MB和CMC液體培養基對禾谷鐮孢產孢水平的影響
畢亞琪 王禹賀 羅泗川

摘要 禾谷鐮孢分生孢子定量接種是研究作物抗禾谷鐮孢的必要手段。本試驗利用綠豆(mung bean, MB)與羧甲基纖維素(carboxylmethyl cellulose, CMC)液體培養基在相同條件下培養禾谷鐮孢,比較兩種培養基對禾谷鐮孢產孢效率的影響;并用兩種培養基誘導分生孢子制成相同濃度孢子懸浮液,對75個玉米家系進行人工接種鑒定,檢測不同培養基所產分生孢子致病性差異。結果表明,CMC培養基誘導分生孢子增長率(k2=1.125)大于MB培養基誘導增長率(k1=0.844)。在培養第14天,MB培養基誘導獲得的分生孢子平均濃度為2.51×105個/mL,CMC培養基誘導的為3.62×105個/mL,比MB培養基誘導多44.22%。用兩種培養基誘生的孢子進行田間接種,玉米穗腐病發病程度無明顯差異。CMC培養基具有產孢快、孢子濃度高的優點,是一種適宜禾谷鐮孢分生孢子誘生的高效液體培養基。
關鍵詞 禾谷鐮孢; 玉米穗腐病; 分生孢子; 培養基
中圖分類號: S 435.121
文獻標識碼: A
DOI: 10.16688/j.zwbh.2019393
Abstract Artificial inoculation with the conidial suspension of Fusarium graminearum is an important technique for studying the diseases of maize ear and wheat related to F.graminearum. In this study, we used mung bean (MB) medium and carboxylmethyl cellulose (CMC) medium to cultivate the conidia of F.graminearum to compare their cultivating efficiencies. The two kinds of media were used to prepare conidial suspensions for artificial inoculation. The suspensions of F.graminearum from MB and CMC media were separately used to inoculate 75 families of maize recombinant inbred lines (RIL), in order to observe the differences in the infectious potential of the two suspensions. The results showed that, under the same conditions, the growth rates of F.graminearum spores in CMC medium (k2) and MB medium (k1) were 1.125 and 0.844, respectively. Higher growth rate led to higher spore concentration. After 14 days of incubation, the concentrations of F.graminearum conidia in MB medium and CMC medium were 2.51×105 spores/mL and 3.62×105 spores/mL, respectively, and the first was 44.22% higher than the latter. The ear rot identification after artificial inoculation indicated that there was no significant difference in maize ear rot infection between MB and CMC spore suspensions for the same concentration. Therefore, CMC medium was a more effective culture for producing F.graminearum conidia, because more spores could be harvested from CMC medium in a shorter time than from MB medium.
Key words Fusarium graminearum; maize ear rot; conidium; culture medium
禾谷鐮孢Fusarium graminearum是玉米穗腐病的主要致病菌[1],也是小麥赤霉病和莖基腐病的重要病原菌[2-6]。禾谷鐮孢主要侵染小麥、大麥、玉米和其他禾本科作物,在世界范圍內均可造成病害發生,嚴重影響作物產量和品質[3-4]。在我國,禾谷鐮孢可造成玉米10%~20%的田間損失率,高感品種損失率甚至高達65%[5];我國北方冬小麥夏玉米一年兩作輪作區或兩年三作區內,小麥赤霉病和玉米莖基腐病均有發生,經濟損失巨大[6]。利用禾谷鐮孢分生孢子進行定量接種是研究玉米穗腐病和小麥赤霉病等以禾谷鐮孢為致病菌的病害發生規律、品種抗性鑒定和防治技術的必不可少的手段。禾谷鐮孢一般通過分生孢子侵染宿主,因此獲得適宜的禾谷鐮孢分生孢子懸浮液是進行禾谷鐮孢相關病害定量接種和抗性鑒定的基礎。禾谷鐮孢在馬鈴薯葡萄糖瓊脂培養基(potato dextrose agar,PDA)上只能進行營養生長而幾乎不產生分生孢子[7],其大型分生孢子的誘生需采用其他培養基。目前常見的禾谷鐮孢分生孢子誘生培養基主要有康乃馨葉瓊脂(carnation leaf agar,CLA)培養基[8]、綠豆(mung bean,MB)液體培養基[9-11]和羧甲基纖維素(carboxylmethyl cellulose, CMC)培養基。生產上利用CLA培養基產孢需要近紫外光的誘導,且產生的大型分生孢子數量少,難以達到田間接種時的菌液濃度要求,故實際應用不多。本文將利用MB培養基和CMC培養基對禾谷鐮孢進行分生孢子產孢培養,比較兩種培養基的禾谷鐮孢產孢效率,并用兩種培養基獲得的禾谷鐮孢分生孢子配制相同濃度孢子懸浮液進行田間接種,研究兩種培養基所誘生的禾谷鐮孢分生孢子對玉米的致病性差異。
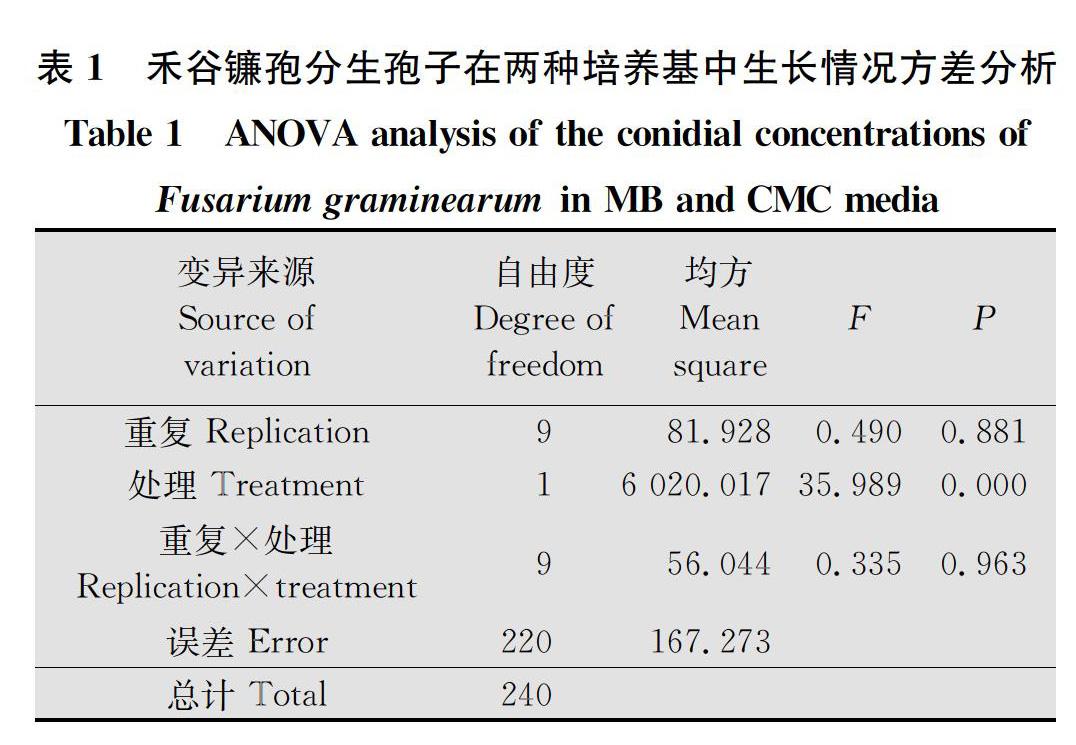
由兩個函數可見,MB培養基增長曲線中增長率k1=0.844,而CMC培養基增長曲線中增長率k2=1.125, k2>k1,即CMC培養基中禾谷鐮孢分生孢子增長速度大于MB培養基。至14 d觀察期結束時,CMC培養基內分生孢子平均濃度為3.62×105個/mL,比MB培養基(2.51×105個/mL)產孢多44.22%。
方差分析結果顯示,禾谷鐮孢分生孢子濃度在兩種培養基中差異顯著(P<0.05),相同處理下重復間差異不顯著(表1)。結果表明,用CMC培養基培養禾谷鐮孢分生孢子比用MB培養基更高效,同等環境條件下可在更短時間內培養出更高濃度的禾谷鐮孢分生孢子。
2.2 兩種孢子懸浮液對玉米致病力比較
由表2可見,75個家系經兩種相同濃度的孢子懸浮液接種后,各有平均7.5個家系因未出苗、未出穗和錯過接種時期造成數據缺失,其余的平均67.5個家系,經CMC培養的孢子懸浮液接種后,抗性對應1、3、5、7級和9級的家系在兩個重復間平均有7.0、4.5、7.0、11.5個和37.5個,而經MB孢子懸浮液接種后,表現為1級至9級抗性的家系分別有4.5、4.5、3.5、12個和43個(表2)。MB培養基誘導的分生孢子懸浮液接種后玉米群體平均表型為感病(7級)和高感(9級)的家系略多于CMC培養基誘導的分生孢子懸浮液接種后的家系;而群體表型平均為高抗(1級)、
抗病(3級)和中抗(5級)的家系略少于CMC培養的分生孢子接種的家系(表2)。利用Mann-Whitney U檢驗分析各家系用兩種孢子懸浮液接種后穗腐病病情指數,結果顯示用MB和CMC培養基制備的禾谷鐮孢分生孢子懸浮液在田間接種試驗中對玉米穗腐病致病性無顯著差異(P>0.05)(表3)。綜上所述,利用CMC培養基進行禾谷鐮孢分生孢子培養,耗時短、濃度高,且致病性與MB培養基培養的相同濃度的禾谷鐮孢相同。因此,CMC培養基是一種可以替代MB培養基進行禾谷鐮孢分生孢子誘導培養、并用于玉米穗腐病田間接種的高效培養基。
3 結論與討論
利用液體培養基進行禾谷鐮孢分生孢子誘導培養并配制孢子懸浮液進行人工定量接種是玉米穗腐病相關研究的關鍵技術。由于禾谷鐮孢在多種培養基上主要以營養生長為主,不易產生分生孢子,而不適宜的培養條件容易引起病原菌的變異及致病力的退化[9]。常用的禾谷鐮孢大型分生孢子培養方法中,康乃馨葉瓊脂培養基(CLA)需要近紫外燈光的誘導才能產孢,培養難度大,一般在病原菌接種上利用較少。當前大部分用于玉米田間接種的禾谷鐮孢分生孢子誘導多采用MB液體培養基[9-11],而MB培養基產孢速度慢、孢子濃度低,難以滿足田間大規模試驗定量接種的要求。因此,探索禾谷鐮孢大量產生大型分生孢子的培養方法是進行禾谷鐮孢相關病害研究的基礎。本研究利用CMC和MB液體培養基分別于相同條件下進行禾谷鐮孢大型分生孢子培養,根據孢子濃度計算兩種培養基的產孢增長率,結果表明,CMC培養基孢子增長率k2=1.125,MB培養基孢子增長率k1=0.844,說明禾谷鐮孢分生孢子在CMC培養基內比MB培養基增長快,且分生孢子可達到的最大濃度更高。CMC培養基中分生飽和狀態。浸根法根部直接暴露在菌液中,沒有土壤環境的干擾,利于發病,且不需要控制濕度。與灌根法和果實接種法相比,本研究建立的接種方法具有菌需求量小,需求空間小,對接種環境要求小,鑒定時間短等優點,適于大批量種質資源的篩選。本研究可為辣椒抗病育種工作提供技術支持。
參考文獻
[1] ZHU Chunyuan, YANG Xiaoyan, L Rongfei, et al. Phytophthora capsici homologue of the cell cycle regulator SDA1 is required for sporangial morphology, mycelial growth and plant infection [J]. Molecular Plant Pathology, 2016, 17(3): 369-387.
[2] ZHANG Huaixia, ALI M, FENG Xiaohui, et al. A novel transcription factor CaSBP12 gene negatively regulates the defense response against Phytophthora capsici in pepper (Capsicum annuum L.) [J/OL]. International Journal of Molecular Sciences, 2019, 20(1):48. DOI:10.3390/ijms20010048.
[3] TIAN Yuee, SUN Di, YANG Jinming, et al. Synthesis of sulfonate derivatives of maltol and their biological activity against Phytophthora capsici and Bursaphelenchus xylophilus in vitro [J]. Journal of Asian Natural Products Research, 2020,22(6):578-587.
[4] WANG Qiujun, MA Yan, YANG Hao, et al. Effect of biofumigation and chemical fumigation on soil microbial community structure and control of pepper phytophthora blight [J]. World Journal of Microbiology and Biotechnology, 2014, 30(2):507-518.
[5] 譚清群, 袁潔, 楊學輝, 等. 貴州省辣椒新品種對疫病和青枯病的抗性鑒定研究[J]. 種子, 2014, 33(11):82-85.
[6] 關天舒, 劉長遠, 王麗萍, 等. 辣椒抗疫病材料的篩選[J]. 江西農業學報, 2011, 23(11):100-102.
[7] NAEGELE R P, TOMLINSON A J, HAUSBECK M K. Evaluation of a diverse, worldwide collection of wild, cultivated, and landrace pepper (Capsicum annuum) for resistance to phytophthora fruit rot, genetic diversity, and population structure [J]. Phytopathology, 2015, 105(1): 110-118.
[8] LIU W Y, KANG J H, JEONG H S, et al. Combined use of bulked segregant analysis and microarrays reveals SNP markers pinpointing a major QTL for resistance to Phytophthora capsici in pepper [J]. Theoretical and Applied Genetics, 2014, 127(11): 2503-2513.
[9] YU J, SHEN D, DAI T, et al. Rapid and equipment-free detection of Phytophthora capsici using lateral flow strip-based recombinase polymerase amplification assay [J]. Letters in Applied Microbiology, 2019, 69(1): 64-70.
[10]赫衛, 張慧, 王瑩. 辣椒對疫霉抗性的快速鑒定[J]. 植物保護, 2018, 44(2): 145-148.
(責任編輯:楊明麗)

