海草床沉積物有機(jī)碳研究綜述
葉嘉暉, 邱崇玉, 曾文軒, 史云峰, 趙牧秋, 韓秋影
海草床沉積物有機(jī)碳研究綜述
葉嘉暉, 邱崇玉, 曾文軒, 史云峰, 趙牧秋, 韓秋影
(海南熱帶海洋學(xué)院崖州灣創(chuàng)新研究院, 熱帶海洋生物資源利用與保護(hù)教育部重點實驗室, 海南省近岸海洋生態(tài)環(huán)境過程與碳匯重點實驗室, 海南 三亞 572022)
海草床具有重要的生態(tài)系統(tǒng)服務(wù)功能, 可以為海洋生物提供棲息地和食物來源, 同時還具有重要的碳儲存功能, 海草床“藍(lán)碳”功能日益受到學(xué)術(shù)界的重視, 據(jù)研究全球每年海草床的碳埋藏量高達(dá)(2.7~4.4)×107MgC。近年來, 由于人類活動的影響, 世界范圍內(nèi)海草床衰退嚴(yán)重, 導(dǎo)致海草床沉積物有機(jī)碳儲量降低。本文綜述了全球海草床沉積物有機(jī)碳的來源、組分、儲量以及指示作用; 從物理、化學(xué)和生物三方面討論了影響海草床碳儲量的環(huán)境因素。最后提出了未來主要研究方向, 主要包括加強(qiáng)海草床碳通量普查, 分析全球氣候變化背景下海草床沉積物有機(jī)碳的變化機(jī)制, 明確海草床碳儲量流失速率, 研究海岸帶工程對海草床沉積物有機(jī)碳的影響。評估海草床沉積物有機(jī)碳儲量及變化機(jī)制可以為全球海洋藍(lán)碳研究提供科學(xué)依據(jù)。
海草床; 沉積物有機(jī)碳; 來源; 儲量; 環(huán)境因素
海草通常生活在潮間帶和潮下帶的淺水區(qū)域, 是一種廣泛分布于熱帶以及溫帶海域的沉水性被子植物[1]。印度-太平洋區(qū)、熱帶大西洋區(qū)、溫帶北大西洋區(qū)、溫帶北太平洋區(qū)、溫帶南大洋區(qū)和地中海區(qū)為全球6個主要海草分布區(qū)[1], 共有6科72種海草[2]。熱帶地區(qū)海草種類較多[1], 熱帶印度-太平洋地區(qū)的海草種類多達(dá)25種, 而在溫帶北大西洋區(qū), 僅有5種海草[3]。我國海草床主要有南海海草分布區(qū)和黃渤海海草分布區(qū), 共有10屬22種, 大約為全球海草種類的30%[4-5]。全球海草床覆蓋面積約為(3~6)×105km2[6-7],據(jù)估算其生態(tài)系統(tǒng)服務(wù)價值約為每年每公頃34 000美元[8]。海草生態(tài)系統(tǒng)具有極其復(fù)雜的結(jié)構(gòu), 可以提供多種生態(tài)功能[9], 為海洋生物提供棲息地[10-11]和食物來源[12]。海草床還具有重要的碳儲存功能, 近年來, 海草床“藍(lán)碳”功能越來越受到學(xué)術(shù)界的重視[13-16]。
海草是沿海生態(tài)系統(tǒng)中重要的碳匯[17], 可以通過光合作用吸收CO2[18]。通常情況下, 海草所固定的碳含量大于其代謝需要[19], 多余的有機(jī)碳大部分被運(yùn)輸?shù)胶2莸母案鶢钋o, 最終通過環(huán)境作用將有機(jī)碳固存于沉積物中[20]。海草床可以通過釋放生物質(zhì)或者從凋落物釋放溶解有機(jī)碳[21-22], 并通過水流作用輸運(yùn)到其他生態(tài)系統(tǒng)[23], 全球海草床年輸出的溶解性有機(jī)碳高達(dá)(1.6~3.3)×108MgC[19], 約占全球海草凈初級生產(chǎn)力的46%[24]。Su等對廣西珍珠灣海草床及其周圍沉積物有機(jī)碳儲量進(jìn)行分析, 發(fā)現(xiàn)海草床沉積物有機(jī)碳含量顯著高于無海草區(qū)域[25]。海草碎屑具有大量穩(wěn)定組分和高沉積速率, 沉積物中的厭氧環(huán)境不利于微生物的生長, 使得沉積物有機(jī)碳長期儲存[17, 26-28]。全球海草床不到海洋總面積的0.2%, 但全球海草床沉積物有機(jī)碳儲量為139.7 MgC/ha, 并且每年碳埋藏量為(2.7~4.4)×107MgC, 占到每年全球海洋碳匯的10%~18%[7, 20, 29], 顯著高于大部分陸地生態(tài)系統(tǒng)[30], 可以緩解全球氣候變化及其帶來的負(fù)面影響[31]。我國海草床每年碳匯量約為(3.2~5.7)× 105MgC[32]。山東桑溝灣鰻草海草床生態(tài)系統(tǒng)每年總固碳量約為290 MgC, 吸收碳的形式包括海草固碳、附生植物固碳、海草床捕獲顆粒碳等, 其中, 海草固定的碳占到總固碳量的46%, 為54.35 MgC/ha[33]。通常溫帶地區(qū)海草床有機(jī)碳儲量要高于熱帶地區(qū), 可能是因為熱帶地區(qū)海草可以為更多生物提供食物和更高的海草碎屑分解速率[34-35]。我國海南島沿岸現(xiàn)存海草床面積約48.646 7 km2[36], 表層5 cm沉積物有機(jī)碳總儲量為40858.5 MgC, CO2吸附量為(1.44± 0.03) MgC/ha, 其中東水、抱才、黃龍、鶯歌等8個海草床沉積物平均碳儲量為7.02 MgC/ha, 總儲量約為1306.45 MgC[37-38]。本文根據(jù)海草床沉積物有機(jī)碳的相關(guān)研究, 分別從海草床沉積物有機(jī)碳來源、組分以及儲量進(jìn)行綜述, 討論影響海草床沉積物有機(jī)碳的主要環(huán)境因素, 結(jié)果將為海草床沉積物有機(jī)碳相關(guān)研究提供科學(xué)依據(jù)。
1 海草床沉積物有機(jī)碳研究進(jìn)展
1.1 沉積物有機(jī)碳來源
1.1.1 海洋沉積物有機(jī)碳來源
沉積物有機(jī)碳不僅是水體污染物遷移的重要媒介, 還參與地球化學(xué)循環(huán), 對生物地球化學(xué)循環(huán)、沉積物演變等有重要的指示作用[39]。沉積物有機(jī)碳參數(shù)主要包括碳氮比、碳同位素等, 儲存著氣候、環(huán)境變化的信息等[40-41]。對于海洋中沉積物來源, 科學(xué)家一般采用碳氮比(C/N)、碳穩(wěn)定同位素法(δ13C)以及生物標(biāo)志法(如脂類和木質(zhì)素)等進(jìn)行研究。研究發(fā)現(xiàn)陸源和海源有機(jī)碳具有一定差別, 陸源C/N比大于12, δ13C為–28‰ ~ –25‰, 海源C/N比值為6~9, δ13C為–19‰ ~ –12‰[42, 43]。Liu等(2020) 采用碳氮穩(wěn)定同位素法和碳氮比法對黃海南部表層沉積物進(jìn)行研究, 發(fā)現(xiàn)該地區(qū)沉積物有機(jī)碳來源組成為海洋、陸地及人為輸入, 且黃河三角洲北部沉積物有機(jī)質(zhì)陸源貢獻(xiàn)較高(>50%), 而在近海泥區(qū)有機(jī)質(zhì)貢獻(xiàn)主要來源于海洋(>70%)[44]。紅樹林生態(tài)系統(tǒng)的碳儲存通常采用穩(wěn)定同位素法和表層沉積物碳氮比方法進(jìn)行研究[45], 紅樹林對來源于陸地的土壤礦物質(zhì)有較好的沉降作用, 在河流侵蝕率高的地區(qū), 紅樹林沉積物有機(jī)碳有三分之二來源于陸地[46]; 而在侵蝕率低、河流輸入少的環(huán)境下, 紅樹林有機(jī)碳有三分之二來源于其本身[45]。Tanaka等(2011)對珊瑚礁溶解有機(jī)碳研究, 發(fā)現(xiàn)有機(jī)質(zhì)是從底棲生物群落中釋放的[47]。而珊瑚礁中幾種有機(jī)質(zhì)的來源主要包括珊瑚-蟲黃藻共生群落[48-49]、海草[50]、底棲藻類[51]的釋放以及細(xì)菌溶解沉積物有機(jī)質(zhì)釋放[52]。科學(xué)家還發(fā)現(xiàn)海源和陸源有機(jī)質(zhì)中的溴元素(Br)存在顯著差異[53-54], 相關(guān)研究采用溴與有機(jī)碳(Br/TOC)的關(guān)聯(lián), 分析海源及陸源對沉積物有機(jī)碳的貢獻(xiàn)[55-56]。通常湖泊地質(zhì)、土壤、河床的Br/TOC比值為0.02~2.8 mg Br/g TOC, 而海岸帶沉積物的Br/TOC要顯著高于陸源沉積物, 高達(dá)7.6 mg Br/g TOC[57]。
1.1.2 海草床沉積物有機(jī)碳來源
海草床沉積物中的有機(jī)碳不僅來自于海草, 還來源于陸生植物碎屑和海洋生物, 如浮游植物、大型藻類、附生植物和底棲藻類[58-59]。天然碳同位素的差異是由于植物在進(jìn)行光合作用的過程中對碳的吸收機(jī)制不同所引起的, 由這種機(jī)制差異將植物分為C3、C4和CAM植物[60], 因此, 可以通過其本身的同位素特征值(δ13C和δ15N)來測定沉積物有機(jī)碳的來源及不同植物的貢獻(xiàn)[61-62]。有關(guān)研究發(fā)現(xiàn), 海草的δ13C為–8.99‰, 大型海藻為–13.61‰[63]。Liu等利用碳穩(wěn)定同位素方法對新村灣海草床有機(jī)碳來源進(jìn)行分析, 發(fā)現(xiàn)沉積物有機(jī)碳穩(wěn)定同位素值介于–20.39‰ ~ –7.39‰之間, 并且從營養(yǎng)鹽濃度相對較低的海草床到高營養(yǎng)鹽海草床的沉積物中, 大型海藻及附生藻類對沉積物有機(jī)碳的貢獻(xiàn)增加了16%, 表明大型海藻及附生藻類對沉積物有機(jī)碳的貢獻(xiàn)與營養(yǎng)鹽濃度呈正相關(guān)[63]。但是, 同位素法本身存在一定缺陷, 海草與其他藻類可能存在δ13C值重疊的情況[63-64], 導(dǎo)致分析結(jié)果偏差。Rahayu等(2019)采用穩(wěn)定同位素標(biāo)記法及碳氮比分析, 對印度尼西亞群島的海草床研究發(fā)現(xiàn): Barranglompo、Sarappokeke和Kapoposang島的海草床沉積物有機(jī)碳具有相似特征, 并且來源于海草的有機(jī)碳占到了75%[65]。但在同一研究中, Bauluang島與其他3個島嶼海草床沉積物有機(jī)碳主要來源不同, 浮游植物對沉積物有機(jī)碳貢獻(xiàn)最大, 約為44%。初級生產(chǎn)者合成的脂肪酸有一些是特定的, 可以用于區(qū)分微藻[66]、大型海藻[67]、被子植物[68]以及原核生物[69], 通過脂肪酸標(biāo)記法來確定初級生產(chǎn)者到初級消費者的食物鏈結(jié)構(gòu)日益受到關(guān)注[70], 采用脂肪酸標(biāo)記法與穩(wěn)定同位素法聯(lián)用以克服δ13C重疊的問題[71-72], 不同植物的碳、氮穩(wěn)定同位素特征值及特征脂肪酸詳見表1。海草葉片主要由多糖組成, 其余物質(zhì)主要為木質(zhì)素、單寧和游離的脂所結(jié)合成的酚酸[73-74]。現(xiàn)有研究采用PY-GC-MS和THM-GC-MS兩種熱解技術(shù)對大洋波喜蕩草進(jìn)行有機(jī)質(zhì)解析, 發(fā)現(xiàn)海草不僅由碳水化合物及木質(zhì)素組成, 還主要由在維管植物中不常見的對羥基苯甲酸(p-HBA)類物質(zhì)組成。同樣, 該區(qū)域海草床沉積物碎屑中主要由酚類物質(zhì)p-HBA及碳水化合物組成, 證實海草床沉積物碎屑主要來源于海草的根、莖、葉[75]。
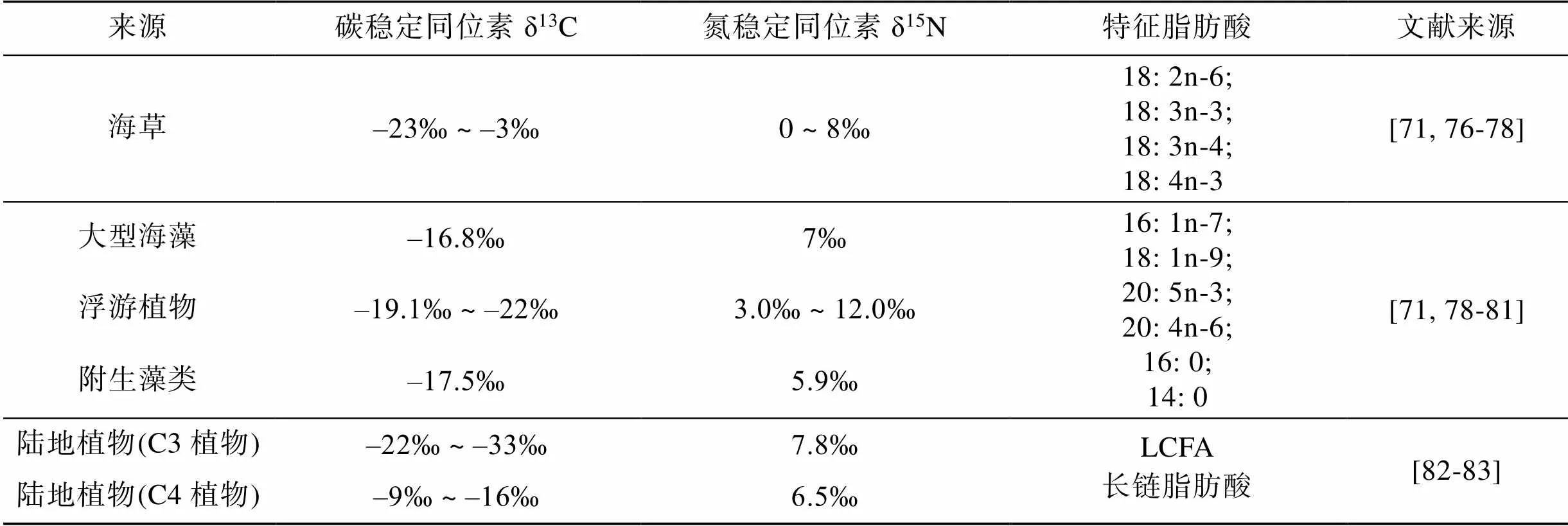
表1 海草、海藻及陸地植物碳、氮穩(wěn)定同位素特征值及特征脂肪酸
1.2 海草床沉積物有機(jī)碳分類
沉積物有機(jī)碳可以根據(jù)物理、化學(xué)、生物(微生物降解性)方法分組。沉積物有機(jī)碳分類方法詳見表2。粒度分組法自20世紀(jì)60年代開始出現(xiàn), 按照與有機(jī)碳結(jié)合的顆粒大小, 可分為砂礫(53~2 000 μm)、粗粉粒(5~53 μm)、細(xì)粉粒(2~5 μm)、粗黏粒(0.2~2 μm)和黏粒(<0.2 μm)[84]。將有機(jī)碳按照密度分, 可分為輕組碳和重組碳[85]。通過化學(xué)方法將沉積物有機(jī)碳分為活性有機(jī)碳(Labile organic carbon, LOC)和惰性有機(jī)碳(Recalcitrant organic carbon, ROC), 活性有機(jī)碳的生物活性高, 礦化速率高而惰性有機(jī)碳則較低[86]。活性有機(jī)碳按照提取方式可以分為鹽提取碳、水提取碳、氯仿提取碳、酸提取碳[87]。根據(jù)其溶解性和水解性又分為溶解有機(jī)碳(Dissolved organic carbon, DOC)、酸水解有機(jī)碳[86]。生物分組法通常將有機(jī)碳分為微生物量碳(Microbial biomass carbon, MBC)和可礦化碳。沉積物中的細(xì)菌、真菌、藻類等含有的碳稱為微生物量碳[88], 那些可以被微生物分解且向大氣中釋放CO2的有機(jī)碳稱為可礦化碳[89]。多數(shù)研究中根據(jù)其礦化速率將其分為活性有機(jī)碳和惰性有機(jī)碳[90], 有機(jī)碳是否容易降解是區(qū)分活性有機(jī)碳和惰性有機(jī)碳的依據(jù), 有機(jī)碳礦化速率對沉積物有機(jī)碳來源變化響應(yīng)迅速[91]。表示海草床有機(jī)碳活性的指標(biāo)通常用微生物量碳和溶解有機(jī)碳[63, 92]。海草地下生物量含有相對較高的碳氮比值、生物可利用性較差, 因此, 海草床固定的碳一般為惰性有機(jī)碳[93]。
1.3 海草床沉積物有機(jī)碳儲量
學(xué)術(shù)界將海草和海草床沉積物中的有機(jī)碳儲量進(jìn)行了量化研究(表3)。估算海草床沉積物有機(jī)碳埋藏速率主要利用的是14C和210Pb測年技術(shù)或通過海草床年際生產(chǎn)力調(diào)查等方法[94]。國內(nèi)外通用的海草床沉積物有機(jī)碳儲量計算方法為: 采集一定深度的沉積物樣品, 將其分為相同厚度的子樣、測量容重、沉積物有機(jī)碳含量測定、沉積物有機(jī)碳密度計算、相同厚度子樣有機(jī)碳儲量計算、總樣品有機(jī)碳儲量計算。容重的測量是將一定深度的風(fēng)干沉積物樣本放入到固定體積的容器中, 測定其質(zhì)量, 計算方法為:
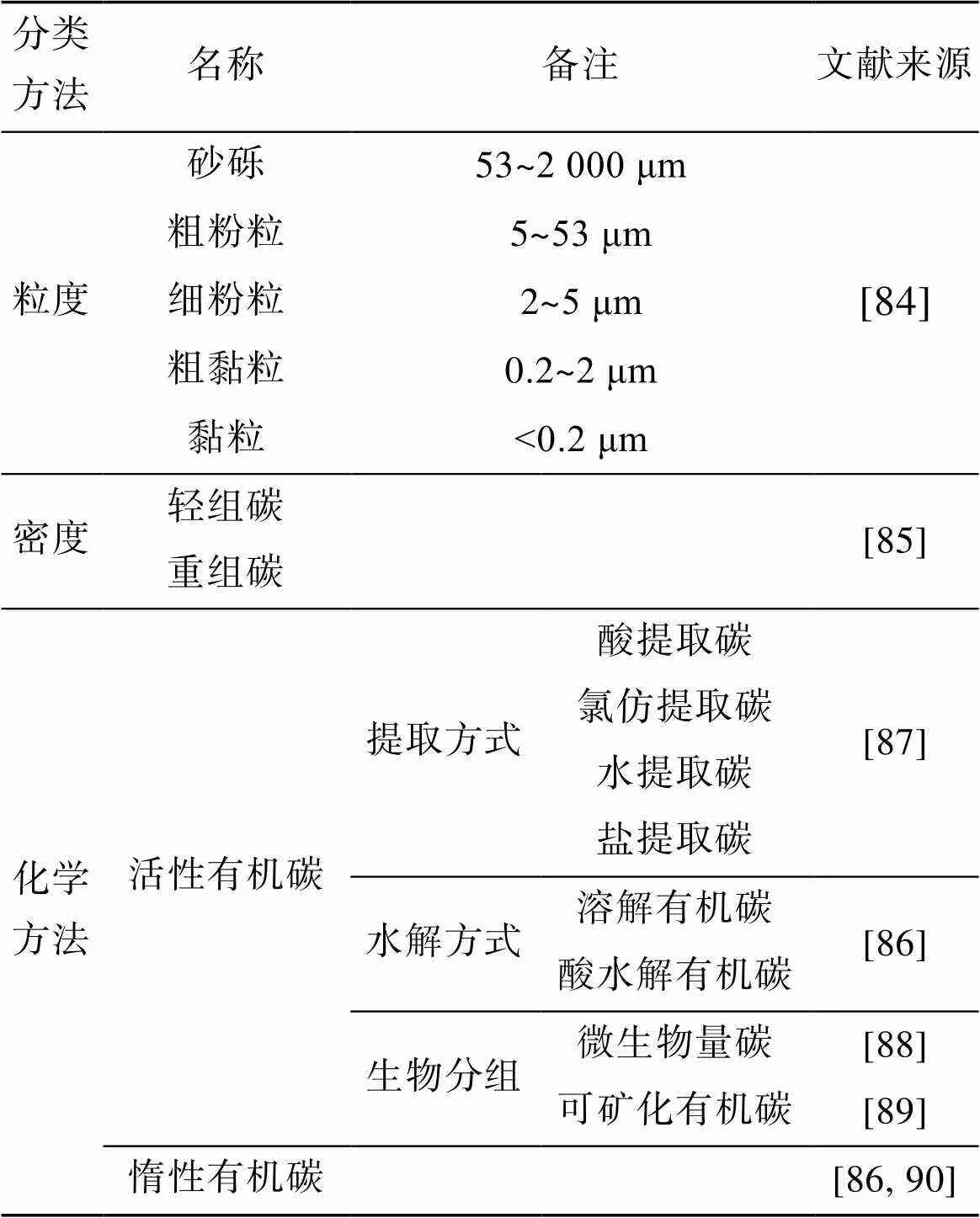
表2 沉積物有機(jī)碳分類
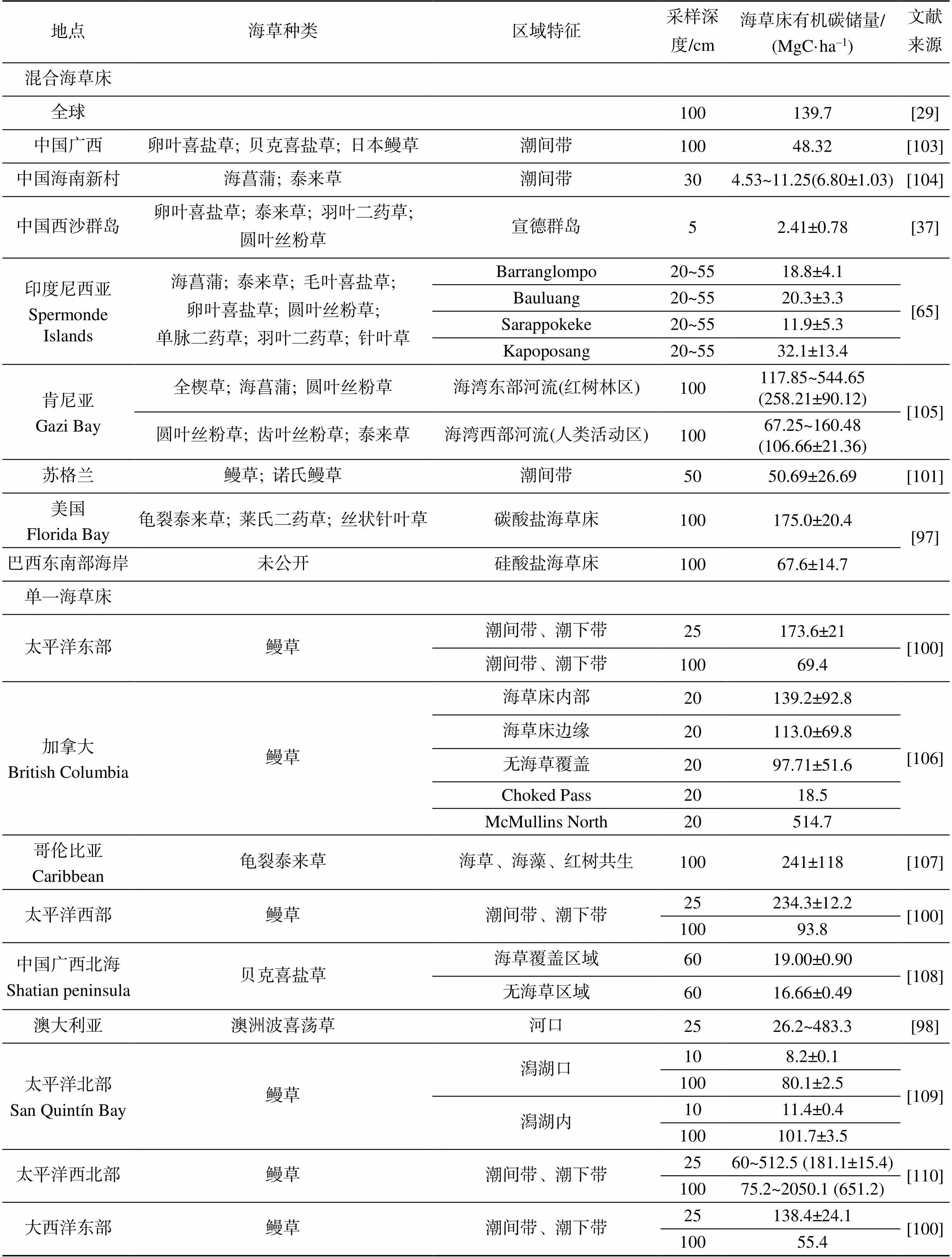
表3 全球海草床沉積物有機(jī)碳儲量
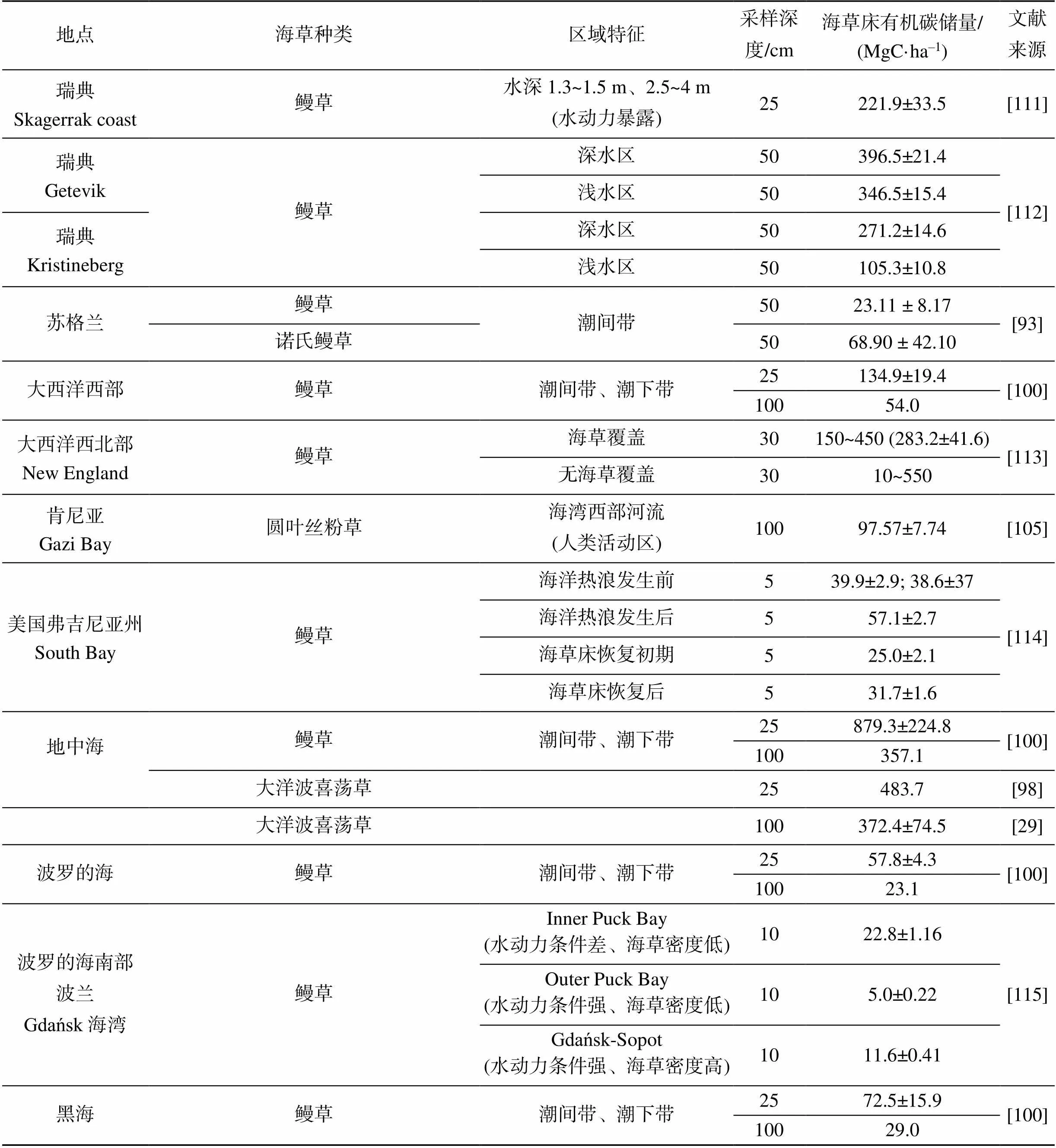
續(xù)表
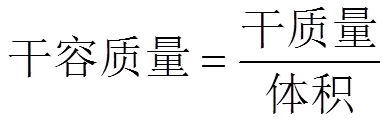
使用元素分析儀測定沉積物中有機(jī)碳含量, 計算一定深度下沉積物有機(jī)碳的密度, 計算方法為:
沉積物有機(jī)碳密度=有機(jī)碳含量×干容質(zhì)量.
一定深度沉積物有機(jī)碳儲量的計算方法為:
沉積物有機(jī)碳儲量=沉積物有機(jī)碳密度×沉積物子樣厚度
通過對某一柱狀樣所有沉積物子樣有機(jī)碳儲量的總和, 得到采樣地區(qū)該深度下沉積物有機(jī)碳的總儲量[95-96]。
1.3.1 不同地區(qū)海草床沉積物有機(jī)碳儲量
地中海海草床沉積物有機(jī)碳儲量較高, 為372.4 MgC/ha[29]; 佛羅里達(dá)灣的海草床沉積物有機(jī)碳略高于全球平均值(139.7MgC/ha), 約為175.0 MgC/ha[97]; 而巴西南海岸、海南新村灣與宣德礁有機(jī)碳儲量約為67.6 MgC/ha, 顯著低于全球平均值; 東亞、東南亞和澳大利亞海草床的沉積物有機(jī)碳儲量約為全球平均水平的25%[98-99]。不同地區(qū)同種海草之間的有機(jī)碳儲量也存在顯著差異, R?hr等對溫帶鰻草海草床沉積物有機(jī)碳儲量研究發(fā)現(xiàn), 地中海鰻草海草床沉積物有機(jī)碳儲量高達(dá)357.1 MgC/ha; 太平洋東部和西部鰻草海草床沉積物有機(jī)碳儲量分別為69.4 MgC/ha和93.8 MgC/ha; 大西洋東部和西部鰻草海草床沉積物有機(jī)碳與太平洋東部相近, 分別為55.4 MgC/ha和54.0 MgC/ha; 而波羅的海鰻草海草床沉積物有機(jī)碳儲量最低, 僅為23.1 MgC/ha[100]。
1.3.2 相近區(qū)域不同種類海草床沉積物有機(jī)碳儲量
研究發(fā)現(xiàn), 相近區(qū)域不同海草種類的沉積物碳儲量不同。例如, Lavery等(2013)對澳大利亞不同種類海草床進(jìn)行調(diào)查研究發(fā)現(xiàn), 澳洲波喜蕩草的沉積物有機(jī)碳含量相對較高, 卵葉喜鹽草、牟氏鰻草、齒葉絲粉草和單脈二藥草的沉積物有機(jī)碳含量相對較低, 而泰來草和圓葉絲粉草等顯著低于以上海草[98]。Potouroglou等(2021)對英格蘭海草床沉積物有機(jī)碳進(jìn)行調(diào)查, 牟氏鰻草海草床沉積物有機(jī)碳含量為68.90±42.10 MgC/ha, 要高于鰻草海草床(23.11±8.17) MgC/ha[101]。而對地中海區(qū)域的研究發(fā)現(xiàn), 大洋波喜蕩草海草床沉積物有機(jī)碳含量相比于鰻草海草床相對較高[29, 100]。位于印度尼西亞群島的Kapoposang島和Sarappokeke島海草床沉積物有機(jī)碳儲量存在明顯的差異, Sarappokeke島的海草優(yōu)勢種為圓葉絲粉草和單脈二藥草, 沉積物有機(jī)碳儲量顯著低于以海菖蒲和泰來草為優(yōu)勢種的Kapoposang島[65, 102]。
2 海草床沉積物有機(jī)碳影響因素
2.1 物理因素
2.1.1 沉積物類型
沉積物類型可能會影響沉積物有機(jī)碳儲量[116]。美國佛羅里達(dá)海草床沉積物有機(jī)碳儲量顯著高于巴西東南部海岸, 這主要是因為佛羅里達(dá)與巴西東南部海岸沉積物類型分別為碳酸鹽與硅酸鹽[97]。鈣化與沉積作用會加速碳酸鹽沉積物的缺氧, 增強(qiáng)有機(jī)碳的保存, 并且當(dāng)海草凋落物上覆蓋礦物基質(zhì)時, 有機(jī)碳更難被分解[117, 118]。巴西東南部海岸缺乏鈣化和碳酸鈣的儲備, 使得有機(jī)碳的代謝與大氣二氧化碳交換、碳酸鹽流動之間存在直接聯(lián)系[97]。
2.1.2 空間分布
海草床的水平屬性(相對邊緣的距離)是海草生態(tài)系統(tǒng)碳儲量空間異質(zhì)性的重要決定因素, 研究發(fā)現(xiàn), 海草床邊緣區(qū)域沉積物有機(jī)碳儲量高于裸露沉積物約3倍, 而海草床內(nèi)部沉積物有機(jī)碳儲量更要顯著高于邊緣[119]。大型海草沉積物有機(jī)碳儲量要大于小型海草或無海草區(qū)域[120], 這主要是因為結(jié)構(gòu)較大、埋藏較深的根莖組織可以對沉積物起到保護(hù)作用以保存有機(jī)碳和截獲更多懸浮顆粒[121-123], 有效光照輻射是影響海草碳儲存能力的關(guān)鍵因子, Collier等研究發(fā)現(xiàn)生長在2 m水深的波狀波喜蕩草地上部分生物量(899 gDW/m2)、地下部分生物量(1 028 gDW/m2)以及海草密度(1 435 shoots/m2)均顯著高于8 m水深處海草(47 gDW/m2; 43 gDW/m2; 80 shoots/m2)[123]。海草床沉積物有機(jī)碳儲量與所在區(qū)域的深度呈現(xiàn)顯著相關(guān)性, 生長在2~4 m水深的波狀波喜蕩草海草床沉積物有機(jī)碳儲量為生長于6~8 m水深區(qū)域的4倍, 而位于水深 2 m和32 m處的大洋波喜蕩草海草床沉積物有機(jī)碳儲量相差10倍以上[116]。
2.1.3 溫度升高
全球氣溫升高會對海草床有機(jī)碳儲量產(chǎn)生一定影響。全球溫度升高將顯著提高沉積物有機(jī)碳的礦化速率[124-125]。研究發(fā)現(xiàn)溫度每上升10 ℃, 碳的礦化速率可提升4.5倍[124]。自養(yǎng)生物的呼吸速率要小于其吸收二氧化碳的速率, 異養(yǎng)生物則相反[126], 溫度升高的情況下, 呼吸速率的增加量要顯著高于二氧化碳的吸收速率[127-128], 氣候變暖可能使得自養(yǎng)生態(tài)系統(tǒng)向異養(yǎng)生態(tài)系統(tǒng)轉(zhuǎn)變, 從而發(fā)生碳匯到碳源的轉(zhuǎn)變[127]。海草生態(tài)系統(tǒng)的甲烷年排放量達(dá)0.09~2.7 Tg, 海草床沉積物甲烷的釋放速率隨著海水溫度的升高而增加[129]。紅海的海草生態(tài)系統(tǒng)已經(jīng)在溫度較高的夏季從自養(yǎng)狀態(tài)向異養(yǎng)狀態(tài)改變[130]。Burkholz等研究發(fā)現(xiàn), 在溫度從25℃上升到37 ℃的過程中, 有海草覆蓋區(qū)域的沉積物甲烷和二氧化碳釋放速率為無海草覆蓋區(qū)域的10~100倍, 并且溫度升高導(dǎo)致甲烷和二氧化碳通量顯著增加[131]。另外, 海洋沉積物微生物活性隨著溫度升高而增強(qiáng), 導(dǎo)致在較高的溫度下沉積物有機(jī)碳水解和發(fā)酵速率都超過了正常條件[132]。
2.1.4 自然與人為擾動
臺風(fēng)伴隨的強(qiáng)降雨會對沉積物表面造成明顯的擾動[133-134], 降雨對沉積物造成的擾動為正常情況下的100倍[135], 并且暴雨會導(dǎo)致沉積物中有機(jī)碳的氧化方式發(fā)生改變, 從而造成沉積物有機(jī)碳加速分解。Sampere等對大陸邊緣表層沉積物中有機(jī)質(zhì)的木質(zhì)素研究, 發(fā)現(xiàn)颶風(fēng)過后來自海灣和沿海濕地的有機(jī)碳輸入可能會迅速分解[136]。海平面上升會導(dǎo)致沿海地區(qū)沉積物有機(jī)碳大量釋放到臨近河口及開闊水域[137-138], 這可能會改變河口及開闊水域微生物群落及活性, 進(jìn)一步造成沉積物有機(jī)碳降解。Aoki等對美國弗吉尼亞州的鰻草海草床沉積物調(diào)查發(fā)現(xiàn), 海洋熱浪發(fā)生3年后沉積物有機(jī)碳含量下降近20%, 海草密度下降90%, 并且海草床衰退后沉積物有機(jī)碳的恢復(fù)呈現(xiàn)滯后性[114]。
人為的干擾也會造成海草床沉積物有機(jī)碳損失。例如, 疏浚工程、挖沙以及船只活動會引起海水沉積物擾動, 導(dǎo)致海水渾濁度升高, 從而危害海草生長[139-141]。海草床衰退導(dǎo)致海草床碳儲存功能減弱, 使得原本存儲于海草床中碳再次釋放, 釋放量高達(dá)(1.5~9.0) ×107MgC[142]。船只擱淺所造成的有機(jī)碳損失量最高, 約為57.1 MgC/ha[143]。海草床內(nèi)頻繁的灘涂漁業(yè)活動會擾動沉積物, 造成海草床沉積物有機(jī)碳儲量降低[144]。海草床沉積物有機(jī)碳含量與沉積物深度呈顯著負(fù)相關(guān), Macreadie等發(fā)現(xiàn)活性有機(jī)碳含量與沉積物深度呈顯著負(fù)相關(guān), 活性有機(jī)碳含量從表層的43%下降至深層(80 cm)的3%, 深層的有機(jī)碳暴露于空氣中會顯著增加微生物豐度, 加速有機(jī)碳礦化和周轉(zhuǎn), 表明沉積物的擾動會引起海草床有機(jī)碳減少[145]。Thorhaug等對墨西哥近岸海草床進(jìn)行調(diào)查, 發(fā)現(xiàn)人為干擾后海草床沉積物有機(jī)碳損失量平均值為(20.98±7.14) MgC/ha, 并且在海草床修復(fù)工程中所恢復(fù)的有機(jī)碳平均值高達(dá)(20.96±8.59) MgC/ha[143]。得克薩斯州Predator地區(qū)的海草床修復(fù)過程海草存活率高達(dá)90.7%, 顯著增加了當(dāng)?shù)睾2莞采w度[146], 但該地區(qū)海草修復(fù)工程對有機(jī)碳的恢復(fù)效果并不顯著, 其每年對沉積物有機(jī)碳的固定量僅為0.5 MgC/ha[143]。學(xué)術(shù)界需要對海草床修復(fù)工程運(yùn)行過程及后期可能對海草床碳通量產(chǎn)生的影響進(jìn)行評估, 為政府平衡投入與收益間的關(guān)系提供依據(jù)。
2.2 化學(xué)因素
2.2.1 海洋酸化
海洋酸化可以引起海草生物量和密度增加, 從而加強(qiáng)其對有機(jī)碳的埋藏能力[147]。在溫帶以及熱帶的高二氧化碳區(qū)域, 都出現(xiàn)了海草密度以及生物量上升的情況[148]。但是, Apostolaki等研究發(fā)現(xiàn), 與較低的二氧化碳區(qū)域相比, 地中海中高二氧化碳區(qū)域海神草生物量反而減少[149]。Vizzini等通過結(jié)合海草床植物以及沉積物性質(zhì)對希臘Milos島和意大利Vulcano島的2個高二氧化碳區(qū)域進(jìn)行調(diào)查, 發(fā)現(xiàn)Vulcano島的海草生物量以及葉片面積減小, 可能會對沉積物表層有機(jī)碳的積累造成負(fù)面影響; 而在Milos島, 雖然海草的生物量、葉面積均上升, 但是表層沉積物有機(jī)碳含量下降[150]。在較低pH值情況下, 細(xì)菌胞外酶活性增加, 加速高分子有機(jī)物向低分子有機(jī)物分解的過程, 可能降低海草床的碳儲存能力[151, 152]。
2.2.2 富營養(yǎng)化
沿海水域的養(yǎng)分富集會降低海草床的碳匯能力[153]。營養(yǎng)鹽濃度過高會導(dǎo)致海草氨中毒, 或者引起大型海藻爆發(fā)限制海草的光合作用[154-155], 降低海草生物量[156], 使得海草對沉積物有機(jī)碳的貢獻(xiàn)減少[157]。營養(yǎng)鹽濃度增高會影響浮游細(xì)菌的活動, 改變細(xì)菌群落, 加速溶解性有機(jī)碳的分解[158-159]。Liu等發(fā)現(xiàn), 當(dāng)海草床處于高營養(yǎng)鹽濃度環(huán)境下, 具有降解難降解化合物能力的微生物如酸微菌(Acidi-mi--cro-biia)、疣微菌(Verrucomicrobiales)以及微球菌(Micro-coc-ca-les)的豐度增加, 從而減弱海草床長期固存有機(jī)碳的能力[44]。
2.3 生物因素
2.3.1 微生物因素
沉積物中有機(jī)碳長期儲存的因素主要是因為厭氧環(huán)境不利于微生物生長以及海草碎屑不易分解[17, 26-28]。然而, 全球海草床每年的有機(jī)碳損失高達(dá)2.99×108MgC[29]。大量研究表明, 富營養(yǎng)化、全球變暖、植物入侵、人為干擾都會影響海草床中微生物群落特征[160-161], 微生物控制著關(guān)鍵的生物地球化學(xué)途徑, 因此, 微生物活性和群落結(jié)構(gòu)的變化會影響藍(lán)碳的穩(wěn)定性, 微生物的呼吸以及活性的增強(qiáng)會導(dǎo)致有機(jī)碳礦化速率提高, 從而加速碳的流失[162-164]。
2.3.2 底棲生物
小型底棲動物對沉積物的擾動會增加沉積物的孔隙度與含氧率, 并且小型底棲動物如線蟲會釋放粘液, 為細(xì)菌的生長發(fā)育創(chuàng)造條件[165], 顯著提高微生物的豐度與活性[166-167]。大型底棲動物會通過抑制或激活微生物基團(tuán)來影響沉積物中微生物群落[168]。Lacoste等發(fā)現(xiàn), 大型底棲動物對沉積物的擾動會造成細(xì)菌活性的增強(qiáng), 這可能會加速有機(jī)碳的降解[169]。
2.3.3 藻類爆發(fā)
由富營養(yǎng)化和全球氣候變化協(xié)同影響下引起的附生藻類的大量繁殖會在一定程度上保護(hù)海草, 并且增加海草床沉降懸浮顆粒物的能力[170-171], 但是附生藻類和大型海藻暴發(fā), 會通過與海草競爭營養(yǎng)鹽、形成缺氧環(huán)境、影響光照等途徑造成海草衰退[157]。由于海草床的加速減少, 近岸海域更容易受到氣流和波浪的影響, 這會導(dǎo)致海草床中儲存的有機(jī)碳大量減少[172]。當(dāng)營養(yǎng)鹽濃度升高時, 大型海藻和附生藻類對沉積物有機(jī)碳的貢獻(xiàn)短時間內(nèi)會相對增加[27], 向水體中大量釋放碳水化合物與氨基酸[173-174], 導(dǎo)致微生物所能利用有機(jī)碳的源發(fā)生改變[153], 引起海草床長期存儲有機(jī)碳的能力降低。與海草相比, 來源于附生藻類和大型海藻的有機(jī)碳更容易分解[175], 會在幾天內(nèi)被細(xì)菌迅速利用[176], 大量多糖及纖維素的加入, 會引起沉積物中蔗糖酶與纖維素酶活性的顯著上升[177], 增加原有有機(jī)碳的分解, 導(dǎo)致海草床碳儲量減少[178-179]。
3 展望
綜上所述, 國內(nèi)外學(xué)術(shù)界對海草床沉積物有機(jī)碳來源、儲量以及影響因素等方面已經(jīng)展開了很多研究, 但是相關(guān)研究仍有待加強(qiáng)。未來海草床沉積物有機(jī)碳研究應(yīng)該在以下幾個方面展開:
(1) 加強(qiáng)海草床碳通量普查和海草床調(diào)查。調(diào)查全國各海草床海草地上地下部分生物量和沉積物中有機(jī)碳的來源、組份及儲量, 明確全國海草床沉積物碳儲存的基本情況。
(2) 分析全球氣候變化背景下沉積物有機(jī)碳的變化機(jī)制。在全球氣候變化背景下, 研究海草床有機(jī)碳來源、組分, 沉積物中微生物、酶活性變化等, 明確海草床中有機(jī)碳的變化機(jī)制, 為海草床沉積物有機(jī)碳的科學(xué)管理提供科學(xué)對策。
(3) 研究影響海草床碳儲量的主要環(huán)境因素。對處于富營養(yǎng)化以及其它人類活動影響下的海草床沉積物進(jìn)行碳儲量的長期觀測, 利用野外操控實驗和室內(nèi)模擬實驗, 明確環(huán)境因素對沉積物有機(jī)碳儲量的影響機(jī)制。
(4) 明確海草床修復(fù)工程對沉積物有機(jī)碳儲存的長期響應(yīng), 尤其是對海草床修復(fù)工程運(yùn)行過程及后期對海草床碳通量的可能影響進(jìn)行評估, 分析海草床修復(fù)工程在碳匯方面的實際收益, 為平衡海草床修復(fù)工程的投入與收益提供科學(xué)依據(jù)。
[1] Short F T, Carruthers T J B, Dennison W C, et al. Global seagrass distribution and diversity: a bioregional model[J]. Journal of Experimental Marine Biology and Ecology, 2007, 350(1/2): 3-20.
[2] International Union for the Conservation of Nature (IUCN). IUCN red list of threatened species[M]. Gland: IUCN Conservation Centre, 2010.
[3] Short F T, Polidoro B, Livingstone S R, et al. Extinction risk assessment of the world’s seagrass species[J]. Biological Conservation, 2011, 144: 1961-1971.
[4] 鄭鳳英, 邱廣龍, 范航清, 等. 中國海草的多樣性、分布及保護(hù)[J]. 生物多樣性, 2013, 21(5): 517-526.
Zheng Fengyin, Qiu Guanglong, Fan Hangqing, et al. Diversity, distribution and conservation of Chinese seagrass species[J]. Biodiversity Science, 2013, 21(5): 517-526.
[5] 黃小平, 江志堅, 范航青, 等. 中國海草的“藻”名更改[J]. 海洋與湖沼, 2016, 47(1): 290-294.
Huang Xiaoping, Jiang Zhijian, Fan Hangqing, et al. The nomenclature of the “Algae” name of seagrasses in China[J]. Oceanologia et Limnologia Sinica, 2016, 47(1): 290-294.
[6] Charpy-Roubaud C, Sournia A. The comparative estimation of phytoplanktonic and microphytobenthic production in the oceans[J]. Marine Microbial Food Webs, 1990, 4: 31-57.
[7] Duarte C M, Middelburg J J, Caraco N. Major role of marine vegetation on the oceanic carbon cycle[J]. Biogeoscience, 2005, 2(6): 1-8.
[8] Costanza R, D’Arge R, de Groot R, et al. The value of the world’s ecosystem services and natural capital[J]. Nature, 1997, 38(7): 253-260.
[9] Cullen-Unsworth L C, Nordlund L M, Paddock J, et al. Seagrass meadows globally as a coupled social-ecological system: Implications for human wellbeing[J]. Marine Pollution Bulletin, 2014, 83(2): 387-397.
[10] Jackson E L, Rowden A A, Attrill M J, et al. The importance of seagrass beds as a habitat for fishery species[J]. Oceanology and Marine Biology-An Annual Review, 2001, 39: 269-303.
[11] Waycott M, Longstaff B J, Mellors J. Seagrass population dynamics and water quality in the Great Barrier Reef region: a review and future research directions[J]. Marine Pollution Bulletin, 2005, 51(1): 343-350.
[12] Burn D M. The digestive strategy and efficiency of the West Indian manatee,[J]. Comparative Biochemistry and Physiology, 1986, 85(1): 139-142.
[13] Hopkinson C S, Cai W J, Hu X. Carbon sequestration in wetland dominated coastal systems - A global sink of rapidly diminishing magnitude[J]. Current Opi-nion in Environmental Sustainability, 2012, 4(2): 186- 194.
[14] DUARTE C M, CEBRIAN J. The fate of marine autotrophic production[J]. Limnology and Oceanography, 1996, 44: 103-110.
[15] Angrelina I, Sartimbul A, Wahyudi A J. The potential of seagrass beds on the coast of Putri Menjangan as a carbon sequestration ecosystem on Bali Island[J]. IOP Conference Series: Earth and Environmental Science, 2019, 241(1): 012010.
[16] Quevedo J M D, Uchiyama Y, Kohsaka R. Perceptions of the seagrass ecosystems for the local communities of Eastern Samar, Philippines: Preliminary results and prospects of blue carbon services[J]. Ocean and Coastal Management, 2020, 191: 105181.
[17] Mcleod E, Chmura G L, Bouillon S, et al. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2[J]. Frontiers in Ecology and the Environment, 2011, 9(10): 552-560.
[18] Phang V X, Chou L, Friess D A. Ecosystem carbon stocks across a tropical intertidal habitat mosaic of mangrove forest, seagrass meadow, mudflat and sandbar[J]. Earth Surface Processes and Landforms, 2015, 40(10): 1387-1400.
[19] Duarte C M, Marbà N, Gacia E, et al. Seagrass community metabolism: assessing the carbon sink capacity of seagrass meadows[J]. Global Biogeochemical Cycles, 2010, 24(4): GB4032.
[20] Kennedy H, Beggins J, Duarte C M, et al. Seagrass sediments as a global carbon sink: isotopic constraints[J]. Global Biogeocemical Cycles, 2010, 24(4): 6696-6705.
[21] Barrón C, Apostolaki E, Duarte C. Dissolved organic carbon release by marine macrophytes[J]. Biogeosciences Discussions, 2012, 9(2): 1529-1555.
[22] Liu S L, Jiang Z J, Zhou C Y, et al. Leaching of dissolved organic matter from seagrass leaf litter and its biogeochemical implications[J]. Acta Oceanologica Sinica, 2018, 37(8): 84-90.
[23] Bouillon S, Moens T, Dehairs F. Carbon sources supporting benthic mineralization in mangrove and adjacent seagrass sediments (Gazi Bay, Kenya)[J]. Biogeosciences Discussions, 2004, 1(5): 311-333.
[24] Barrón C, Apostolaki E T, Duarte C M. Dissolved organic carbon fluxes by seagrass meadows and macroalgal beds[J]. Frontiers in Marine Science, 2014, 1: 42.
[25] Su Z N, Qiu G L, Fan H Q, et al. Seagrass beds store less carbon but support more macrobenthos than mangrove forests[J]. Marine Enviromental Research, 2020, 162: 105162.
[26] Duarte C M, Kennedy H, Marbà N, et al. Assessing the capacity of seagrass meadows for carbon burial: Current limitations and future strategies[J]. Ocean & Coastal Management, 2013, 83: 32-38.
[27] Liu S L, Jiang Z J, Zhang J P, et al. Sediment microbes mediate the impact of nutrient loading on blue carbon sequestration by mixed seagrass meadows[J]. Science of the total environment, 2017, 599/600: 1478- 1484.
[28] Alongi D M. Blue carbon coastal sequestration for climate change mitigation[M]. Australia: Springer Brie-fs in Climate Studies. 2018: 88.
[29] Fourqurean J W, Duarte C M, Kennedy H, et al. Seagrass ecosystems as a globally signifificant carbon stock[J]. Nature Geoscience, 2012, 5(7): 505-509.
[30] Pidgeon E. Carbon sequestration by coastal marine habitats: Missing sinks[C]// Laffoley D. The Management of Natural Coastal Carbon Sinks. Gland: IUCN, 2009: 47-51.
[31] Saintilan N, Rogers K, Mazumder D, et al. Allochthonous and autochthonous contributions to car-bon accumulation and carbon store in southeastern Aus-tralian coastal wetlands[J]. Estuarine Coastal and Shelf Science, 2013, 128(10): 84-92.
[32] 李捷, 劉譯蔓, 孫輝, 等. 中國海岸帶藍(lán)碳現(xiàn)狀分析[J]. 環(huán)境科學(xué)與技術(shù), 2019, 42(10): 207-216.
Li Jie, Liu Yiman, Sun Hui, et al. Analysis of blue carbon in China’s coastal zone[J]. Environmental Science and Technology, 2019, 42(10): 207-216.
[33] 高亞平, 方建光, 唐望, 等. 桑溝灣大葉藻海草床生態(tài)系統(tǒng)碳匯擴(kuò)增力的估算[J]. 漁業(yè)科學(xué)進(jìn)展, 2013, 34(1): 17-21.
Gao Yaping, Fang Jianguang, Tang Wang, et al. Seagrass meadow carbon sink and amplification of the carbon sink for eelgrass bed in Sanggou Bay[J]. Progress in Fishery Sciences, 2013, 34(1): 17-21.
[34] Huang Y H, Lee C L, Chung C Y, et al. Carbon budgets of multispecies seagrass beds at Dongsha Island in the South China Sea[J]. Marine Environmental Research, 2015, 106: 92-102.
[35] Heck K L, Valentine J F. Plant-herbivore interactions in seagrass meadows[J].Journal of Experimental Marine Biology and Ecology, 2006, 330: 420-436.
[36] 吳鐘解, 陳石泉, 蔡澤富, 等. 海南島海草床分布變化及恢復(fù)建議[J]. 海洋環(huán)境科學(xué), 2021, 40(4): 542- 549.
Wu Zhongjie, Chen Shiquan, Cai Zefu, et al. Analysis of distribution change and restoration suggestion of theseagrass beds in Hainan Island[J]. Marine Environmental Science, 2021, 40(4): 542-549.
[37] Jiang Z J, Zhao C Y, Yu S, et al. Contrasting root length, nutrient content and carbon sequestration of seagrass growing in offshore carbonate and onshore terrigenous sediments in the South China Sea[J]. Science of the Total Environment, 2019, 662: 151-159.
[38] Jiang Z J, Liu S L, Zhang J P, et al. Newly discovered seagrass beds and their potential for blue carbon in the coastal seas of Hainan Island, South China Sea[J]. Marine Pollution Bulletin, 2017, 125(1/2): 513-521.
[39] Hendry M J, Wassenaar L I. Transport and geochemical controls on the distribution of solutes and stable isotopes in a thick clay-rich till aquitard, Canada[J]. Isotopes in Environmental and Health Studies, 2004, 40(1): 3-19.
[40] Lucke A, Brauer A. Blogeochemical and micro-facial fingerprints of ecosystem response to rapid Late Glacial climatlic changes in varved sediments of Meerfelder Maar (Germany)[J]. Palaeogeography Palaeoclimatology Palaeoecology, 2004, 211: 139-155.
[41] Wu Y, Luecke A, Jin Z, et al. Holocene climate de-velopment on the central Tibetan Plateau: a sedimen-tary record from Cuoe Lake[J]. Palaeogeography Palaeoclimatology Palaeoecology, 2006, 234(2/4): 328-340.
[42] Cifuentes L, Coffin R, Solorzano L.Isotopic and elemental variations of carbon and nitrogen in a mangrove estuary[J]. Estuarine Coastal and Shelf Science, 1996, 43(6): 781-800.
[43] Fry B, Sherr E B.13C measurements as indicators of carbon flow in marine and freshwater ecosystems[C]// Rundel P W. Stable Isotopes in Ecological Research. New York: Springer, 1989: 196-229.
[44] Liu X J, Tang D H, Ge C D. Distribution and sources of organic carbon, nitrogen and their isotopic composition in surface sediments from the southern Yellow Sea, China[J]. Marine Pollution Bulletin, 2020, 150: 110716.
[45] Jennerjahn T C. Relevance and magnitude of ‘Blue Carbon’ storage in mangrove sediments: Carbon accumulation rates vs. stocks, sources vs. Sinks[J]. Estuarine, Coastal and Shelf Science, 2020, 248: 107156.
[46] Kusumaningtyas M A, Hutahaean A A, Fischer H W, et al. Variability in the organic carbon stocks, sources, and accumulation rates of Indonesian mangrove ecosystems[J]. Estuarine, Coastal and Shelf Science, 2019, 218: 310-323.
[47] Tanaka Y, Miyajima T, Watanabe A, et al. Distribution of dissolved organic carbon and nitrogen in a coral reef[J]. Coral Reefs, 2011, 30(2): 533-541.
[48] Wild C, Mayr C, Wehrmann L, et al. Organic matter release by cold water corals and its implication for fauna-microbe interaction[J]. Marine Ecology Progress Series, 2008, 372: 67-75.
[49] Tanaka Y, Miyajima T, Ogawa H. Effects of nutrient enrichment on the release of dissolved organic carbon and nitrogen by the scleractinian coral[J]. Coral Reefs, 2010, 29: 675-682.
[50] Ziegler S, Benner R. Dissolved organic carbon cycling in a subtropical seagrass-dominated lagoon[J]. Marine Ecology Progress Series, 1999, 180: 149-160.
[51] Haas A F, Wild C. Composition analysis of organic matter released by cosmopolitan coral reef-associated green algae[J]. Aquatic Biology, 2010, 10(2): 131-138.
[52] Urban-Rich J. Release of dissolved organic carbon from copepod fecal pellets in the Greenland Sea[J]. Journal of Experimental Marine Biology and Ecology, 1999, 232(1): 107-124.
[53] Mayer L M, Macko S A, Mook W H, et al. The distribution of bromine in coastal sediments and its use as a source indicator for organic matter[J]. Organic Geochemistry, 1981, 3(1/2): 37-42.
[54] Malcolm S J, Price N B. The behavior of iodine and bromine in estuarine surface sediments[J]. Marine Chemistry, 1984, 15: 263-271.
[55] Leri A C, Hakala J A, Marcus M A, et al. Natural organobromine in marine sediments: New evidence of biogeochemical Br cycling[J]. Global Biogeochemical Cycles, 2010, 24(4): GB4017.
[56] Leri A C, Myneni S C B. Natural organobromine in terrestrial ecosystems[J]. Geochimica et Cosmochimica Acta, 2012, 77: 1-10.
[57] Kandasamy S, Lin B, Lou J Y, et al. Estimation of Marine Versus Terrigenous Organic Carbon in Sediments Off Southwestern Taiwan Using the Bromine to Total Organic Carbon Ratio as a Proxy[J]. Journal of Geophysical Research: Biogeosciences, 2018, 123(10): 3387-3402.
[58] R?hr M E, Bostr?m C, Canal-Vergés P, et al. Blue carbon stocks in Baltic Sea eelgrass () meadows[J]. Biogeosciences, 2016, 13(22): 6139- 6153.
[59] Ricart A M, Pérez M, Romero J. Landscape configuration modulates carbon storage in seagrass sediments[J]. Estuarine, Coastal and Shelf Science, 2017, 185: 69-76
[60] Smith B N, Epstein S. Two categories of13C/12C ratios for higher plants[J]. Plant Physiology, 1971, 47(3): 380-384.
[61] Gacia E, Duarte C M, Middelburg J J. Carbon and nutrient deposition in a Mediterranean seagrass () meadow[J]. Limnology and Oceanography, 2002, 47(1): 23-32.
[62] Kennedy H, Gacia E, Kennedy D P, et al. Organic carbon sources to SE Asian coastal sediments[J]. Estuarine Coastal and Shelf Science, 2004, 60(1): 59-68.
[63] Liu S L, Jiang Z J, Zhang J P. Effect of nutrient enrichment on the source and composition of sediment organic carbon in tropical seagrass beds in the South China Sea[J]. Marine Pollution Bulletin, 2016, 110(1): 274-280.
[64] Lebreton B, Richard P, Galois R, et al. Trophic importance of diatoms in an intertidalseagrass bed: evidence from stable isotope and fatty acid analyses[J]. Estuarine, Coastal and Shelf Science, 2011, 92(1): 140-153.
[65] Rahayu Y P, Solihuddin T, Kusumaningtyas M A, et al. The sources of organic matter in seagrass sediments and their contribution to carbon stocks in the Spermonde Islands, Indonesia[J]. Aquatic Geochemistry, 2019, 25(3/4): 161-178.
[66] VolKman J K, Barrett S M, Blackburn S I, et al. Microalgal biomarkers: a review of recent research developments[J]. Organic Geochemistry, 1998, 29(5/7): 1163-1179.
[67] Meziane T, Tsuchuya M. Fatty acids as tracers of organic matter in the sediment and food web of a mangrove/intertidal flat ecosystem, Okinawa, Japan[J]. Marine Ecology Progress Series, 2000, 200: 49-57.
[68] Khotimchenko S V. Fatty acids and polar lipids of seagrasses from the sea of Japan[J]. Phytochemistry, 1993, 33(2): 369-372.
[69] Rajendran N, Suwa Y, Urushigawa Y. Distribution of phospholipid ester-linked fattyacid biomarkers for bacteria in the sediment of Ise Bay, Japan[J]. Marine Chemistry, 1993, 42(1): 39-56.
[70] Kelly J R, Scheibling R E. Fatty acids as dietary tracers in benthic food webs[J]. Marine Ecology Progress Series, 2012, 446: 1-22.
[71] Park H, Choy E, Lee K. Trophic transfer between coastal habitats in a seagrass-dominated marcrotidal embayment system as determined by stable isotope and fatty acid signatures[J]. Marine and Freshwater Research, 2013, 64(12): 1169-1183.
[72] Dubois S, Blanchet H, Garcia A. Trophic resource use by macrozoobenthic primary consumers within a semi-enclosed coastal ecosystem: Stable isotope and fatty acid assessment[J]. Journal of Sea Research, 2014, 88: 87-99.
[73] Arnold T M, Targett N M. Marine tannins: the importance of a mechanistic framework for predicting ecological roles[J]. Journal of Chemical Ecology, 2002, 28(10): 1919-1934.
[74] Torbatinejad N M, Annison G, Rutherfurd- Markwick K, et al. Structural constituents of the seagrass[J]. Journal of Agricultural and Food Chemistry, 2007, 55(10): 4021-4026.
[75] Kaal J, Serrano O, Nierop K G J, et al. Molecular composition of plant parts and sediment organic matter in a Mediterranean seagrass () mat[J]. Aquatic Botany, 2016, 133: 50-61.
[76] Hemminga M A, Mateo M A. Stable carbon isotopes in seagrasses: Variability in ratio and use in eco-logy studies[J]. Marine Ecology Progress Series, 1996, 140(1/3): 285-298.
[77] Anderson W T, Fourqurean J W. Intra-and interannual variability in seagrass carbon and nitrogen stable isotopes from south Florida, a preliminary study[J]. Organic Geochemistry, 2003, 34(2): 185-194.
[78] Jaschinski S, Hansen T, Sommer U. Effects of acidification in multiple stable isotope analyses[J]. Limnology and Oceanography, 2008, 6(1): 12-15.
[79] Gearing J N, Gearing P J, Rudnick D T, et al. Isotopic variability of organic carbon in a phytoplankton-based, temperate estuary[J]. Geochimica et Cosmochimica Acta, 1984, 48(5): 1089-1098.
[80] Khotimchenko S V, Vaskovsky V E. Distribution of C20polyenoic fatty acids in red macrophytic algae[J]. Botanica Marine, 1990, 33(6): 525-528.
[81] Moncreiff C A, Sullivan M J. Trophic importance of epiphytic algae in subtropical seagrass beds: evidence from multiple stable isotope analyses[J]. Marine Ecology Progress Series, 2001, 215: 93-106.
[82] Pancost R D, Boot C S. The palaeoclimatic utility of terrestrial biomarkers in marine sediments[J]. Marine Chemistry, 2004, 92(1/4): 239-261.
[83] 梁越, 肖化云, 劉小真, 等. 碳氮穩(wěn)定同位素示蹤鄱陽湖流域蚌湖豐水期的氮污染[J]. 湖泊科學(xué), 2018, 30(4): 957-966.
Liang Yu, Xiao Huayun, Liu Xiaozhen, et al. Carbon and nitrogen stable isotopes tracing nitrogen pollution in major flooding season in Lake Bang, Lake Poyang Basin[J]. Journal of Lake Sciences, 2018, 30(4): 957-966.
[84] 佟小剛, 徐明崗, 張文菊, 等. 長期施肥對紅壤和潮土顆粒有機(jī)碳含量與分布的影響[J]. 中國農(nóng)業(yè)科學(xué), 2008, 41(11): 3664-3671.
Tong Xiaogang, Xu Minggang, Zhang Wenju, et al. Influence of long-term fertilization on content and distribution of organic carbon in particle-size fractions of red soil and fluvo-aquic soil in China[J]. Scientia Agricultura Sinica, 2008, 41(11): 3664-3671.
[85] 林曉東, 漆智平, 唐樹梅, 等. 海南人工林地, 人工草地土壤易氧化有機(jī)碳和輕組碳含量初探[J]. 熱帶作物學(xué)報, 2012, 33(1): 171-177.
Lin Xiaodong, Qi Zhiping, Tang Shumei, et al. Oxidizable organic Carbon and light fraction organic carbon of artificial plantation land and artificial grassland in Hainan Province[J]., 2012, 33(1): 171-177.
[86] ROVIRA P, VALLEJO V R. Labile and recalcitrant pools of carbon and nitrogen in organic matter decomposing at different depths in soil: an acid hydrolysis approach[J]. Geoderma, 2002, 107(1/2): 109-141.
[87] 連忠廉, 江志堅, 黃小平, 等. 珠江口表層沉積物有機(jī)碳不同浸提組分的空間分布特征[J]. 海洋環(huán)境科學(xué), 2019, 38(3): 391-398.
Lian Zhonglian, Jiang Zhijian, Huang Xiaoping, et al. Distribution of labile organic carbon using different extract method in the surface sediments of Pearl River Estuary[J]. Marine Environmental Science, 2019, 38(3): 391-398.
[88] 周腳根, 黃道友. 土壤微生物量碳周轉(zhuǎn)分析方法及其影響因素[J]. 中國生態(tài)農(nóng)業(yè)學(xué)報, 2006, 14(2): 131-134.
Zhou Jiaogen, Huang Daoyou. Research methods of soil microbial biomass carbon turnover and its influencing factors[J]. Chinese Journal of Eco-Agriculture, 2006, 14(2): 131-134.
[89] 張國, 曹志平, 胡嬋娟. 土壤有機(jī)碳分組方法及其在農(nóng)田生態(tài)系統(tǒng)研究中的應(yīng)用[J]. 應(yīng)用生態(tài)學(xué)報, 2011, 22(7): 1921-1930.
Zhang Guo, Cao Zhiping, Hu Chanjuan. Soil organic carbon fractionation methods and their applications in farmland ecosystem research: A review[J]. Chinese Journal of Applied Ecology, 2011, 22(7): 1921-1930.
[90] Hicks C E.Sediment organic carbon pools and sources in a recently constructed mangrove and seagrass ecosystem[D]. Gainesville: University of Florida, 2007.
[91] Dodla S K, Wang J J, Delaune R D. Characterization of labile organic carbon in coastal wetland soils of the Mississippi River deltaic plain: Relationships to carbon functionalities[J]. Science Total Environment, 2012, 435: 151-158.
[92] Fang C M, Smith P, Moncrieff J B. Similar response of labile and resistant soil organic matter pools to changes in temperature. Nature, 2005, 436: 881.
[93] Mateo M A, Cebrin J, Dunton K. Carbon flux in seagrass ecosystems[C]//Larkum A W D, ed. Seagrasses: Biology, Ecology and Conservation. Dordrecht: Springer, 2006: 159-192.
[94] 劉松林, 江志堅, 吳云超, 等. 海草床沉積物儲碳機(jī)制及其對富營養(yǎng)化的響應(yīng)[J]. 科學(xué)通報, 2012, 62(Z2): 3309-3318.
Liu Songlin, Jiang Zhijian, Wu Yunchao, et al. Mechanisms of sediment carbon sequestration in seagrass meadows and its responses to eutrophication[J]. Chinese Science Bulletin, 2012, 62(Z2): 3309-3318.
[95] Howard J, Hoyt S, Isensee K, et al. Coastal Blue Carbon: Methods for Assessing Carbon Stocks and Emissions Factors in Mangroves, Tidal Salt Marshes, and Seagrasses[J]. Journal of American History, 2014, 14(4): 4-7.
[96] FOURQUREAN J W, RUTTEN L M. The impact of hurricane Georges on soft-bottom, back reef commu-ni-ties: Site- and species-specific effects in South Florida seagrass beds[J]. Bulletin of Marine Science, 2004, 75: 239-257.
[97] Howard J L, Creed J C, Aguiar M V, et al. CO2released by carbonate sediment production in some coastal areas may offset the benefits of seagrass “blue carbon” storage[J]. Limnology and Oceanography, 2017, 63(1): 160-172.
[98] Lavery P S, Mateo M A, Serrano O. Variability in the carbon storage of seagrass habitats and its implications for global estimates of blue carbon ecosystem service[J]. PLoS One, 2013, 8: e73748.
[99] Miyajima T, Hori M, Hamaguchi M. Geographic variability in organic carbon stock and accumulation rate in sediments of East and Southeast Asian seagrass meadows[J]. Global Biogeochemistry Cycles, 2015, 29(4): 397-415.
[100] R?hr M E, Holmer M, Baum J K, et al. Blue carbon storage capacity of temperate eelgrass () meadows[J]. Global Biogeochemical Cycles, 2018, 32: 1-18.
[101] Potouroglou M, Whitlock D, Milatovic L, et al. The sediment carbon stocks of intertidal seagrass meadows in Scotland[J]. Estuarine, Coastal and Shelf Science, 2021, 258: 107442.
[102] Rustam A, Sudirman N, Ati R N, et al. Seagrass ecosystem carbon stock in the small islands: case study in Spermonde island, South Sulawesi, Indonesia[J]. Jurnal Segara, 2017, 13(2): 97-106.
[103] 李夢. 廣西海草床沉積物碳儲量研究[D]. 桂林: 廣西師范大學(xué), 2018.
LI Meng. Carbon Storage in the seagrass sediments of Guangxi, China[D]. Guilin: Guangxi Teachers Education University, 2018.
[104] Jiang Z J, Liu S L, Zhang J P, et al. Eutrophication indirectly reduced carbon sequestration in a tropical seagrass bed[J]. Plant and Soil, 2018, 426: 135-152.
[105] Juma G A, Magana A M, Michael G N, et al. Variation in Seagrass Carbon Stocks Between Tropical Estuarine and Marine Mangrove-Fringed Creeks[J]. Frontiers in Marine Science, 2020, 7: 696.
[106] Prentice C I. Reduced water motion enhances organic carbon stocks in temperate eelgrass meadows[D]. British Columbia: Simon Fraser University, 2019.
[107] Serrano O, Gómez-López D I, Sánchez- Valencia L, et al. Seagrass blue carbon stocks and sequestration rates in the Colombian Caribbean[J]. Scientific Reports, 2021, 11(1): 11067.
[108] Su Z N, Qiu G L, Fan H Q, et al. Changes in carbon storage and macrobenthic communities in a mangrove-seagrass ecosystem after the invasion of smooth cordgrass in southern China[J]. Marine Pollution Bulletin, 2020, 152: 110887.
[109] Cuellar-Martinez T, Ruiz-Fernández A C, Sanchez-Cabeza J A, et al. Relevance of carbon burial and storage in two contrasting blue carbon ecosystems of a north-east Pacific coastal lagoon[J]. Science of the Total Environment, 2019, 675: 581-593.
[110] Prentice C, Poppe K L, Lutz M, et al. A Synthesis of Blue Carbon Stocks, Sources, and Accumulation Rates in Eelgrass () Meadows in the Northeast Pacific[J]. Global Biogeochemical Cycles, 2020, 34(2): e2019GB006345.
[111] Dahl M, Asplund M E, Bj?rk M, et al. The influence of hydrodynamic exposure on carbon storage and nutrient retention in eelgrass (L.) meadowson the Swedish Skagerrak coast[J]. Scientific Reports, 2020, 10: 13666.
[112] Dahl M, Asplund M e, Dayanova D, et al. High seasonal variability in sediment carbon stocks of cold-temperate seagrass meadows[J]. Journal of Geophysical Research: Biogeosciences, 2020, 125(1): e2019JG005430.
[113] Novak A B, Pelletier M C, Colarusso P, et alFactors Influencing Carbon Stocks and Accumulation Rates in Eelgrass Meadows Across New England, USA[J]. Estuaries and Coasts, 2020, 43(8): 2076-2091.
[114] Aoki L R, McGlathery K J, Wiberg P L, et al. Seagrass recovery following marine heat wave influences sediment carbon stocks[J]. Frontiers in Marine Science, 2021, 7: 576784.
[115] Jankowska E, Michel L N, Zaborska A, et al. Sediment carbon sink in low-density temperate eelgrass meadows (Baltic Sea)[J]. Journal of Geophysical Research: Biogeosciences, 2016, 121(12): 2918-2934.
[116] Serrano O, Lavery P S, Rozaimi M. Influence of water depth on the carbon sequestration capacity of seagrasses[J]. Global Biogeochemistry Cycles, 2014, 28(9): 950-961.
[117] Keil R G, Hedges J I. Sorption of organic matter to mineral surfaces and the preservation of organic matter in coastal marine sediments[J]. Chemical Geology, 1993, 107(3/4): 385-388.
[118] Arndt S, J?rgensen B B, LaRowe D E, et al. Quantifying the degradation of organic matter in marine sediments: A review and synthesis[J]. Earth-Science Reviews, 2013, 123(4): 53-86.
[119] Ricart A M, York P H, Rasheed M A. Variability of sedimentary organic carbon in patchy seagrass landscapes[J]. Marine Pollution Bulletin, 2015, 100(1): 476-482.
[120] Gruber R K, Kemp W M. Feedback effects in a coastal canopy-forming submersed plant bed[J]. Limnology and Oceanography, 2010, 55(6): 2285-2298.
[121] Agawin N, Duarte C. Evidence of direct particle trapping by a tropical seagrass meadow[J]. Estuaries, 2002, 25(6): 1205-1209.
[122] Burdige D J. Preservation of organic matter in marine sediments: controls, mechanisms, and an imbalance in sediment organic carbon budgets?[J] Chemical reviews, 2007, 107(2): 467-485.
[123] Collier C, Lavery P, Masini R, et al. Morphological, growth and meadow characteristics of the seagrassalong a depth-related gradient of light availability[J]. Marine Ecology Progress Series, 2007, 337: 103-115.
[124] Pedersen M ?, Serrano O, Mateo M A, et al. Temperature effects on decomposition of amat[J]. Aquatic Microbial Ecology, 2011, 65: 169-182.
[125] Lefevre R, Barre P, Moyano F E. Higher temperature sensitivity for stable than for labile soil organic carbon-evidence from incubations of long-term bare fallow soil[J]. Global Change Biology, 2013, 20: 1087-1095.
[126] Duarte C M, Agusti S, Regaudie-De-Gioux A. The role of marine biota in the biogeochemical and geological cycles of carbon[C]// Duarte CM, ed. The role of marine biota in the functioning of the biosphere. Marid: Fundación BBVA, 2011: 21-37.
[127] Harris L A, Duarte C M, Nixon S W. Allometric laws and prediction in estuarine and coastal ecology[J]. Estuaries and Coasts, 2006, 29(2): 340-344.
[128] Regaudie-De-Gioux A, Duarte C M. Temperature dependence of planktonic metabolism in the ocean[J]. Global Biogeochemical Cycles, 2012, 26(1): 1-10.
[129] Garcias-Bonet N, Duarte C M. Methane production by seagrass ecosystems in the Red Sea[J]. Frontiers in Marine Science, 2017, 4: 1-10.
[130] Burkholz C, Duarte C M, Garcias-Bonet N. Thermal dependence of seagrass ecosystem metabolism in the Red Sea[J]. Marine Ecology Progress Series, 2019, 614: 79-90.
[131] Burkholz C, Garcias-Bonet N, Duarte C M. Warming enhances carbon dioxide and methane fluxes from Red Sea seagrass () sediments[J]. Biogeosciences Discuss, 2019, 17: 1-20.
[132] Weston N B, Joye S B. Temperature-driven decoupling of key phases of organic matter degradation in marine sediments[J]. Proceedings of the National Aca-demy of Sciences, 2005, 102(47): 17036-17040.
[133] Gao B, Walter M T, Steenhuis T S, et al. Investigating raindrop effects on transport of sediment and non-sorbed chemicals from soil to surface runoff[J]. Journal of Hydrology, 2005, 308(1/4): 313-320.
[134] Tolhurst T J, Watts C W, Vardy S, et al. The effects of simulated rain on the erosion threshold and biogeochemical properties of intertidal sediments[J]. Continental Shelf Research, 2008, 28(10): 1271-1230.
[135] Hartley D M, Alonso C V. Numerical study of the maximum boundary shear stress induced by raindrop impact[J]. Water Resources Research, 1991, 27(8): 1819-1826.
[136] Sampere T A, Bianchi T S, Wakeham S G, et al. Source of organic matter in surface sediment of the Louisiana continental margin: Effect of major depositional/transport pathways and Hurricane Ivan[J]. Continental Shelf Research, 2008, 28: 247-248.
[137] Li C, Swenson E, Weeks E, et al. Asymmetric tidal straining across an inlet: Lateral inversion and variability over a tidal cycle[J]. Estuarine, Coastal and Shelf Science, 2009, 85(4): 651-660.
[138] Li C, White J R, Chen C, et al. Summertime tidal flushing of Barataria bay: transports of water and suspended sediments[J]. Journal of Geophysical Research, 2011, 116: C04009.
[139] Erftemeijer P L A, Lewis III R R R. Environment impacts of dredging on seagrasses: A review[J]. Marine Pollution Bulletin, 2006, 52: 1553-1572.
[140] Kirkman H. Baseline and monitoring methods for seagrass meadows[J]. Journal of Environmental Management, 1996, 47: 191-201.
[141] Pasqualini V, Pergent-Martini C, Pergent G. Environmental impact identification along the Corsican coast (Mediterranean sea) using image processing[J]. Aquatic Botany, 1999, 65: 311-320.
[142] Pendleton L, Donato D C, Murray B C, et al. Estimating global “Blue Carbon” emissions from conversion and degradation of vegetated coastal ecosystems[J]. PLoS One, 2012, 9(7): e43542.
[143] Thorhaug A, Poulos H M, López-Portillo J, et al. Seagrass blue carbon dynamics in the Gulf of Mexico: Stocks, losses fromanthropogenic disturbance, and gains through seagrass restoration[J]. Science of the Total Environment, 2017, 606: 1-12.
[144] 郭雨昕. 廣西北部灣海草床生態(tài)經(jīng)濟(jì)價值評估與保護(hù)對策[J]. 現(xiàn)代農(nóng)業(yè)科技, 2019, 2: 170-173.
Guo Yuxin. Eco-economic value evaluation of seagrass beds in Guangxi Beibu Gulf and protection countermeasures[J]. Modern Agricultural Science and Technology, 2019, 2: 170-173.
[145] Macreadie P I, Atwood T B, Seymour J R, et al. Vulnerability of seagrass blue carbon to microbial attack following exposure to warming and oxygen[J]. Science of the Total Environment, 2019, 686: 264-275.
[146] Thorhaug A. Petroleum industry’s use of seagrass restoration as mitigation for construction and as a potential cleanup tool[J]. International Oil Spill Conference Proceedings, 2001, 1: 385-389.
[147] Garrard S L, Beaumont N J. The effect of ocean acidification on carbon storageand sequestration in sea-grass beds; a global and UK context[J]. Marine Pol-lu-tion Bulletin, 2014, 86(1/2): 138-146.
[148] Hall-Spencer J M, Rodolfo-Metalpa R, Martin S, et al. Volcanic carbon dioxide vents show ecosystem effects of ocean acidification[J]. Nature, 2008, 454: 96-99.
[149] Apostolaki E t, Vizzini S, Hendriks L E, et al. Seagrass ecosystem response to long-term high CO2in a Mediterranean volcanic vent[J]. Marine Environmental Research, 2014, 99: 9-15.
[150] Vizzini S, Apostolaki E T, Ricevuto E, et alPlant and sediment properties in seagrass meadows from two Mediterranean O2vents: Implications for carbon storage capacity of acidified oceans[J]. Marine Environmental Research, 2019, 146: 101-108.
[151] Ravaglioli C, Bulleri F, Rühl S, et al. Ocean acidification and hypoxia alter organic carbon fluxes in marine soft sediments[J]. Global Change Biology, 2019, 1: 1-14.
[152] Molari M, Guilini K, Lott C, et al. CO2leakage alters biogeochemical and ecological functions of subma-rine sands[J]. Science Advances, 2018, 4(2): eaao2040.
[153] Macreadie P I, Allen K, Kelaher B P, et al. Paleoreconstruction of estuarine sediments reveal human-induced weakening of coastal carbon sinks[J]. Global Change Biology, 2012, 18(3): 891-901.
[154] Ralph P, Durako M, Enriquez S. Impact of light limitation on seagrasses[J]. Journal of Experimental marine biology and ecology, 2007, 350(1/2): 176-193.
[155] Tanaka Y, Go G A, Watanaba A, et al. 17-year change in species composition of mixed seagrass beds around Santiago Island, Bolinao, the northwestern Phi-lippines[J]. Marine Pollution Bulletin, 2014, 88(1/2): 81-85.
[156] Jiang Z J, Huang X P, Zhang J P. Effect of nitrate enrichment and salinity reduction on the seagrasspreviously grown in low light[J]. Journal of Experimental Marine Biology and Ecology, 2013, 443: 114-122.
[157] Han Q Y, Liu D Y. Macroalgae blooms and their effe-cts on seagrass ecosystems[J]. Journal of Ocean Uni-versity of China, 2014, 13(5): 791-798.
[158] Romera-Castillo C, Sarmento H, Alvarez- Salgado X A, et alNet production and consumption of fluorescent colored dissolved organic matter by na-tural bacterial assemblages growing on marine phytoplankton exudates[J]. Applied and Environmental Microbiology, 2011, 77(21): 7490-7498.
[159] Olsen L M, Hernández K I, van Ardelan M, et al. Responses in bacterial community structure to waste nutrients from aquaculture: an in situ microcosm experiment in a Chilean fjord[J]. Aquaculture Environ-ment Interactions, 2017, 9: 21-32.
[160] Racchetti E, Bartoli M, Soana E, et al. Inf-luence of hydrological connectivity of riverine wetlands on nitrogen removal via denitrification[J]. Biogeo-che-mi-stry, 2011, 103(1/3): 335-354.
[161] Ardón M, Morse J L, Colman B P, et al. Drought- induced saltwater incursion leads to increased wetland nitrogen export[J]. Global Change Biology, 2013, 19(10): 2976-2985.
[162] Cleveland C C, Townsend A R. Nutrient addi-tions to a tropical rain forest drive substantial soil carbon dioxide losses to the atmosphere[J]. Proceedings of the National Academy of Sciences of the United Stated of America, 2006, 103(27): 10316-10321.
[163] Kirwan M L, Blum L K. Enhanced decomposition offsets enhanced productivity and soil carbon accumu-lation in coastal wetlands responding to climate change[J]. Biogeosciences, 2011, 8(4): 987-993.
[164] Kearns P J, Angell J H, Howard E M, et al. Nutrient enrichment induces dormancy and decreases diversity of active bacteria in salt marsh sediments[J]. Nature Communications, 2016, 7: 12881.
[165] Hubas C, Sachidhanandam C, Rybarczyk H, et al. Bacterivorous nematodes stimulate microbial growth and exopolymer production in marine sediment microcosms[J]. Marine Ecology Progress Series, 2010, 419(6): 85-94.
[166] Aller R C, Aller J Y. Meiofauna and solute trans-port in marine muds[J]. Limnology and Oceanography, 1992, 37(5): 1018-1033.
[167] Bradshaw C, Kumblad L, Fagrell A. The use of tracers to evaluate the importance of bioturbation in remobilising contaminants in Baltic sediments[J]. Estuarine, Coastal and Shelf Science, 2006, 66(1/2): 123-134.
[168] Mermillod-Blondin F, Rosenberg R, Fran?ois-Carcaillet F, et al. Influence of bioturbation by three benthic infaunal species on microbial commu-ni-ties and biogeochemical processes in marine sedi-ment[J]. Aquatic Microbial Ecology, 2004, 36(3): 271-284.
[169] Lacoste E, Piot A, ArchambauLt P, et al. Bioturbation activity of three macrofaunal species and the presence of meiofauna affect the abundance and composition of benthic bacterial communities[J]. Marine Environmental Research, 2018, 136: 62-70.
[170] 張景平, 黃小平. 海草附生藻類生物量的主要影響因子[J]. 生態(tài)學(xué)報, 2009, 29(10): 5611-5617.
Zhang Jingping, Huang Xiaoping. Effect factors on the abundance of epiphytic algae on seagrasses[J]. Acta Ecologica Sinica, 2009, 29(10): 5611-5617.
[171] 邱廣龍, 林幸助, 李宗善, 等. 海草生態(tài)系統(tǒng)的固碳機(jī)理及貢獻(xiàn)[J]. 應(yīng)用生態(tài)學(xué)報, 2014, 25(6): 1825-1832.
Qiu Guanglong, Lin Xingzhu, Li Zongshan, et al. Seagrass ecosystems: Contributions to and mechanisms of carbon sequestration[J]. Chinese Journal of Applied Ecology, 2014, 25(6): 1825-1832.
[172] Marbà N, Arias-Ortiz A, Masqué P, et al. Impact of seagrass loss and subsequent revegetation on carbon sequestration and stocks[J]. Journal of Ecology, 2015, 103: 296-302.
[173] Vichkovitten T, Holmer M. Microbial community response to nitrogen deposition in northern forest eco-systems[J]. Soil Biology and Biochemistry, 2004, 36(9): 1443-1451.
[174] Lavery P S, Mcmahon K, Weyers J, et al. Release of dissolved organic carbon from seagrass wrack and its implications for trophic connectivity[J]. Marine Ecology Progress Series, 2013, 494(3): 121-133.
[175] Holmer M, Duarte C, Boschker H. Carbon cycling and bacterial carbon sources in pristine and impacted Mediterranean seagrass sediments[J]. Aquatic Microbial Ecology, 2004, 36(3): 227-237.
[176] Zhang T, Wang X C. Release and microbial degra-dation of dissolved organic matter (DOM) from the macroalgae[J]. Marine Pollution Bulletin, 2017, 125: 192-198.
[177] Liu S L, Jiang Z J, Wu Y, et al. Effects of nutrient load on microbial activities within a seagrass-dominated ecosystem: implications of changes in seagrass blue carbon[J]. Marine Pollution Bulletin, 2017, 117(1/2): 214-221.
[178] Banta G T, Pedersen M F, Nielsen S L. Deco-m-position of marine primary producers: consequences for nutrient recycling and retention in coastal ecosys-te-ms[C]// Nielsen S L, ed. Estuarine Nutrient Cycling: The Influence of Primary Producers. Dordrecht: Kluwer Academic Publishers, 2004: 187-216.
[179] Blagodatskaya E V, Blagodatsky S A, Anderson T H, et al. Priming effects in Chernozem induced by glucose and N in relation to microbial growth strategies[J]. Applied Soil Ecology, 2007, 37(1/2): 95-105.
Review of organic carbon in seagrass bed sediment
YE Jia-hui, QIU Chong-yu, ZENG Wen-xuan, SHI Yun-feng, ZHAO Mu-qiu, HAN Qiu-ying
(Yazhou Bay Innovation Institute, Key Laboratory of Utilization and Conservation for Tropical Marine Biore-sources of Ministry of Education, Key Laboratory for Coastal Marine Eco-environment Process and Carbon Sink of Hainan Province, Hainan Tropical Ocean University, Sanya 572022, China)
Seagrass beds provide important ecosystem services, such as supporting biodiversity and providing carbon storage. Several scientists have studied the carbon storage mechanisms of seagrass beds. The annual carbon sequestration of seagrass beds is (2.7~4.4)×107MgC. Recently, seagrass beds have declined worldwide due to human activities, resulting in organic carbon storage reduction in seagrass sediment. This paper reviewed the research advancements of sediment organic carbon in seagrass beds, including the sources, components, storage, and environmental indicators. The environmental variations affecting carbon storage in seagrass beds were discussed from the three aspects of physics, chemistry, and biology. Finally, the primary research directions for the future study were proposed, including strengthening the carbon flux survey of seagrass beds, exploring the mechanism of sediment organic carbon change due to global climate change, defining the rate of carbon storage loss in seagrass beds, and studying the impact of coastal zone engineering on sediment organic carbon. Evaluating carbon storage mechanisms will provide the scientific basis for the blue carbon study in the oceans globally.
seagrass beds; sediment organic carbon; source; storage; environmental effects
Aug. 15, 2021
P76
A
1000-3096(2022)09-0130-16
10.11759/hykx20210815001
2021-08-15;
2022-01-18
海南省高層次人才項目(420RC657); 國家自然科學(xué)基金(41730529, 41766004); 海南熱帶海洋學(xué)院科研啟動項目(RHDXB201710)
[the High-level Talents Project of Hainan Province, No. 420RC657; the National Natural Science Foundation of China, Nos. 41730529, 41766004; the Project of Hainan Tropical Ocean University, No. RHDXB201710]
葉嘉暉(1997—), 男, 浙江余姚人, 碩士研究生, 主要從事海洋生態(tài)學(xué)研究, 電話: 15958810658, E-mail: 850273277@qq.com; 韓秋影(1980—),通信作者, 女, 吉林德惠人, 博士, 研究員, 研究方向: 海洋生態(tài)學(xué), 電話: 13006036262, E-mail: hanqiuying0312@sina.com
(本文編輯: 康亦兼)

