Diagnostic role of fractional exhaled nitric oxide in pediatric eosinophilic esophagitis, relationship with gastric and duodenal eosinophils
Panamdeep Kaur, Rachel Chevalier, Craig Friesen, Jamie Ryan, Ashley Sherman, Stephanie Page
Panamdeep Kaur, Department of Pediatric Gastroenterology, Connecticut Children’s Medical Center, University of Connecticut School of Medicine, Hartford, Connecticut, CT 06106,United States
Rachel Chevalier, Craig Friesen, Jamie Ryan, Department of Pediatric Gastroenterology,Children's Mercy Kansas City, University of Missouri-Kansas City School of Medicine, Kansas City, Missouri, MO 64108, United States
Rachel Chevalier, Craig Friesen, Department of Pediatrics, University of Kansas School of Medicine, Kansas City, Kansas, KS 66160, United States
Ashley Sherman, Department of Biostatistics, Children's Mercy Kansas City, Kansas City,Missouri, MO 64108, United States
Stephanie Page, Department of Pediatric Gastroenterology, Midwest Pediatric Specialists,Overland Park, Kansas, KS 66215, United States
Abstract
BACKGROUND
Eosinophilic esophagitis (EoE) is an eosinophilic-predominant inflammation of the esophagus diagnosed by upper endoscopy and biopsies. A non-invasive and cost-effective alternative for management of EoE is being researched. Previous studies assessing utility of fractional exhaled nitric oxide (FeNO) in EoE were low powered. None investigated the contribution of eosinophilic inflammation of the stomach and duodenum to FeNO.
AIM
To assess the utility of FeNO as a non-invasive biomarker of esophageal eosinophilic inflammation for monitoring disease activity.
METHODS
Patients aged 6-21 years undergoing scheduled upper endoscopy with biopsy for suspected EoE were recruited in our observational study. Patients on steroids and with persistent asthma requiring daily controller medication were excluded. FeNO measurements were obtained in duplicate using a chemiluminescence nitric oxide analyzer (NIOX MINO, Aerocrine, Inc.;Stockholm, Sweden) prior to endoscopy. Based on the esophageal peak eosinophil count(PEC)/high power field on biopsy, patients were classified as EoE (PEC ≥ 15) or control (PEC ≤ 14).Mean FeNO levels were correlated with presence or absence of EoE, eosinophil counts on esophageal biopsy, and abnormal downstream eosinophilia in the stomach (PEC ≥ 10) and duodenum (PEC ≥ 20). Wilcoxon rank-sum test, Spearman correlation, and logistic regression were used for analysis. P value < 0.05 was considered significant.
RESULTS
We recruited a total of 134 patients, of which 45 were diagnosed with EoE by histopathology. The median interquartile range FeNO level was 17 parts per billion (11-37, range: 7-81) in the EoE group and 12 parts per billion (8-19, range: 5-71) in the control group. After adjusting for atopic diseases, EoE patients had significantly higher FeNO levels as compared to patients without EoE (Z = 3.33, P < 0.001). A weak yet statistically significant positive association was found between the number of esophageal eosinophils and FeNO levels (r = 0.30, P < 0.005). On subgroup analysis within the EoE cohort, higher FeNO levels were noted in patients with abnormal gastric (n = 23, 18 vs 15) and duodenal eosinophilia (n = 28, 21 vs 14); however, the difference was not statistically significant.
CONCLUSION
After ruling out atopy as possible confounder, we found significantly higher FeNO levels in the EoE cohort than in the control group.
Key Words: Nitric oxide; Fractional exhaled nitric oxide; Eosinophilic esophagitis; Esophagus; Pediatric;Gastroenterology
INTRODUCTION
Eosinophilic esophagitis (EoE) is an immune-mediated chronic inflammatory disease of the esophagus histologically characterized by an eosinophil-predominant inflammation of the esophageal mucosa[1].Active inflammation leads to dysphagia, odynophagia and, in younger patients, vomiting, abdominal pain, and poor growth[1]. Chronic inflammation results in fibrosis, causing strictures and dysmotility.Stricturing requires repeated, invasive dilations to maintain adequate swallowing[2]
In vitroandin vivostudies have demonstrated the role of IL-4, 5, and 13 in promoting eosinophilic inflammation, loss of barrier function, and tissue remodeling in the esophagus[3]. A subset of EoE is responsive to proton-pump inhibitors; the remaining cases are managed with either topical glucocorticoids or dietary food group eliminations[4]. The gold standard for diagnosis is endoscopic biopsy where the degree of eosinophil infiltration in the esophageal mucosa is quantified as the number of eosinophilsperhigh power field (HPF). Any patient with ≥ 15 eosinophils/HPF meets criteria for diagnosis of EoE[5].
EoE and asthma are both considered atopic conditions and frequently occur concurrently in patients[6]. The diagnosis of asthma is largely based upon the observation of symptoms of airway hyperresponsiveness and their response to bronchodilators. The degree of airflow obstruction is demonstrated using spirometry along with subjective assessment standardized questionnaires to assess limitation and severity of asthma symptoms. The presence of eosinophils in the bronchi is an integral part of the inflammatory process and is responsible for the production of exhaled nitric oxide from the pulmonary epithelium[7]. The advent of exhaled nitric oxide testing as a Food and Drug Administration (FDA)-approved device has brought forth a new tool capable of capturing the degree of pulmonary inflammation in exhaled breath[8,9]. Clinical studies have validated the concept of FeNO as a surrogate marker of eosinophilic airway inflammation[10].
The abundance of eosinophils in the esophageal mucosa in EoE prompts evaluation of their contribution to exhaled nitric oxide in individuals with EoE. Previous studies assessing correlation of fractional exhaled nitric oxide (FeNO) with degree of esophageal eosinophilic inflammation were low powered but noted a trend for association. If it could serve as a robust marker of disease activity in EoE,FeNO could potentially replace the need to perform periodic, invasive, and cumbersome endoscopies.
MATERIALS AND METHODS
Study design and study participants
We performed a cross -sectional study that enrolled patients aged 6-19 years seen in the Gastroenterology Clinic at Children’s Mercy Kansas City between July 2011 and July 2016. Patients 6 years and older were most likely to be able to use the chemiluminescence nitric oxide analyzer (NIOX MINO,Aerocrine, Inc.; Stockholm, Sweden) machine as instructed. All patients with upper gastrointestinal complaints (dysphagia, food impactions, vomiting, upper abdominal pain, or reflux) who were scheduled to undergo esophagogastroduodenoscopy (EGD) with biopsies were eligible. Patients taking swallowed, inhaled, or systemic corticosteroids within a month prior to enrollment in the study were excluded to decrease the confounding factors that would affect the FeNO scores. Given the high prevalence of concurrent atopic disorders with EoE, only patients with persistent asthma requiring use of daily controller medications including corticosteroids or leukotriene modifiers were excluded from the study. Other exclusions included history of tobacco use, history of celiac disease, inflammatory bowel disease, diabetes, or other multi-system inflammatory diseases. Patients were excluded if they had ingested caffeine or nitrate-containing food 3 hours prior to the procedure as this could potentially modify FeNO scores. Data was collected retroactively by chart review to include the clinical characteristics of patients including symptoms, endoscopic, and histology findings.
FeNO
Each patient provided 2 exhaled nitric oxide samples, measured in partsperbillion (ppb), using a chemiluminescence analyzer (NIOX MINO, Aerocrine, Inc.; Stockholm, Sweden) prior to endoscopy.The NIOX MINO unit was stationed in the endoscopy suite. A member of the study group trained on the use of the NIOX MINO unit based on FDA-approved technique and specifications instructed subjects to breathe deeply then blow into the NIOX MINO’s plastic mouthpiece for approximately 10-15 s. This procedure was then repeated in order to meet the 2005 American Thoracic Society guidelines[11]. Mean value of two FeNO readings was used for purpose of analysis.
Atopy
Atopy was assessedviaa 11-point questionnaire (Tables 1 and 2) developed collaboratively between the Pediatric Gastroenterology and Pediatric Allergy divisions. The questionnaire consisted of elementary reading level questions designed to screen and identify patients with symptoms suggestive of or a known diagnosis of atopic disease (e.g., allergic rhinitis, eczema, asthma) that may falsely elevate the FeNO score. Patients were considered to be atopic if they answered positively to 1 or more questions.The presence of atopy was also controlled for and analyzed in a multivariate logistic regression model to discern its effects on FeNO in EoE patients.

Table 1 Atopy screening questionnaire

Cough Nighttime wakening from cough Exercise that required the use of an inhaler to help breathe(11) Has the patient taken any of the following medications in the past year? (Check all that apply)
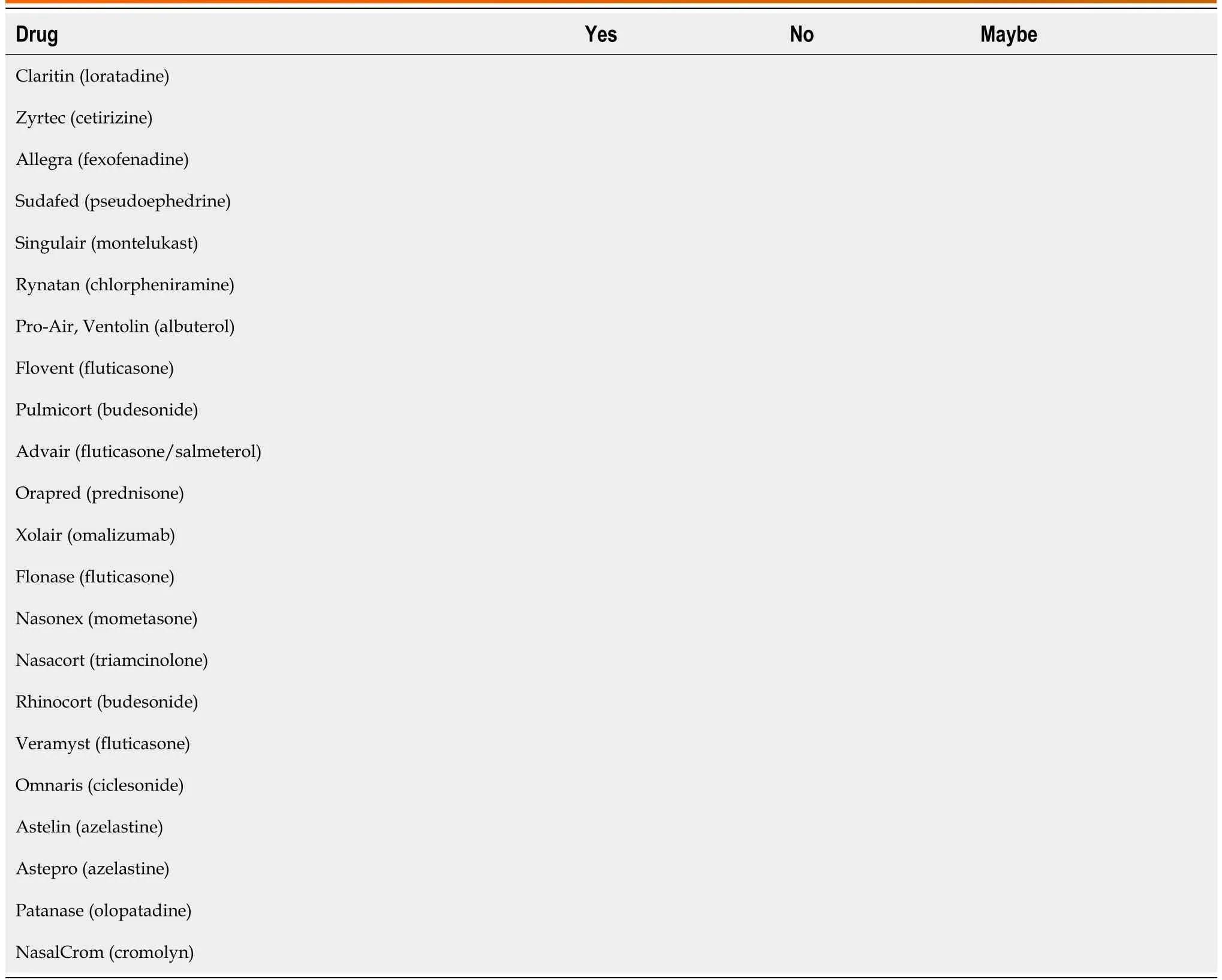
Table 2 Has the patient taken any of the following medications in the past year
Esophageal eosinophils
All subjects underwent standard-of-care EGD with two biopsies in the mid and distal esophagus, two in stomach antrum, and duodenum. A trained pathologist performed eosinophil counts on hematoxylin and eosin-stained mucosa. EoE was defined as ≥ 15 eosinophils/HPF at either of the esophageal locations. Patients with eosinophils ≥ 15/HPF were included in the EoE group; patients with esophageal eosinophils ≤ 14/HPF were in the control group.
Downstream eosinophils
Eosinophils in the stomach (antrum) and/or duodenum were considered “downstream.” The eosinophils in stomach and duodenum were verified by 2 gastroenterologists in the EoE patient cohort.To determine eosinophil density, hematoxylin and eosin-stained sections were initially scanned at a low magnification (10 x objective magnification) to determine areas of maximal density. Then, using 40 x objective magnification, the eosinophils were counted in 5 consecutive non-overlapping HPF.Eosinophils were counted separately for the stomach and duodenum. The 5 counts were averaged to determine final eosinophil cell count for each location. Cutoff values for normal eosinophils (≤ 10 eos/HPF in the stomach and ≤ 20 eos/HPF in the duodenum) were derived from a control group of 10 patients previously identified[12]. This control group consisted of patients with a chief complaint of constipation who had an EGD as part of their clinical evaluation and whose pathology showed no diagnostic abnormality. The EoE patient cohort was then divided into 2 groups – with and without abnormal downstream eosinophils.
Ethical considerations and patient safety
The study was approved by the Children’s Mercy Institutional Review Board. Prior to enrollment, an informed consent was obtained from the subjects and the caregivers, and assent was obtained from minors when appropriate.
Statistical analysis
All analysis was performed using SPSS (version 24) and SAS (version 9.4). The statistical methods of this study were reviewed by a statistician from Children’s Mercy Kansas City. Patients were classified into EoE and control (non-EoE) groups. Median FeNO levels with interquartile range (IQR) are reported for both groups. Peak eosinophil count (PEC) was the absolute number from mid and distal esophagus.Wilcoxon rank-sum test was used to determine if there were any differences in FeNO levels between EoE and non-EoE subjects. Similarly, differences in FeNO were ascertained in reference to downstream eosinophilia. Receiver operator curves (ROC) were used to further assess the best cutoff for FeNO in terms of predicting eosinophilic esophageal inflammation. A Spearman’s rank-order correlation was run to analyze the relationship between FeNO and PEC. A logistic regression model was used to ascertain the effects of atopy on FeNO scores.Pvalue < 0.05 was considered statistically significant.
RESULTS
The demographics of the study population are described using mean and standard deviation and summarized in Table 3. The patients ranged from age 6 to 19 years (mean age was 13.3 years ± 3.2, 55% females, 84% Caucasians). Overall, 124 patients were recruited with 134 discrete encounters between July 2012 and July 2016. Eight patients had repeat encounters for upper endoscopies. Ten patients were excluded for being on corticosteroids at the time of EGD or for other comorbidities not noted in the preassessment. Four withdrew from the study (Figure 1). Of the 134 encounters, 45 were diagnosed with EoE by histopathology. The clinical characteristics of the study subjects including symptoms, visual esophageal endoscopy findings and esophageal pathology are summarized in Table 4. The peak eosinophils in mid and distal esophagus ranged from 0 to 120 eos/HPF. The eosinophils ranged from 0 to 29 eos/HPF in the stomach and from 15 to 50 eos/HPF in the duodenum for the EoE cohort.

Table 3 Patient demographics, n (%)
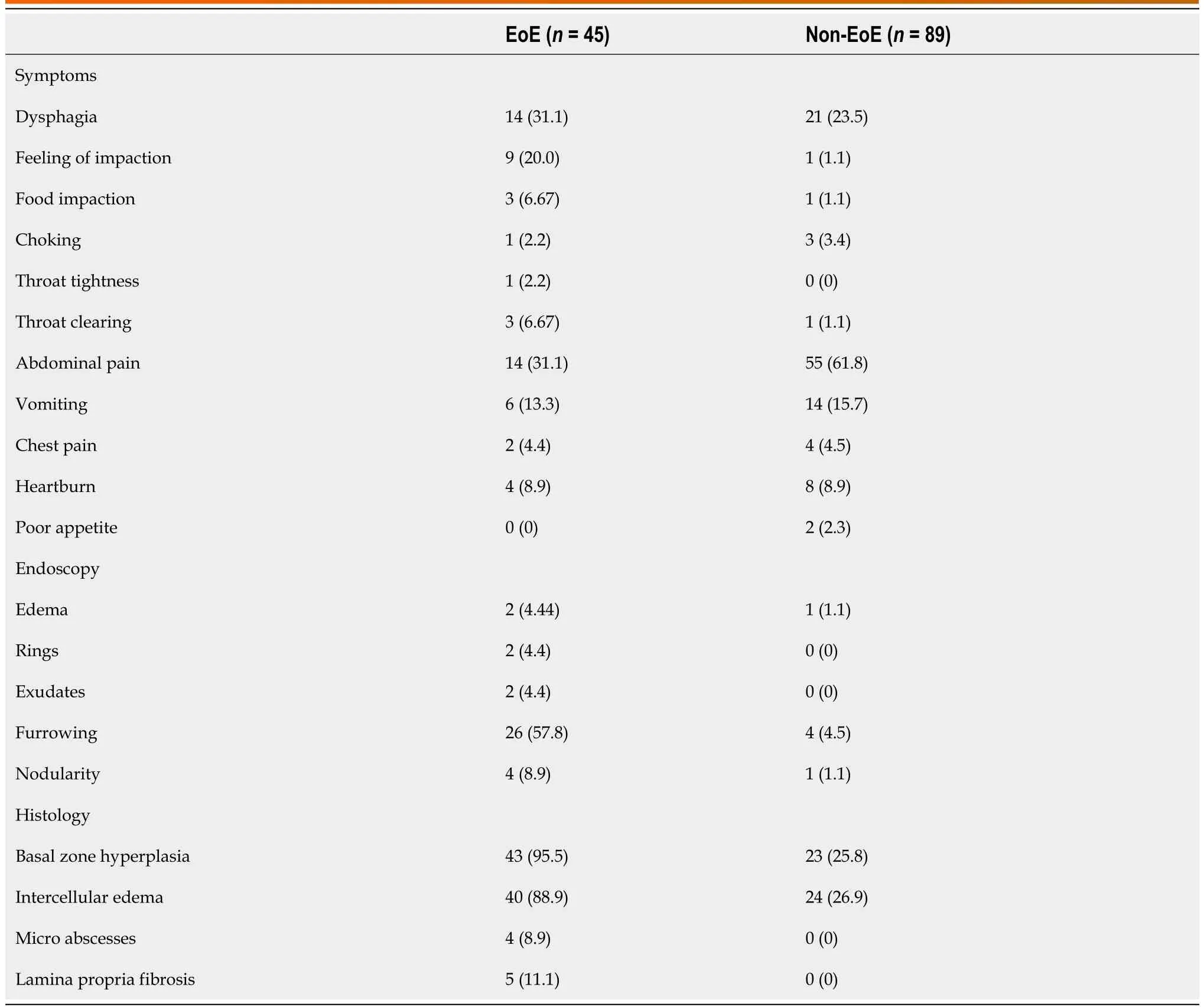
Table 4 Clinical patient characteristics, n (%)
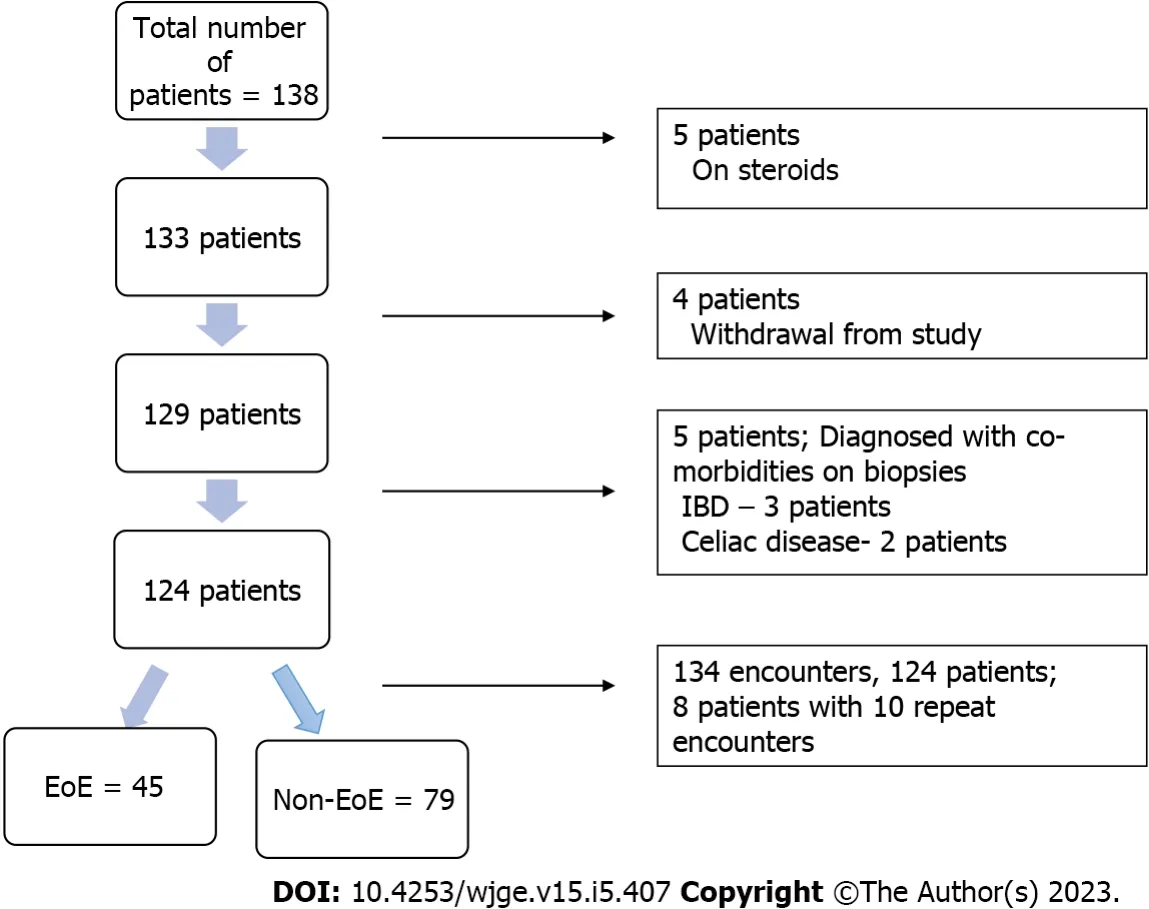
Figure 1 Flow diagram of patient selection. IBD: Inflammatory bowel disease; EoE: Eosinophilic esophagitis.
FeNO and EoE
The EoE group had higher FeNO levels with a median of 17 ppb (IQR: 11-37, range: 7-81) as compared to the control group, which had a median of 12 ppb (IQR: 8-19, range: 5-71),P= 0.001 (Figure 2). On multivariate analysis adjusting for presence of atopy, similar relation between FeNO and EoE was noted withPvalue of 0.003 (Supplementary Table 1). To predict the best cutoff for FeNO in terms of predicting EoE, ROC analysis was done (Figure 3), which indicated the area under the curve (AUC) as 0.677. With FeNO cutoff of ≥ 14 ppb, sensitivity is 60% and specificity is 57.3%, with positive predictive value (PPV) of 41.5 and negative predictive value (NPV) of 73.9%. If FeNO cutoff is increased to ≥ 30,sensitivity decreases to 35.6%, and specificity significantly increases to 92.1%, with PPV of 69.6% and NPV of 73.9%.

Figure 2 Wilcoxon rank-sum test to assess fractional exhaled nitric oxide levels in eosinophilic esophagitis group compared to control.FeNO: Fractional exhaled nitric oxide; EoE: Eosinophilic esophagitis.

Figure 3 Receiver operating characteristics analysis to predict fractional exhaled nitric oxide cut off. ROC: Receiver operating characteristics;FeNO: Fractional exhaled nitric oxide; EoE: Eosinophilic esophagitis.
FeNO and esophageal eosinophilia
A Spearman’s rank-order correlation to ascertain the relationship between FeNO and esophageal eosinophilia demonstrated weakly positive, but statistically significant, correlation, rs= 0.30,P< 0.005(Figure 4).
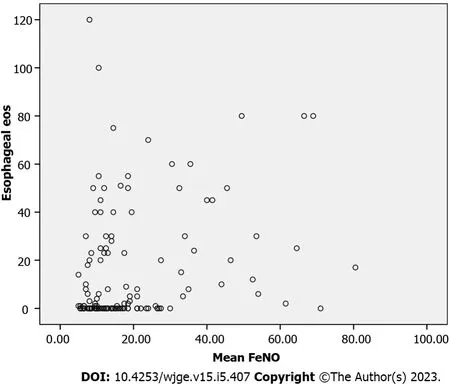
Figure 4 Spearman correlation analysis between fractional exhaled nitric oxide and esophageal eosinophils. FeNO: Fractional exhaled nitric oxide; eos: Eosinophils.
FeNO and downstream eosinophils
We further analyzed the EoE cohort to determine differences between FeNO levels in patients with and without elevated downstream eosinophilia. Out of 45 EoE patients, 23 patients had elevated gastric eosinophils and 28 patients had elevated duodenal eosinophils. Higher FeNO levels were noted in patients with elevated gastric [n= 23, median 18 (IQR: 12-34)vs15 (IQR: 11-42)] and duodenal eosinophilia [n= 28, median 21 (IQR 12-43)vs14 (IQR 11-17)]; however, the difference was not statistically significant.
DISCUSSION
Upper gastrointestinal endoscopy with mucosal biopsy remains the histological gold standard in diagnosis and management of EoE[13-15]. Endoscopic evaluation is needed at every step of management in EoE patients as it is currently the only way to assess response. Apart from being an invasive modality, repeat endoscopy carries its own risks along with rare anesthesia complications. Additionally,the cumulative cost of the procedures over the years is a financial burden for families. Multiple studies in the literature have evaluated different biomarkers as an objective measure to monitor esophageal inflammation associated with EoE, but none have been conclusive[16]. Measurement of nitric oxide in exhaled breath FeNO is a clinically useful non-invasive test in measuring airway inflammation in pulmonary inflammatory disorders like asthma and other atopic disorders, as FeNO has been noted to correlate with pulmonary eosinophils. Exhaled nitric oxide (NO) is understood to be a marker of Thelper cell type 2-mediated immune response, which is seen in chronic airway or allergic inflammation[17-19].
Based on a similar concept, a few previous studies have looked at FeNO as a non-invasive alternative to assess any correlation with esophageal inflammation in EoE patients[20]. A prospective multicenter study looked at change in FeNO levels in response to corticosteroid treatment in 11 non-asthmatic patients with EoE[21]. Although the difference between pre- and post-treatment FeNO levels were noted to be statistically significant, they did not predict a clinical or histological response. Another study measured exhaled nitric oxide in 55 pediatric patients with chronic upper gastrointestinal symptoms,out of which 18 were diagnosed with EoE, half of which had elevated FeNO[22]. The authors concluded that a normal FeNO level (15 ppb) may be used to rule out EoE with high specificity (> 87%), and NPV(78%); however, they did not correlate well enough to use for diagnostic purposes. Similarly, a more recent prospective study in adults demonstrated a weak relationship between FeNO and esophageal eosinophilia, deeming limited clinical utility of FeNO in EoE except for patients with high FeNO levels(> 40 ppb)[23].
Our study examined the relationship between FeNO levels and histological diagnosis of EoE,esophageal eosinophilia, and any contributory effect of downstream eosinophils in pediatric patients.The EoE cohort in our study was noted to have a higher FeNO level as compared to the patients who histologically did not have EoE. Since patients with EoE have a high incidence of atopic diseases, a subgroup analysis was performed to control for atopy, which still produced similar correlation results between FeNO and presence of EoE. These findings have not been noted in the previous studies and may attribute to an adequately powered study. Similar to a study by Johnsonet al[23], our study also noted high FeNO levels (> 30 ppb) to be more specific in ROC analysis and may have a clinical role in predicting active esophageal inflammation.
American Thoracic Society (ATS) clinical practice guidelines for asthma suggest to use cut-off points as opposed to reference values to interpret FeNO in a clinically useful way due to multiple confounding factors and overlap between normal populations and those with asthma. Cut-off point of < 20 ppb was considered low in children and indicated less likelihood of eosinophilic inflammation and responsiveness to corticosteroids[11]. In a study that looked at FeNO measurements in healthy children of 4 to 17 years of age concluded their FeNO values to be below 15-25 ppb depending on age and atopy[24]. A value > 35 ppb was considered elevated and provided higher specificity for eosinophilic inflammation[11]. Our data suggests that given the specificity of high FeNO levels (> 30 ppb) in prediction of histological diagnosis of EoE, a similar FeNO cutoff could be established for surveillance in EoE patients, particularly those with high initial FeNO levels. Following an individual patient’s FENO levels over time could allow for monitoring of esophageal inflammation in this subgroup of EoE with high FeNO scores. The ATS guidelines further suggest that a reduction of at least 20% in FeNO for values >50 ppb (or > 10 ppb for values lower than 50 ppb) be used as the cutoff point to indicate a significant response to anti-inflammatory therapy[11]. New ATS guidelines suggest that FeNO should be combined with other clinical markers to assess disease control[25]. Potentially, a similar reduction value in FeNO scores can be established for EoE patients that can be integrated with other clinical characteristics to demonstrate response to therapy.
This is the first study to evaluate any elevations in FeNO levels that could be contributed by the eosinophils in the stomach and duodenum (downstream eosinophils). FeNO levels were noted to be elevated in patients with high gastric and duodenal eosinophilia, which had a trend towards significance. Previous studies in patients with inflammatory bowel disease have shown elevations in NO levels from intestinal inflammation[26,27]. Since intestinal inflammation downstream may affect the FeNO levels, monitoring esophageal inflammation by FeNO might not be reliable in patients with systemic inflammatory disease. Further studies are needed to assess if perhaps a higher FeNO cut off can be utilized for EoE surveillance in patients with high downstream eosinophils.
This study is novel as it includes a large pediatric cohort, which allows us more power to assess patients with high FeNO levels. Overall, a greater percentage of our cohort had high FeNO levels than in previously published studies, indicating there might be a difference in FeNO product of pediatric EoE patients as compared to adults. Additionally, our study is the first to evaluate downstream eosinophils as a potential confounder of FeNO levels.
This study is limited by being conducted at a single institution. To reduce confounding factors, the study did not include patients with asthma which limits assessment of the group of patients that have both EoE and asthma. Due to the study design, EoE patients being treated and in remission could not be assessed for more accurate FeNO correlation. More patients with high (> 50 ppb) FeNO levels would have improved the ability to assess this subgroup. Future studies would benefit from larger sample sizes, particularly patients with higher eosinophil counts and, including patients with existing diagnosis of EoE being treated and in remission to predict more precisely whether a higher FeNO cutoff can be used to predict changes in esophageal inflammation.
CONCLUSION
In conclusion, EoE cohort was noted to have higher FeNO levels compared to control. FeNO levels of more than 30ppb were found to be more specific for eosinophilic esophageal inflammation. FeNO may have a clinical role in assessing treatment response in a subset of EoE patients.
ARTICLE HIGHLIGHTS
Research background
Eosinophilic esophagitis (EoE) is characterized by eosinophilic inflammation of esophageal mucosa and symptoms of esophageal dysfunction. To avoid the burden of multiple endoscopies and associated risks of procedures, search for a surrogate marker for esophageal inflammation has been ongoing and inconclusive till date. Previous low powered studies assessing Fractional exhaled nitric oxide (FeNO)’s utility in EoE were noted to have a trend for association. No previous studies investigated the effect of eosinophilia in stomach and duodenum on FeNO.
Research motivation
To identify a non-invasive marker of disease activity in EoE that could be a low-risk, low-cost alternative to endoscopic evaluation. FeNO measurements have been successfully utilized in management of eosinophilic airway inflammatory disorders such as asthma. Our study assessed FeNO as a potential biomarker to monitor esophageal eosinophilic inflammation in EoE.
Research objectives
Main objective of our study is to evaluate utility of FeNO in management of Pediatric EoE. Our study also analyzed if gastric and duodenal eosinophils (downstream eosinophilia) have any effect on FeNO scores.
Research methods
Pediatric patients with upper gastrointestinal symptoms and suspected EoE were enrolled in this crosssectional study. Chemiluminescence nitric oxide analyzer (NIOX MINO, Aerocrine, Inc.; Stockholm,Sweden) machine was used to obtain FeNO measurements prior to endoscopy. Clinical characteristics data for all EoE and non-EoE patients was collected. Correlation of FeNO levels with esophageal eosinophils, EoE and abnormal downstream eosinophilia in the stomach and duodenum was analyzed.A comprehensive atopy questionnaire was utilized for presence of atopy, which was controlled for in a separate logistic regression analysis to assess its effect on FeNO in EoE patients.
Research results
Higher FeNO levels were found in patients with EoE compared to the non-EoE cohort, after adjusting for atopy. FeNO levels more than 30 ppb were noted to be more specific for active esophageal inflammation. Elevated FeNO levels were also noted in patients with high gastric and duodenal eosinophils,with a trend towards significance.
Research conclusions
Given the specificity of high FeNO levels (> 30 ppb) in prediction of histological diagnosis of EoE, a FeNO cutoff could be established for surveillance in EoE patients, particularly those with high initial FeNO levels. Cautious interpretation or perhaps a higher FeNO cut off may be needed in patients with high downstream eosinophils. FeNO may have a clinical role in management of EoE to suggest response to therapy in a subset of pediatric EoE patients. Future studies are needed to evaluate this further.
Research perspectives
Future studies should focus on including EoE patients from the time of diagnosis, and in remission while following an individual patient’s FeNO levels over time to allow monitoring of esophageal inflammation. This could provide a precise assessment for utilization of a FeNO cutoff in prediction of esophageal eosinophilic inflammation.
FOOTNOTES
Author contributions:Kaur P modified the study design, collected, analyzed and interpreted the data, wrote and revised the manuscript; Chevalier R modified the study design, collected, analyzed and interpreted the data, wrote and revised the manuscript; Friesen C modified the study design, analyzed and interpreted the data, edited and revised the manuscript; Ryan J interpreted the data and edited the manuscript; Sherman A interpreted data,performed statistical analysis of the data and revised analysis and manuscript; Page S designed and performed the research study, interpreted the data and edited the manuscript; All authors have approved the manuscript.
Institutional review board statement:The study was reviewed and approved by the Children’s Mercy Institutional Review Board (Approval No. 11120665).
Informed consent statement:Informed consent was obtained from patients/caregivers prior to enrollment into the study.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Data sharing statement:No additional data are available.
STROBE statement:The authors have read the STROBE Statement—checklist of items, and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:United States
ORCID number:Panamdeep Kaur 0000-0002-9016-8119.
Corresponding Author's Membership in Professional Societies:American College of Gastroenterology.
S-Editor:Liu GL
L-Editor:A
P-Editor:Liu GL
 World Journal of Gastrointestinal Endoscopy2023年5期
World Journal of Gastrointestinal Endoscopy2023年5期
- World Journal of Gastrointestinal Endoscopy的其它文章
- Effect of music on colonoscopy performance: A propensity scorematched analysis
- Expanding endoscopic boundaries: Endoscopic resection of large appendiceal orifice polyps with endoscopic mucosal resection and endoscopic submucosal dissection
- Effect of modified ShengYangYiwei decoction on painless gastroscopy and gastrointestinal and immune function in gastric cancer patients
- Rectal neuroendocrine tumours and the role of emerging endoscopic techniques
- Improving polyp detection at colonoscopy: Non-technological techniques
- Unlocking quality in endoscopic mucosal resection
