M-doped anatase TiO2 (a) pure anatase TiO2; (b) Sm-doped; (c) Pr-doped; (d) Ga-doped.
As stated above,the doped atoms change the center of the positive charge centers to form internal dipole moment, which is conducive to the separation of photo-generated electron hole pairs, especially in the Sm-doped case. This result is in agreement with the following conclusion from the band structure and DOS analysis.
3.2 Band structure and DOS
To analyze the modifications of electronic properties and discover the origin reason of enhanced visible-light photocatalytic activity, the band structures and density of states (DOS) including projected density of states (PDOS) of pure and M-doped anatase TiO2were calculated. The calculated band gap structures are given in Fig. 3, the DOS and PDOS results are plotted in Fig. 4.
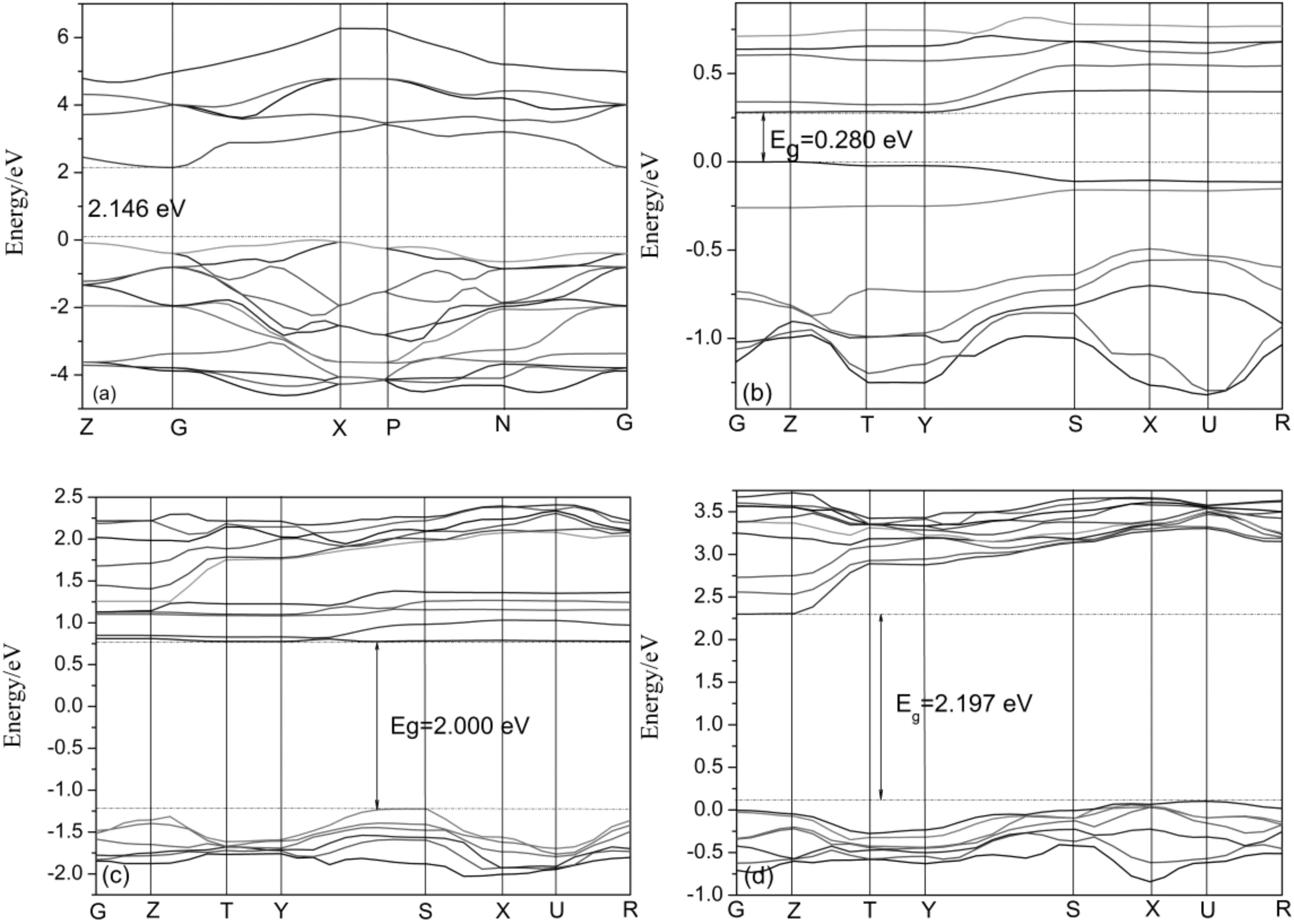
Fig.3 Band structures for all the simulated systems. (a) pure anatase TiO2; (b) Sm-doped; (c) Pr-doped; (d) Ga-doped.
As seen in Fig.3, the modification of Sm to TiO2introduces new states below the conduction band edge compared to the original TiO2, meanwhile, the dopant of Pr introduces both the conduction band and valence band to low edge, and the electronic gap between the highest occupied and lowest unoccupied electronic states is modified. As a result, the band gaps of Sm-doped (0.280 eV) and Pr-doped (2.000 eV) systems were reduced by 1.866 and 0.146 eV compared with that of pure TiO2(2.146 eV) respectively, which led to the red shift of the optical absorption edge and visible light could be absorbed by doped systems. Similar phenomena have been found by other groups both theoretically and experimentally[26, 27]. Whereas, the band gap increases by 0.051 eV when the dopant element being of Ga (2.197 eV).
Although the DFT method underestimates the band gap due to a self-interaction error, and the actual band gap of TiO2should be slightly larger than the calculated values, but the relative order and trend are credible.
The conduction and valence orbitals changes could be analyzed based on the DOS and PDOS of all systems. As shown in Fig. 4a, the valence bands are mainly made up of O-2p between -5.04 and 0 eV, and the small part of which is made up of Ti-3d orbitals between -5.01 eV and 0 eV. Meanwhile, the O-2p orbital hybridized with Ti-3d orbitals between 0 and -5.04 eV in the valence band, and the O-2p orbital hybridized with the Ti-3d orbital between 2.146 and 7.543 eV in the conduction band.
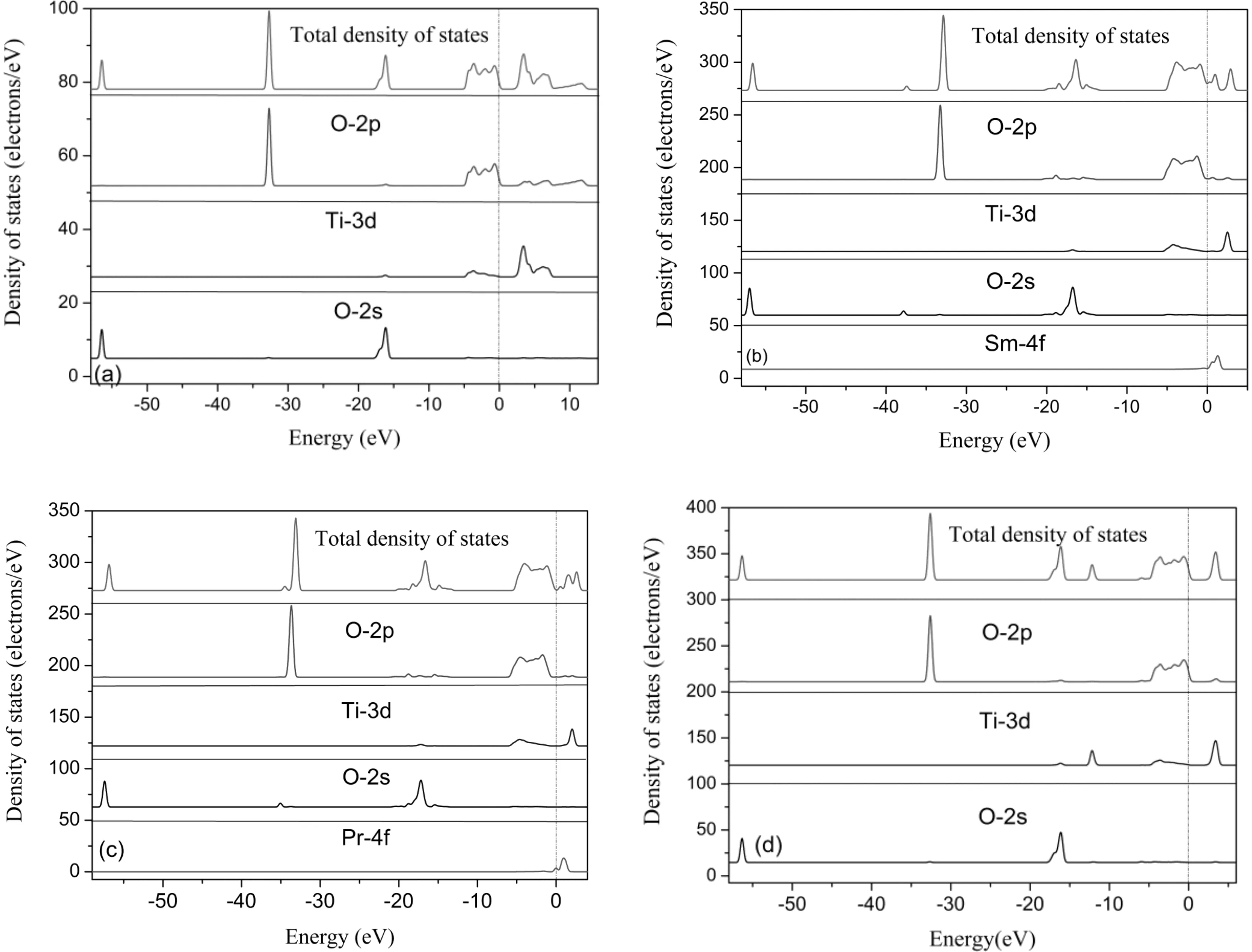
Fig. 4 Density of states for all the simulated systems: (a) pure anatase TiO2; (b) Sm-doped; (c) Pr-doped; (d) Ga-doped.
As shown in Fig.4b, Fig. 4c and Fig. 4d, the compositions of the conduction band and valence band in doped systems are homologous with the pure one. However, the conduction band moved towards the Fermi level, which resulted in the reduction of band gap in Sm-doped and Pr-doped systems. The dopants actually create new energy levels between the conduction and valence bands, known as inter-levels, in the TiO2band-gap. Meanwhile, Ga-doped TiO2behaves differently, when an Ga atom was introduced into TiO2, Ga-4p and Ga-3d orbitals hybridized with the O-2p orbital between -6.88 and 0.102 eV in the valence band and the Ga-4d orbital hybridized with the O-2p orbital between 2.299 and 4.42 eV in the conduction band. The conduction bands moved towards higher energy compared to pure TiO2, which caused the band gap increasing 0.051 eV.
4 Conclusions
In summary, the anatase TiO2systems doped with elements of Sm, Pr, and Ga were studied by first principles calculations. The band gaps of Sm-doped and Pr-doped systems were reduced by 1.866 and 0.146 eV compared with that of pure TiO2respectively, whereas the band gap increases 0.051 eV when the dopant element being of Ga. Anatase TiO2doped by Sm and Pr can be possible efficacy for the visible light photo-catalysis and solar energy conversion. The reasons may be that more electrons transfer from the Sm or Pr atom to adjacent O atoms to affect the strength of the hybrid orbital of M-O, and the hybridized orbitals can form some impurity energy levels, which can reduce the recombination rate of photo-excited carrier and improve the visible-light absorption performance of TiO2gradually.

