凡納濱對蝦不同生長階段腸道可培養細菌耐藥性研究*
楊澤禹 萬夕和 史文軍 王李寶 黎 慧 沈 輝 喬 毅 蔣 葛 成 婕
凡納濱對蝦不同生長階段腸道可培養細菌耐藥性研究*
楊澤禹1,2萬夕和1①史文軍1王李寶1黎 慧1沈 輝1喬 毅1蔣 葛1成 婕1
(1. 江蘇省海洋水產研究所 江蘇 南通 226007;2. 高郵市水產技術指導站 江蘇 高郵 225600)
在凡納濱對蝦()養殖過程中多使用微生態制劑來調節水質,為避免破壞池塘菌群結構,很少使用抗生素。為了解凡納濱對蝦腸道細菌耐藥性與不同生長階段的關系,本研究選取江蘇地區4種主要養殖模式凡納濱對蝦成蝦和蝦苗作為研究對象,利用K-B紙片法和qRT-PCR技術,研究對蝦樣本腸道可培養細菌對四環素等12種抗生素的耐藥性和等9種抗生素耐藥基因(ARGs)的豐度。結果顯示,不同養殖模式中的凡納濱對蝦腸道可培養細菌優勢屬為弧菌屬();成蝦腸道內可培養細菌種類和數量較蝦苗顯著上升(<0.05),耐藥菌(antibiotics resistant bacteria, ARB)占比降低,ARGs豐度顯著下降(<0.05);不同養殖模式之間規律不明顯。研究表明,各模式下成蝦腸道細菌耐藥性和ARGs豐度均低于蝦苗,提示,養殖過程中通過施用微生態制劑來減少抗生素使用量的方法能降低凡納濱對蝦腸道可培養細菌的耐藥性。
凡納濱對蝦;細菌耐藥性;抗生素耐藥基因(ARGs);qRT-PCR
近年來,對蝦養殖已成為我國水產行業的重要組成部分(農業農村部漁業漁政管理局等, 2020)。經過多年的發展,江蘇沿海地區形成了多種凡納濱對蝦()養殖模式,包括池塘魚蝦混養、小型溫棚養殖等,經濟效益顯著。
隨著對蝦養殖業的迅猛發展,各種病害頻發(Lee, 2015)。為推進水產養殖綠色發展,農業農村部大力開展用藥減量行動,益生菌(Chumpol, 2017; 劉文亮等, 2017)、中草藥(朱璐丹等, 2019; Zhang, 2021)等新型無抗化防病技術不斷被運用到生產實踐中。目前,國務院獸醫行政管理部門批準的水產養殖用抗菌藥有12種(農業農村部, 2020)。研究表明,抗生素在水產養殖上使用后部分被養殖對象吸收,不能吸收的部分則被排出體外(張騫月等, 2015),殘留在土壤、水源中(D′costa, 2006),對動物體內以及環境中的細菌耐藥性產生定向選擇作用(?sterblad, 2001),誘導耐藥基因(antibiotic resistance genes, ARGs)的產生(Fridman, 2014; Frimodt-M?ller, 2019)與傳遞(Frost, 2005; Mazel, 2006),改變環境和生物體內微生態結構,最終威脅人類健康與安全。
本課題組前期調研發現,江蘇地區凡納濱對蝦養殖過程多使用微生態制劑來調節水質,為避免破壞池塘菌群結構,生產中很少施用抗生素。本研究選擇4種不同養殖模式的凡納濱對蝦蝦苗和成蝦作為研究對象,利用K-B紙片法研究其腸道可培養細菌對6大類12種抗生素(四環素、多西環素、氟苯尼考、氯霉素、磺胺異惡唑、復方新諾明、新霉素、慶大霉素、環丙沙星、諾氟沙星、頭孢曲松和頭孢噻肟)的耐藥性,并通過qRT-PCR技術檢測腸道細菌總DNA中5大類9種ARGs (、、、、、、、、)的豐度,探究江蘇地區常見4種養殖模式下凡納濱對蝦不同生長階段腸道耐藥菌(antibiotics resistant bacteria, ARB)和ARGs污染的變化情況,為后續防控細菌耐藥性產生以及水產養殖科學用藥提供參考依據。
1 材料與方法
1.1 樣品采集

表1 樣品信息

Tab.1 Sample information (n=10; Mean±SD)
1.2 可培養細菌平板計數、分離、純化及鑒定
對蝦體表用75%酒精擦拭消毒,并用滅菌生理鹽水漂洗后再進行腸道細菌分離培養。每組成蝦樣品取10只成蝦的腸道,混勻稱重后,加入1 mL滅菌生理鹽水充分研磨,取100 μL適當稀釋后的研磨液于2216E平板上均勻涂布,每個樣品做3個重復,28℃培養過夜后進行計數。每組蝦苗樣品取10只蝦苗重復上述操作。取均值作為各組凡納濱對蝦腸道可培養細菌含量。在平板上挑取形態不同的單菌落純化培養2代后,利用細菌通用引物27F和1492R進行PCR擴增,產物經生工生物工程(上海)股份有限公司測序后,通過Blast檢索系統對細菌序列同源性進行分析。
1.3 可培養細菌耐藥性檢測
以大腸埃希氏菌()標準菌株(ATCC 25922)為質控菌,利用K-B紙片法測定上述分離純化的細菌對四環素類、氯霉素類、磺胺類、氨基糖苷類、喹諾酮類和β-內酰胺類6大類12種抗生素的耐藥性,每株菌做3個重復。藥物敏感性實驗方法及判讀標準參考美國臨床與實驗室標準化研究所(CLSI)操作手冊(Clinical and Laboratory Standards Institute, 2017)和《全國臨床檢驗操作規程》(尚紅等,2015)。藥敏紙片和MH培養基購于杭州微生物試劑有限公司。耐藥菌占比計算公式如下:
1.4 ARGs豐度檢測
1.4.1 ARGs標準質粒構建及標準曲線繪制 利用ARGs引物(目的基因信息詳見表2),對本研究室儲存的對蝦源細菌DNA樣品進行PCR擴增,瓊脂糖凝膠電泳法檢測目的條帶。挑選出強陽性樣本,用TaKaRa MiniBEST Agarose Gel DNA Extraction Kit Ver.4.0 (TaKaRa)回收目的條帶。用pMD18-T Vector Cloning Kit (TaKaRa)對目的基因進行克隆。利用藍白斑篩選、通用引物M13普通PCR擴增以及菌液測序的方法來驗證克隆成功與否。用TaKaRa MiniBEST Plasmid Purification Kit Ver.4.0 (TaKaRa)提取克隆成功菌液的質粒,并以此質粒為標準品模板,利用Applied Biosystems Step One Plus Real-Time PCR System進行qRT-PCR分析,繪制標準曲線。
表2 目的基因信息

Tab.2 Target gene information
目的基因PCR程序:95℃ 5 min;95℃ 30 s,m45 s,72℃ 1 min,35個循環72℃ 10 min。qRT-PCR程序:95℃ 30 s;95℃ 5 s,60℃ 30 s,40個循環。溶解曲線程序:95℃ 15 s,60℃ 15 s,95℃ 15 s。20 L反應體系:10 μL TB Green Premix Ex(TaKaRa),上、下游引物各0. 4 μL,ROX 0.4 μL,模板0.2 μL。
1.4.2 樣品DNA提取及qRT-PCR檢測ARGs豐度
用3S DNA Isolation Kit for Environment Samples (上海博彩生物)提取樣品腸道細菌DNA(每組成蝦樣品取10只成蝦的腸道研磨混勻后提取,每組蝦苗樣品取10只蝦苗研磨混勻后提取),1.0%瓊脂糖凝膠電泳和Thermo Nanodrop 2000檢驗DNA質量,以合格的DNA為模板,進行qRT-PCR檢測,條件與反應體系同1.4.1。根據標準曲線計算各基因拷貝數的絕對含量,以為內參基因作歸一化處理,計算各ARGs的相對含量,并以該相對含量進行下一步數據統計和分析。
1.5 數據統計方法
采用Excel 2019進行可培養細菌數量統計、藥物敏感性分析、ARGs標準曲線繪制以及ARGs樣品t值歸一化處理等。用TBtools對基因相對含量進行聚類熱圖分析(Chen, 2020)。
2 結果
2.1 可培養細菌平板計數及分類鑒定結果
苗種樣品中可培養細菌總數最小值為33 CFU/g,最大值為2.73×104CFU/g;成蝦樣品可培養細菌總數最小值為6.33×104CFU/g,最大值為1.12×107CFU/g。按不同養殖模式分類,4種養殖模式苗期至成蝦期細菌總數分別上升1832.1 (M1)、206.1 (M2)、610.0 (M3)和81.8 (M4)倍。各組對蝦腸道可培養細菌含量對數值見圖1。
共分離純化得到17種、49株可培養細菌,來源于8個不同屬,分別為弧菌屬()、發光桿菌屬()、氣單胞菌屬()、西瓦氏菌屬()、芽孢桿菌屬()、微小桿菌屬()、鹽芽孢桿菌屬()、假交替單胞菌屬()(表3)。其中,數量最多的為弧菌屬,共計30株,占61.2%。弧菌屬主要種類為溶藻弧菌()和副溶血弧菌(),分別為11株和9株,占比為36.7%和30%。苗種樣品中分離到3屬,10種,21株細菌。其中,數量最多的為溶藻弧菌(9株),占42.9%。成蝦樣品中共分離到8屬,12種,28株細菌,其中副溶血弧菌8株,占30.8%。

圖1 各組對蝦腸道可培養細菌含量對數值
柱形圖上方不同字母表示差異顯著(<0.05).縱坐標為對應組別3個樣品可培養細菌含量對數值均值
Different letters on the column indicate significant difference (<0.05). The ordinate represents mean log values of three samples’ culturable bacterial quantity in the corresponding group
2.2 可培養細菌耐藥性檢測結果
對蝦腸道耐藥菌占比結果見表4。蝦苗和成蝦腸道耐藥菌占比均較低,對氯霉素、慶大霉素、頭孢曲松等藥物的耐藥菌占比都為0,對四環素類和磺胺類抗生素耐藥的菌株占比稍高,但最大也只有19.05%。同時,成蝦腸道耐藥菌占比均低于蝦苗。
2.3 ARGs豐度檢測結果
2.3.1 ARGs標準曲線 以ARGs拷貝數對數值為橫坐標,樣品t值為縱坐標繪制標準曲線,結果見表5。各ARGs標準曲線斜率()介于–3.388 6~–3.142 8之間,截距()介于40.647~46.469之間,相關系數(2)介于0.991 3~0.998 5之間,引物擴增效率(%)介于97.3~108.1之間,溶解曲線無雜峰。結果表明,標準曲線線性良好,且引物擴增效率高,能夠用于相對拷貝數的計算。
2.3.2 ARGs豐度及分布特征 結果顯示,所有耐藥基因豐度在凡納濱對蝦腸道中表現較低,以樣品P-TTY-2中豐度稍高,為84.6;在C-TTY-1和C-DSM-1兩份樣品中未被檢出;在C-TTX-1、C-XP-1、C-XP-2、C-XP-3、C-DSM-1、C-DSM-2和C-DSM-3七份樣品中未被檢出。在樣品C-DSM-1中未被檢出。樣品中9種ARGs相對含量范圍見表6。
表3 可培養細菌分類及數量

Tab.3 Classification and quantity of culturable dominant bacteria
表4 對蝦腸道耐藥菌占比
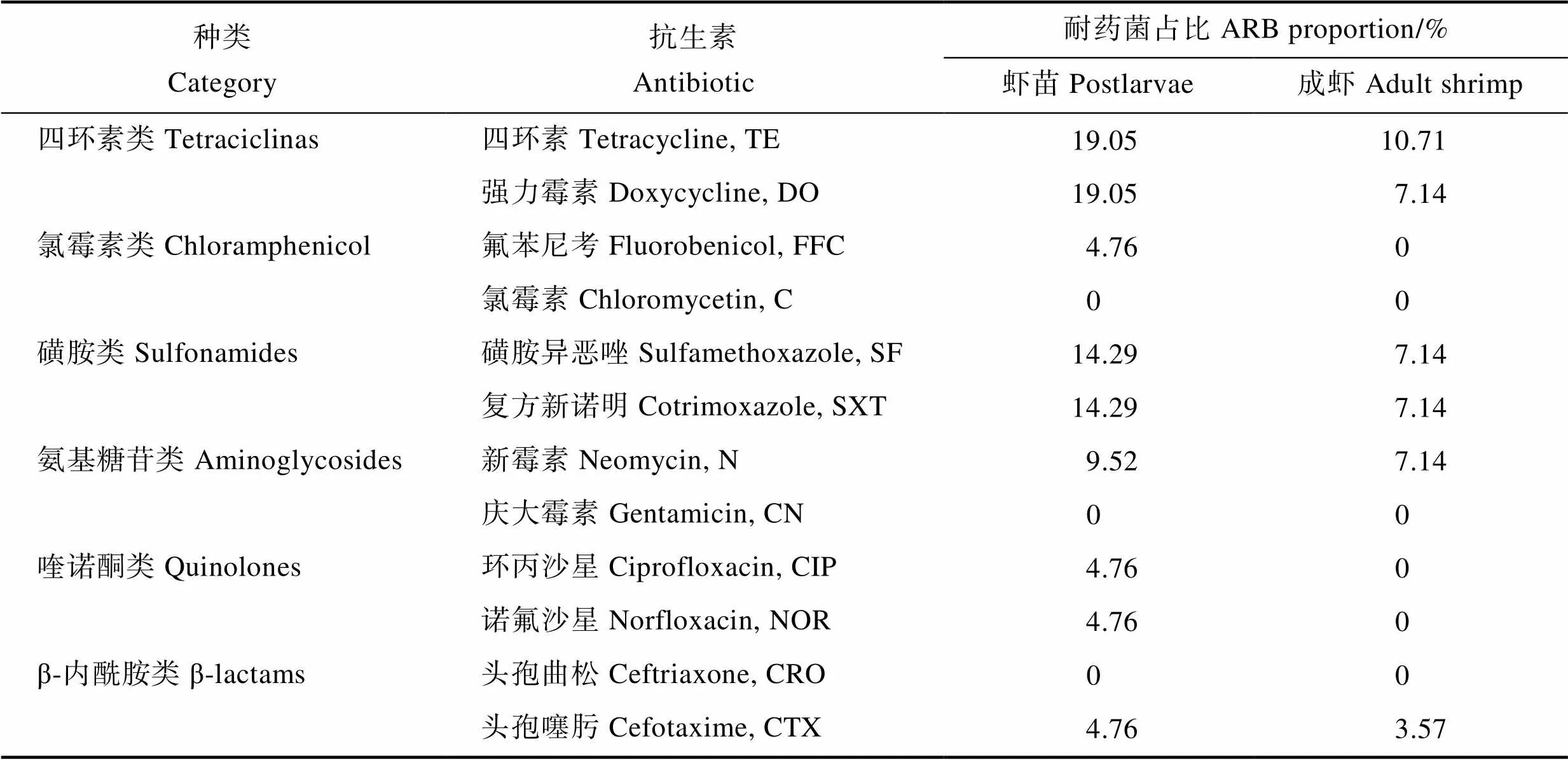
Tab.4 Antibiotics resistant bacteria (ARB) proportion in shrimp intestinal tract
ARGs豐度檢測結果見圖2。由ARGs豐度聚類分析結果可見,苗種和成蝦樣品聚在不同的分支,養成后的對蝦中的ARGs豐度均較養殖前蝦苗中低,表明經過1個養殖周期,ARGs豐度降低。氯霉素類(、)、四環素類()和磺胺類() 4種ARGs在蝦苗樣品中出現。
表5 抗生素ARGs標準曲線

Tab.5 Standard curves of drug antibiotic resistance genes
表6 9種ARGs相對含量范圍
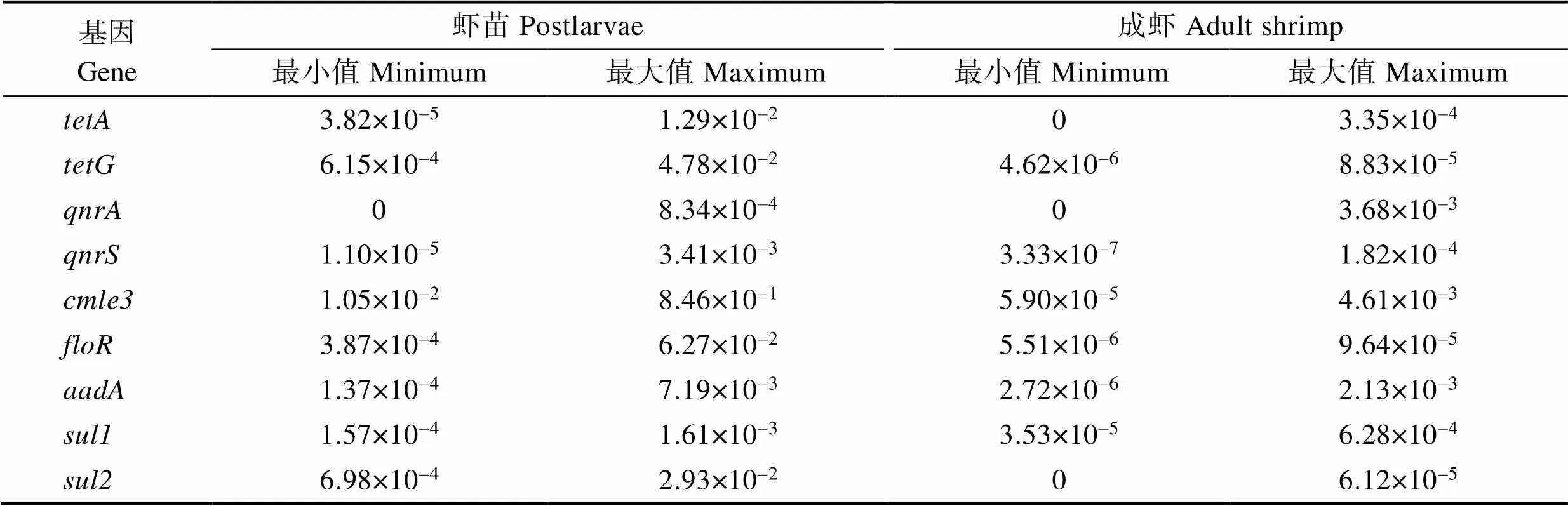
Tab.6 Relative contents range of 9 antimicrobial resistant genes/(copies/16S rDNA)

圖2 9種ARGs的豐度熱圖
3 討論
3.1 凡納濱對蝦腸道可培養細菌
張盛靜等(2015)研究表明,凡納濱對蝦在苗種階段腸道內可培養的細菌屬包括弧菌屬、發光桿菌屬、芽孢桿菌屬和鹽單胞菌屬()等,其中弧菌屬占絕對優勢,養成階段腸道內可培養的細菌屬包括乳球菌屬()、弧菌屬、芽孢桿菌屬、發光桿菌屬、希瓦氏菌屬、節桿菌屬()、微桿菌屬()等。本研究結果與其相似,部分種屬之間的差異可能與養殖過程中微生態制劑使用較多(吳定心, 2016)和抗生素使用較少有關。
3.2 可培養細菌抗生素耐藥性
目前,國內已批準的水產養殖用抗微生物藥物有12種(農業農村部, 2020),在魚類細菌病的防控中起著重要作用。在我國部分養殖區(王志芳等, 2019; 李兆新等, 2018)的環境中能檢出喹諾酮類、磺胺類、四環素類等抗生素殘留,且距離養殖區越近濃度越高,呈現明顯的時空分布特征(連璐璐, 2016)。持續性的低劑量抗生素脅迫,會增強細菌耐受性和耐藥性,降低抗生素治療效率。研究顯示,間歇性將群體暴露于氨芐青霉素(ampicillin)中,其群體會對氨芐青霉素產生耐受性(Fridman, 2014),誘發菌株突變產生耐藥性(Frimodt-M?ller, 2019)。給日本沼蝦()長期投飼含安全劑量抗生素的日糧后,會增加其體內細菌耐藥性選擇壓力(Sun, 2020)。因此,必須重視細菌的耐藥性。
本研究分離所得可培養細菌中對常見抗生素的耐藥性均較低,僅對四環素類和磺胺類抗生素存在一定的耐藥性,這與現實環境抗生素殘留污染趨勢類似(王志芳等, 2019; 李兆新等, 2018; 連璐璐, 2016),提示,這些細菌的耐藥能力可能與自然環境中抗生素污染情況有關。另外,成蝦耐藥菌占比較蝦苗降低,表明成蝦腸道耐藥菌污染程度較蝦苗輕。
3.3 ARGs豐度差異
抗生素使用可增加ARGs豐度和多樣性(Fridman, 2014; Frimodt-M?ller, 2019)。已有文獻報道在對蝦體內、腸道及養殖環境中檢測出多種抗生素ARGs (Su, 2017; 洪斌等, 2019),整合子等一些移動基因原件(mobile genetic element , MGE)可促進ARGs在細菌間的傳播(Mazel, 2006; Frost, 2005)。天津地區水產養殖環境中,磺胺類ARGs和豐度較高(Gao, 2012);對蝦體內和養殖水環境中優勢ARGs分別為、、、(Su, 2017)。本研究結果與前人研究結果類似,其中,和豐度稍高,可能與動物專用抗菌藥氟苯尼考的使用有關,而磺胺類和四環素類ARGs則可能來源于環境污染。另外,研究還發現,成蝦樣品的ARGs豐度均顯著低于苗種樣品(<0.05),提示經過 1個養殖周期,對蝦體內ARGs污染程度下降。
3.4 細菌耐藥性和ARGs關系分析
研究結果顯示,菌株對各大類抗生素的敏感性與其所對應的ARGs豐度并不能呈現完全一一對應的關系,這與閆倩倩等(2020)、牛麗等(2019)和趙姝等(2019)等研究結果類似。如氟苯尼考和氯霉素的耐藥菌占比都很低,但和豐度高。這可能是因為對蝦腸道可培養細菌占腸道菌群的比例低,且本研究僅針對腸道中可培養細菌展開耐藥性研究,因此二者結果存在差異。又如,新霉素耐藥菌占比較高,但豐度較低。這可能是因為細菌受到脂質層(Lambert, 2002)和抗滲屏障(Mcdonnell, 1999)等因素的影響而表現出較強的耐藥性,或者該耐藥能力由氨基糖苷類其他的ARGs所賦予。
研究發現,多數細菌耐藥性和ARGs豐度呈現出對應關系。如磺胺異惡唑、復方新諾明的耐藥菌占比和豐度表現相一致,其來源可能與自然環境污染有關。環丙沙星、諾氟沙星耐藥菌占比和、豐度都較低,提示本地區凡納濱對蝦養殖業中,這 2類藥物可能使用較少,符合國家禁用這2種抗生素的規定,也表明我國自2017年開展的水產養殖用藥減量行動的效果逐步顯現。
4 結論
本研究闡述了江蘇地區4種養殖模式下,凡納濱對蝦腸道細菌耐藥性和ARGs豐度與不同生長階段的關系。研究表明,江蘇地區4種養殖模式凡納濱對蝦腸道可培養細菌屬為弧菌屬;成蝦腸道可培養細菌種類和數量較蝦苗顯著上升(<0.05),耐藥菌占比降低,ARGs豐度顯著降低(<0.05);各模式之間未發見明顯規律。研究結果顯示,各模式下成蝦腸道細菌耐藥性污染程度均小于蝦苗,提示,科學、合理的養殖方法可能會降低凡納濱對蝦腸道可培養細菌的耐藥性。
BACH H J, TOMANOVA J, SCHLOTER M,. Enumeration of total bacteria and bacteria with genes for proteolytic activity in pure cultures and in environmental samples by quantitative PCR mediated amplification. Journal of Microbiological Methods, 2002, 49(3): 235–245
Bureau of Fisheries, Ministry of Agriculture and Rural Affairs, National Fisheries Technology Extension Center, China Society of Fisheries. China fishery statistical yearbook 2020. Beijing: China Agriculture Press, 2020, 21–27 [農業農村部漁業漁政管理局, 全國水產技術推廣總站, 中國水產學會. 2020中國漁業統計年鑒. 北京: 中國農業出版社, 2020, 21–27]
CHEN C, CHEN H, ZHANG Y,. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Molecular Plant, 2020, 13(8): 1194–1202
CHUMPOL S, KANTACHOTE D, RATTANACHUAY P,.andselection of probiotic purple nonsulphur bacteria with an ability to inhibit shrimp pathogens: Acute hepatopancreatic necrosis disease-causingand other vibrios. Aquaculture Research, 2017, 48(6): 3182–3197
Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimricrobial susceptibility testing. 2017, 20–36
D′COSTA V M, MCGRANN K M, HUGHES D W,. Sampling the antibiotic resistome. Science (New York, NY), 2006, 311(5759): 374–377
FRIDMAN O, GOLDBERG A, RONIN I,. Optimization of lag time underlies antibiotic tolerance in evolved bacterial populations. Nature, 2014, 513(7518): 418–421
FRIMODT-M?LLER J, L?BNER-OLESEN A. Efflux-pump upregulation: From tolerance to high-level antibiotic resistance? Trends in Microbiology, 2019, 27(4): 291–293
FROST L S, LEPLAE R, SUMMERS A O,. Mobile genetic elements: The agents of open source evolution. Nature Reviews Microbiology, 2005, 3(9): 722–732
GAO P, MAO D, LUO Y,. Occurrence of sulfonamide and tetracycline-resistant bacteria and resistance genes in aquaculture environment. Water Research, 2012, 46(7): 2355–2364
HE L Y, LIU Y S, SU H C,. Dissemination of antibiotic resistance genes in representative broiler feedlots environments: Identification of indicator ARGs and correlations with environmental variables. Environmental Science and Technology, 2014, 48(22): 13120–13129
HE X L, XU Y B, CHEN J L,. Evolution of corresponding resistance genes in the water of fish tanks with multiple stresses of antibiotics and heavy metals. Water Research, 2017, 124: 39–48
HONG B, NIU B, CHEN P,. Diversity of gut microbiota and antibiotic resistance genes inand. Journal of Fisheries of China, 2019, 43(5): 1347–1358 [洪斌, 牛犇, 陳萍, 等. 凡納濱對蝦和羅氏沼蝦腸道微生物及抗生素抗性基因多樣性分析. 水產學報, 2019, 43(5): 1347–1358]
LAMBERT P A. Cellular impermeability and uptake of biocides and antibiotics in Gram-positive bacteria and mycobacteria. Journal of Applied Microbiology, 2002, 92(S1): 46S–54S
LEE C, CHEN I, YANG Y,. The opportunistic marine pathogenbecomes virulent by acquiring a plasmid that expresses a deadly toxin. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(34): 10798–10803
LI Y, QU L Y, ZHU P F,. Distribution characteristics of antibiotic resistance bacteria and related resistance genes in mariculture area of Shandong. Marine Environmental Science, 2016, 35(1): 55–62 [李壹, 曲凌云, 朱鵬飛, 等. 山東地區海水養殖區常見抗生素耐藥菌及耐藥基因分布特征. 海洋環境科學, 2016, 35(1): 55–62]
LI Z X, DONG X, WU M M,. Quinolone residues in seawater of aquaculture area, Sanggou Bay, Yellow Sea, China. Marine Environmental Science, 2018, 37(2): 182–186, 192 [李兆新, 董曉, 吳蒙蒙, 等. 黃海桑溝灣養殖區海水中喹諾酮類抗生素的殘留狀況. 海洋環境科學, 2018, 37(2): 182–186, 192]
LIAN L L. Distribution of antibiotics in surface and core sediments from aquaculture areas. Master′s Thesis of Dalian University of Technology, 2016, 28–61 [連璐璐. 抗生素在濱海養殖區表層及柱狀沉積物中的分布特征. 大連理工大學碩士研究生學位論文, 2016, 28–61]
LIU W L, XU H, TANG Y,. The effect of diet withbiofilm on the growth rate, disease resistance and intestinal microflora of. Progress in Fishery Sciences, 2017, 38(4): 87–95 [劉文亮, 許華, 唐楊等. 飼料中補充蠟樣芽孢桿菌()生物膜對凡納濱對蝦()生長、抗病力及其腸道微生物組成的影響. 漁業科學進展, 2017, 38(4): 87–95]
MAZEL D. Integrons: Agents of bacterial evolution. Nature Reviews Microbiology, 2006, 4(8): 608–620
MCDONNELL G, RUSSELL A D. Antiseptics and disinfectants: Activity, action, and resistance. Clinical Microbiology Reviews, 1999, 12(1): 147–179
Ministry of Agriculture and Rural Affairs. Guidance on aquaculture drug application, No.1, No.2, 2020. 2020, 3–4 [農業農村部. 水產養殖用藥明白紙2020年1、2號. 2020, 3–4]
NIU L, BA Y B, BAI F J,. Comparison of antimicrobial resistance ofisolated from different sources. Journal of Food Science and Biotechnology, 2019, 38(12): 9–16 [牛麗, 巴永兵, 白鳳佳, 等. 不同來源副溶血性弧菌耐藥情況比較. 食品與生物技術學報, 2019, 38(12): 9–16]
?STERBLAD M, NORRDAHL K, KORPIM?KI E,. How wild are wild mammals? Nature, 2001, 409: 37–38
PEI R, KIM S C, CARLSON K H,. Effect of river landscape on the sediment concentrations of antibiotics and corresponding antibiotic resistance genes (ARG). Water Research, 2006, 40(12): 2427–2435
SHANG H, WANG Y S, SHEN Z Y. National guide for clinical laboratory procedures. Beijing: People’s Medical Publishing House, 2015, 21–33 [尚紅, 王毓三, 申子瑜. 全國臨床檢驗操作規程. 北京: 人民衛生出版社, 2015, 21–33]
SU H C, LIU S, HU X J,. Occurrence and temporal variation of antibiotic resistance genes (ARGs) in shrimp aquaculture: ARGs dissemination from farming source to reared organisms. Science of the Total Environment, 2017, 607–608: 357–366
SUN S, KORHEINA D K A, FU H,. Chronic exposure to dietary antibiotics affects intestinal health and antibiotic resistance gene abundance in oriental river prawn (), and provokes human health risk. Science of the Total Environment, 2020, 720: 137478
WANG H P, YAN H, ZHAO J R,. Quantitative detection of six classes of antibiotic resistance and class Ⅰ integrin genes in aquatic products. Modern Food Science and Technology, 2017, 33(5): 270–276 [王慧平, 閆鶴, 趙俊仁, 等. 水產品中6類抗生素抗性基因和Ⅰ類整合子的定量檢測. 現代食品科技, 2017, 33(5): 270–276]
WANG Z F, LEI Y, XIAO J,. Residue status of antibiotics in aquaculture ponds of main tilapia aquaculture areas in Guangxi. Journal of Southern Agriculture, 2019, 50(4): 891–897 [王志芳, 雷燕, 肖俊, 等. 廣西羅非魚主產區養殖池塘抗生素殘留狀況分析. 南方農業學報, 2019, 50(4): 891–897]
WU D X. Studies of effects onbacterial ecology caused by probiotics application and mechanism of interactin between microalgae and probiotics. Doctoral Dissertation of Huazhong Agricultural University, 2016, 40–72 [吳定心. 微生物制劑對南美白對蝦養殖體系微生態的影響及其與藻類關系的研究. 華中農業大學博士研究生學位論文, 2016, 40–72]
YAN Q Q, LI B, LIAO M J,. Distribution characteristics of antibiotic resistant bacteria and antimicrobial resistant genes in the intestine of cultured sea cucumber () seedlings in Shandong Province. Progress in Fishery Sciences, 2020, 41(4): 134–143 [閆倩倩, 李彬, 廖梅杰, 等. 山東主要刺參養殖區幼參腸道抗生素耐藥菌及耐藥基因分布特征. 漁業科學進展, 2020, 41(4): 134–143]
ZHANG Q Y, ZHAO W W, WU W. Antibiotics resistance gene pollution and its research progress acheived in aquaculture environment. Journal of Agricultural Science and Technology, 2015, 17(6): 125–134 [張騫月, 趙婉婉, 吳偉. 水產養殖環境中抗生素抗性基因污染及其研究進展. 中國農業科技導報, 2015, 17(6): 125–134]
ZHANG S J, ZHAO X J, SONG X L,. Analysis of the culturable bacteria’s quantity and composition in the intestinal tract of cultivation shrimp. Journal of Shanghai Ocean University, 2015, 24(2): 211–218 [張盛靜, 趙小金, 宋曉玲, 等. 人工養殖對蝦腸道內可培養細菌數量及組成分析. 上海海洋大學學報, 2015, 24(2): 211–218]
ZHANG Z, YANG Z Y, WANG Y G,. Effectiveness of garden burnet,L., in controlling acute hepatopancreatic necrosis disease caused by infection ofin shrimp farming. Aquaculture, 2021, 531: 735875
ZHAO S, LI J, MA L C,. Analysis of phenotype and genetype in quinolone resistance infrom mairculture. Marine Fisheries, 2019, 41(4): 463–471 [趙姝, 李健, 馬立才, 等. 海水養殖動物源弧菌喹諾酮類藥物耐藥表型與基因型分析. 海洋漁業, 2019, 41(4): 463–471]
ZHU L D, CHEN K, XI B W,.antibacterial effect of fraxetin on pathogenic. Journal of Fishery Sciences of China, 2019, 26(5): 984–992 [朱璐丹, 陳凱, 習丙文, 等. 秦皮素的抑菌作用及其對嗜水氣單胞菌毒力的影響. 中國水產科學, 2019, 26(5): 984–992]
Study on Bacterial Resistance inIntestinal Culturable Bacteria at Different Growth Stages
YANG Zeyu1,2, WAN Xihe1①, SHI Wenjun1, WANG Libao1, LI Hui1, SHEN Hui1, QIAO Yi1, JIANG Ge1, CHENG Jie1
(1. Jiangsu Institute of Marine Fisheries, Nantong, Jiangsu 226007, China; 2. Aquatic Product Technology Extension Station of Gaoyou, Gaoyou, Jiangsu 225600, China)
culturing industry is an important part of the rural economy in coastal areas. With the development of the shrimp farming industry, diseases breakout frequently. Antibiotic overuse can lead to bacterial antibiotic resistance and antibiotic resistance genes (ARGs). These then threatens human health and safety. Therefore, the evaluation of bacterial antibiotic resistance phenotypes and antibiotic ARG abundance can help to understand antibiotic pollution in specific areas. According to our preliminary investigation, more probiotics were used duringculturing in Jiangsu Province. In order to avoid damaging the microbial community structure in ponds, less antibiotics were used during shrimp farming. Tounderstand the relationship between bacterial resistance inintestinal culturable bacteria and different growth stages, four main culture models ofin Jiangsu Province were selected as research objects. This study investigated the bacterial resistance to 12 antibiotics (tetracycline, doxycycline, fluorobenicol, chloramphenicol, sulfamethoxazole, cotrimoxazole, neomycin, gentamicin, ciprofloxacin, norfloxacin, ceftriaxone, and cefotaxime) and the abundance of nine ARGs (,,,,,,,, and) of the intestinal culturable bacteria in samples using the K-B disc diffusion method and qRT-PCRtechnology. The results showed thatwas the dominant bacterial genus among the shrimp intestinal culturable bacteria in different aquaculture models. The species and quantity of culturable bacteria in adult shrimp intestines increased significantly compared to those in post-larvae intestines (<0.05). The proportion of antibiotic resistant bacteria decreased. The abundance of ARGs declined significantly (<0.05). No obvious regularity was observed among the different aquaculture models. The antimicrobial resistance and abundance of ARGs in the intestinal bacteria of adult shrimp were lower than those in juvenile shrimp under different aquaculture patterns, indicating that reducing the use of antibiotics by administering probiotics may reduce the resistance of culturable bacteria in the intestinal tract of. In future research, the differences in the bacterial antibiotic resistance and ARG abundance in the intestinal tract ofunder different aquaculture models can be further explored by combining the methods of metagenomics.
; Bacterial antibiotic resistance; Antibiotic resistance genes (ARGs); qRT-PCR
WAN Xihe, E-mail: wxh1708@163.com
S945.1
A
2095-9869(2022)02-0175-10
10.19663/j.issn2095-9869.20201209002
* 江蘇省第十六批“六大人才高峰”高層次人才項目(NY-106)和江蘇省農業科技自主創新資金–農產品產業發展關鍵技術創新[CX(18)2010]共同資助 [This work was supported by 16th Batch of “Six Talent Peaks” High-Level Talents Project in Jiangsu Province (NY-106), and Independent Innovation Fund for Agricultural Science and Technology of Jiangsu Province-Key Technological Innovations in the Development of Agricultural Products Industry [CX(18)2010]]. 楊澤禹,E-mail: 779287258@qq.com
萬夕和,研究員,E-mail: wxh1708@163.com
2020-12-09,
2021-01-27
楊澤禹, 萬夕和, 史文軍, 王李寶, 黎慧, 沈輝, 喬毅, 蔣葛, 成婕. 凡納濱對蝦不同生長階段腸道可培養細菌耐藥性研究. 漁業科學進展, 2022, 43(2): 175–184
YANG Z Y, WAN X H, SHI W J, WANG L B, LI H, SHEN H, QIAO Y, JIANG G, CHENG J. Study on bacterial resistance inintestinal culturable bacteria at different growth stages. Progress in Fishery Sciences, 2022, 43(2): 175–184
(編輯 馬璀艷)

