金屬有機骨架(MOFs)基復合質子交換膜的研究進展
摘 要:近年來,金屬有機骨架(MOFs)材料由于具有超大的比表面積和豐富的孔道結構,作為一種新型的質子導體在質子交換膜中的應用受到=來=多的關注。隨著研究的不斷深入,為進一步提升MOFs 復合質子交換膜的各項性能,MOFs 材料在質子交換膜中的物理形態逐漸由顆粒狀向連續相發展,MOFs 材料的組分也呈現出由單一組分到雙組分的發展態勢。本文以質子交換膜中MOFs 材料的物理形態為主線,將其劃分為MOFs 晶體/聚合物質子交換膜、第三相增強MOFs/聚合物復合質子交換膜和MOFs 納米纖維/聚合物復合質子交換膜,全面綜述了MOFs 材料在質子交換膜中的研究進展,并對MOFs 復合質子交換膜的發展方向進行了展望。
關鍵詞: 金屬有機框架; 復合質子交換膜; 質子傳導率; 燃料電池
中圖分類號:TM912 文獻標志碼:A 文章編號:1671-024X(0024)01-0017-11
Research progress of metal-organic frameworks(MOFs)based composite
proton exchange membranesLI Lei1,2,WANG Yuanyuan1,WEI Guolan1
(1. School of Textile Science and Engineering,Tiangong University,Tianjin 300387,China;2. State Key Laboratory of
Separation Membranes and Membrane Processes,Tiangong University,Tianjin 300387,China)
Abstract:In recent years,metal-organic frameworks(MOFs) have attracted more and more attention as a new type ofproton conductor in proton exchange membranes due to their large specific surface area and abundant porestructures. With the continuous deepening of research,to further improve the performance of MOFs compositeproton exchange membrane,the physical morphology of MOFs materials in the proton exchange membrane hasgradually developed from granular to continuous phase,and the composition of MOFs materials has also evolvedfrom a single component to dual component. Based on the physical morphology of MOFs materials in protonexchange membranes,this paper divides them into MOFs crystal/polymer proton exchange membranes,the thirdphase reinforced MOFs/polymer composite proton exchange membranes,and MOFs nanofiber/polymer compositeproton exchange membranes,comprehensively summarizes the research progress of MOFs materials in protonexchange membranes,and prospects the development direction of MOFs composite proton exchange membranes.
Key words:metal-organic frameworks(MOFs); composite proton exchange membrane; proton conductivity; fuel cell
“碳中和碳達峰”國家戰略推動了高效可持續可再生能源轉換和儲存的快速發展。安全高效的儲能系統對利用可再生能源和環境保護至關重要。質子交換膜燃料電池(PEMFCs)因其高能量密度、良好的轉化效率和環境友好性已成為一種最具發展潛力的聚合物電解質膜燃料電池[1-2]。PEMFCs 主要由陰陽極、質子交換膜(PEMs)、催化劑等部分組成,其成本主要取決于質子交換膜和催化劑。質子交換膜作為PEMFCs 的核心部件,起著傳導質子、阻止燃料氣體和氧化劑氣體混合的雙重作用。
目前,應用最廣泛的PEM 是杜邦公司于20 世紀70年代開發的全氟磺酸(Nafion) 膜[3-4]。該膜具有常溫下質子傳導率高、化學穩定性好等特點。然而,由于其制備工藝復雜、成本居高不下、高溫性能嚴重下降(躍80 ℃)和阻醇性差(用于直接甲醇燃料電池中)等缺點成為PEMFCs 商業化的瓶頸[5]。因此,需要具有高質子導電性、低燃料穿透性以及足夠的熱穩定性和機械穩定性來保證PEMFCs 的穩定工作[6]。近年來,磺化芳香族聚合物如磺化聚醚酮(SPEEK)、磺化聚芳醚酮(SPAEK)、 磺化聚酰亞胺(SPI)、磺化聚醚砜(SPES)、磺化聚砜(SPSF)等因價格低、可加工性強等優點被認為是Nafion 膜材料的潛在替代品[7-12]。對于磺化芳香類聚合物,磺酸基的引入能明顯提高膜的導電性,而高質子傳導率往往需要聚合物主鏈中具有高密度的磺酸基團,高密度的磺酸基團和過度±賴水分含量以保證質子傳導率的缺點會導致聚合物的尺寸穩定性下降、機械性能和化學穩定性變差[13-14]。因此,發展具有高質子傳導率且高穩定性的復合質子交換膜對磺化芳香族聚合物的實際應用具有重要意義。
在過去的幾年中,金屬有機骨架(MOFs)作為一種具有一定晶體結構的有機-無機雜化材料因其可調節的結構、孔徑、表面性質、粒徑和可控的客體分子以及豐富的活性位點引起了=來=多的關注[15-18],在儲氣、氣體分離、磁性材料和反應催化領域得到了廣泛應用[19-24]。近年來,研究人員將MOFs 用來修飾PEM,發現將MOFs加載到聚合物基質中作為質子載體可以明顯改善復合膜的性能。MOFs 的大比表面積及本身含有的豐富的質子傳遞位點可以有效提升復合膜的質子傳導率及其他性能[25]。更重要的是,研究人員發現MOFs 材料的不同物理形態會影響復合膜中質子的傳遞路徑,進而影響復合質子交換膜的性能。本文從MOFs 材料的質子傳遞機理入手,重點對不同物理形態的MOFs 材料,如MOFs晶體材料、第三相增強MOFs 材料及MOFs 納米纖維材料對其復合質子交換膜的影響進行了綜述,最后對MOFs 復合質子交換膜的發展方向進行了展望。
1 MOFs 質子傳導機理
2009 年,Sadakiyo 等[26]首次將晶體MOF 用于質子傳導研究,并證實單晶結構可以合理地揭示質子傳導機制。MOFs 作為質子導體根據工作環境可以分為在有水條件工作的質子導體(約100 ℃),質子的傳輸主要±靠水分子搭建的氫鍵網絡進行[27];在無水條件下工作的質子導體(躍100 ℃)向MOFs 孔道負載攜帶質子的客體完成傳遞過程[28]。對應2 種質子導電機理為Grotthuss 機理和Vehicle 機理[29-30]。Grotthuss 機理是指在氫鍵網絡中的質子傳導,設想質子在水分子簇中形成H3O+,發生質子傳遞的同時切斷氫鍵實現水分子的重排,從而形成不間斷的質子遷移軌道。此機理也可以看作質子通過水分子的質子化和去質子化沿著傳導路徑“跳躍”。這種機制的特點是低活化能(0.1~0.4eV)和高質子遷移率。Vehicle 機理則涉及質子通過質子載體的自擴散進行傳導,作為涉及水分子如H3O+,H5O2+的復雜結構的一部分,和電解質中存在的H9O4+(本征陽離子)或其他質子化載體物質(例如NH4+等)擴散相結合。Vehicle 機理涉及較大離子的遷移,需要更大的能量貢獻,活化能通常大于0.4 eV。目前,提高MOFs 質子傳導率的常用方法有:淤通過一鍋法或后處理方法在MOFs 有機配體上修飾特定官能團(—COOH、 —PO3H 和—SO3H 等)作為質子導體[31-33],可以增強有機配體的酸性和親水性,有助于MOFs 形成有效的質子傳輸途徑;于引入客體分子(例如咪唑、三唑、組胺等) 帶入空隙,這將有助于形成復雜的氫鍵網絡,并有利于提高質子電導率[34-36]。
2 MOFs 基復合質子交換膜
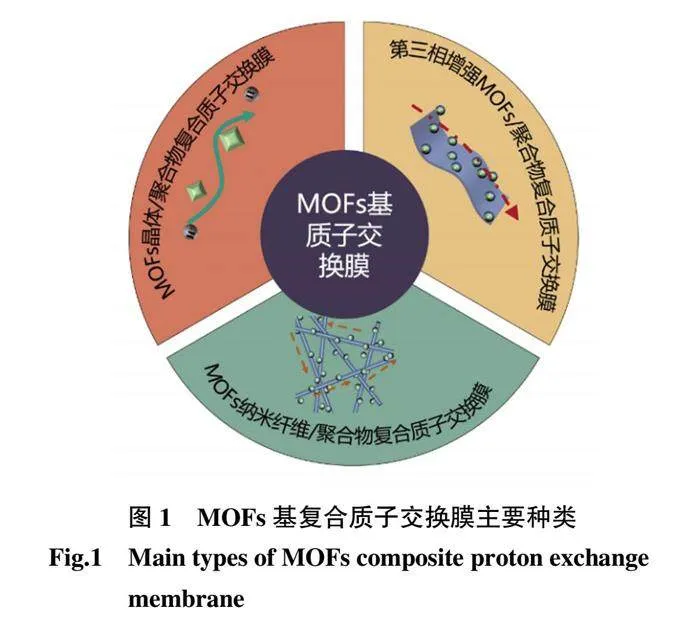
近年來,具有高質子傳導性的MOFs 受到廣泛關注,研究表明,質子可以通過MOFs 中的有機配位骨架進行傳遞。然而,MOFs 的晶界結構限制了質子導體的遷移,導致質子傳導性不足,并且由于MOFs 晶體自身的高結晶和脆性難以直接加工成膜結構,使得MOFs 直接用作質子傳導材料具有很大的挑戰性。隨著研究的不斷深入,研究者們將MOFs 材料與聚合物基質混合形成復合膜,通過設計不同組分和不同物理形態的MOFs材料,豐富MOFs 材料的質子傳遞位點,優化復合膜中質子的傳遞通道。以上策略有效提升了MOFs 復合膜材料的質子傳導性,拓展了MOFs 材料的應用[37-38]。按照聚合物中MOFs 材料的不同物理形態,目前制備的MOFs 復合質子交換膜主要有以下3 種:不同基團修飾的顆粒狀MOFs 復合質子交換膜、引入第三相增強材料的MOFs 復合質子交換膜及以納米纖維為模板制備的MOFs 納米纖維復合質子交換膜(圖1)。本文將以MOFs 增強材料的物理形態為劃分±據,重點對以上3 種MOFs 復合質子交換膜進行綜述,最后對MOFs 基復合質子交換膜的發展進行了展望。
2.1 MOFs 晶體/聚合物復合質子交換膜
制備MOFs 晶體/聚合物復合材料是改善質子交換膜性能的有效策略之一。MOFs 作為一種有機-無機雜化材料,與聚合物基質相容性良好,其剛性的框架結構能夠約束聚合物鏈的運動,改善復合膜的力學性能。MOFs 還含有大量的親水基團,能夠構建密集的氫鍵網絡,此外,MOFs 多孔的結構可以負載客體分子,如咪唑分子、磷鎢酸和離子液體可作為質子源,并為質子提供轉移位點。因此,當MOFs 晶體與聚合物混合時,聚合物填充MOFs 的晶界并支撐MOFs 的機械強度。同時,由于質子傳導性從MOFs 通過界面轉移到聚合物,復合膜的質子傳導性也得到了增強。
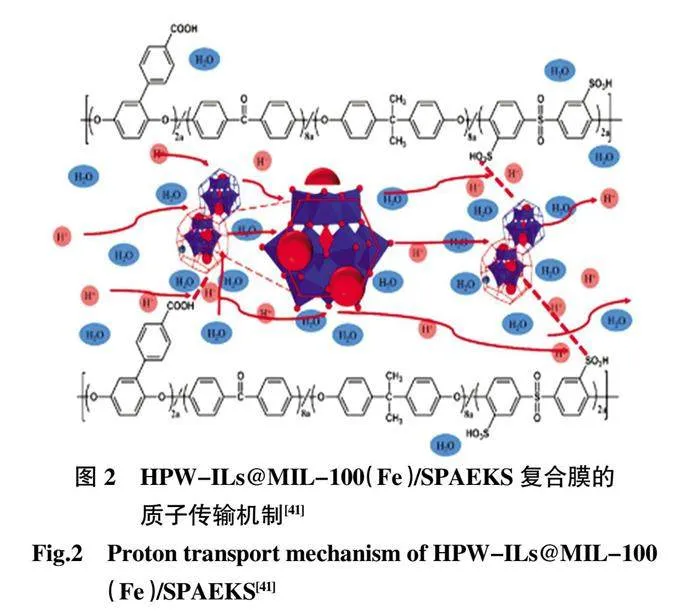
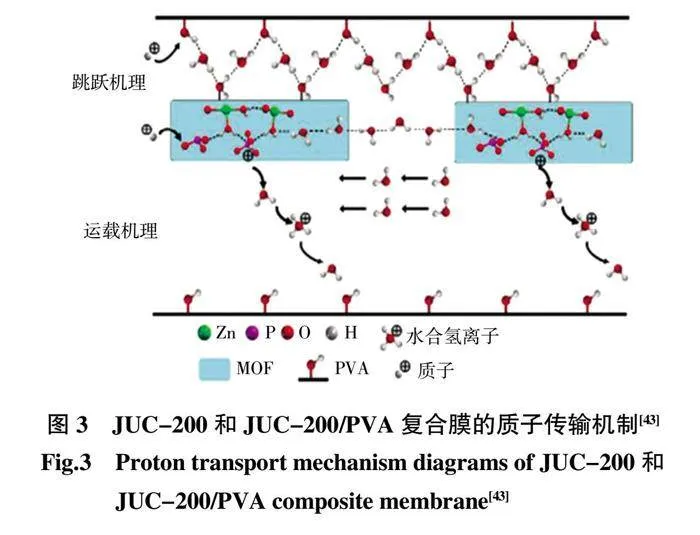
早在2012 年,膦酸鹽和磷酸酯就被指出是MOFs穩定、良好的候選配體基團,因為它們可以與大多數金屬離子形成更強的鍵,并且具有良好的耐水性和耐酸性[39-40]。Zhang 等[41]采用一鍋法將磷鎢酸(HPW)和MIL-100(Fe)混合后制備HPW@MIL-100(Fe),將最終產物引入SPAEKS 聚合物基質中制備復合膜。該復合膜在100 ℃和相對濕度100%的條件下的質子電導率達到0.138 S/cm(圖2)。此后,大量關于MOFs 與聚合物復合的研究應用于PEMFCs。Hu 等[42]設計并制備了不同含量的磷酸硼(BPO4)與HKUST-1 前體的雜化,將其應用于構建SPEEK 的質子交換膜(SPEEK/HB)。由于雜化材料與SPEEK 基質之間的相互作用,SPEEK/HB-10 的質子電導率達到37.4 mS/cm(80℃,相對濕度100%),明顯高于SPEEK。Cai 等[43]使用肌醇六磷酸配體(PA)和Zn(域)成功合成了新型六磷酸酯基MOFJUC-200,合成的結構賦予JUC-200 抗水和酸的高穩定性并在80 ℃的水中表現出良好的質子導電性能,電導率達到1.62×10-3mS/cm(圖3)。這是首次報道制備水和酸穩定的MOF 基質子傳導復合膜,對于PEMFCs 的進一步發展起到一定的開拓作用。
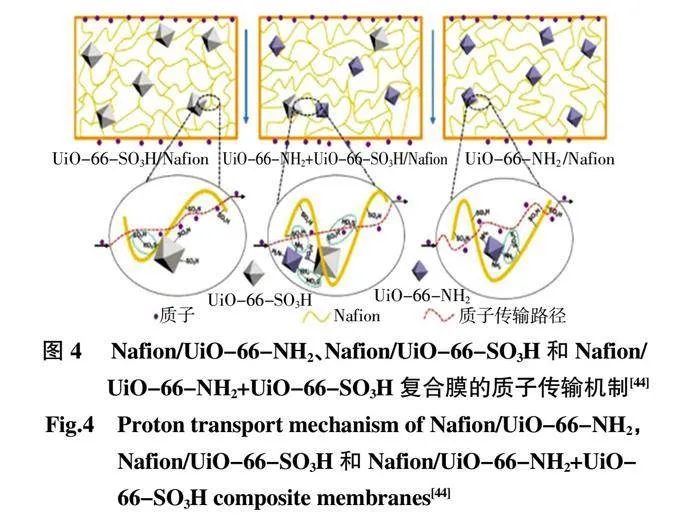
為了更好地提高MOFs 的質子傳導,通過官能團修飾的MOFs 與聚合物基質進行混合制備高性能的復合PEM。磺酸基團可以提供更多的質子傳導位點和轉運途徑,氨基是質子傳導的有效位點,它們的組合極大地改善了復合膜的導電性。Rao 等[44]合成了2 種官能團修飾的MOFs,UiO-66-SO3H 和UiO-66-NH2,隨后分別將這2 種MOF 通過單摻雜和共摻雜制備不同的PEM,發現這2 種具有合適尺寸的MOF 的共摻雜更有利于復合PEM 的質子傳導性增強(圖4)。
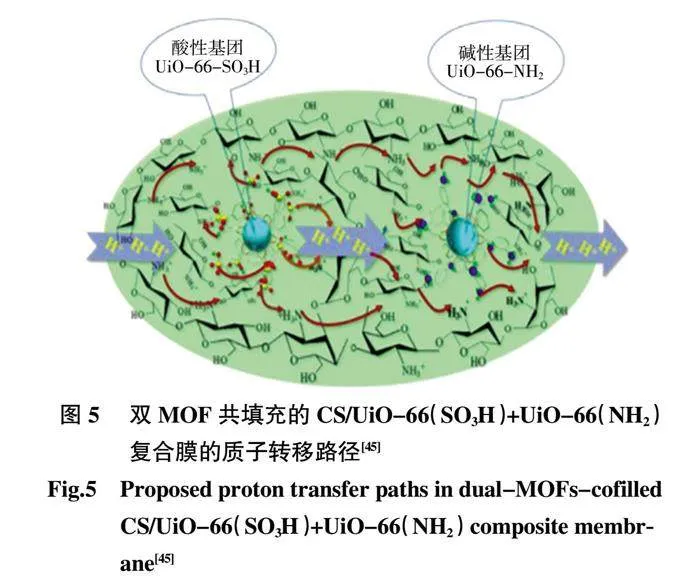
Dong 等[45]制備了由酸性基團(—SO3H)和堿性基團(—NH2)修飾的雙MOFs,即UiO-66-SO3H、UiO-66-NH2 和低成本聚合物殼聚糖(CS)組成的質子導電復合膜(圖5)。
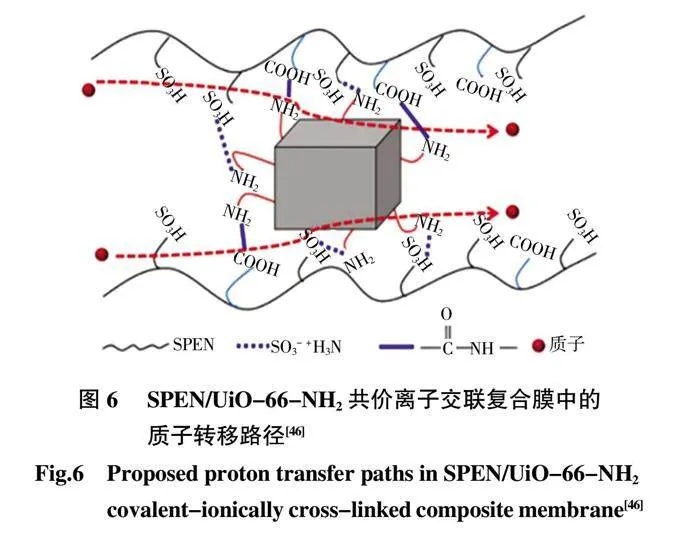
雙MOFs 共填充復合膜的質子電導率在120 ℃和無水條件下為3.78×10-3S/cm,證明了將酸性基團和堿性基團同時引入MOFs 骨架很有可能大大縮短酸堿基團之間的距離,且2 種MOF 之間的協同效應使得在復合膜中形成更連續的水合通道。它進一步極大地促進了復合膜的質子傳導性,更有利于質子跳躍位點的均勻排列,從而有效提高材料的質子電導率。Zheng 等[46在SPEN 中摻雜UiO-66-NH2 質子電導率的提高可歸因于通過分子相互作用沿UiO-66-NH2 和SPEN 基質之間的界面構建離子簇和良好連接的離子納米通道(圖6)。
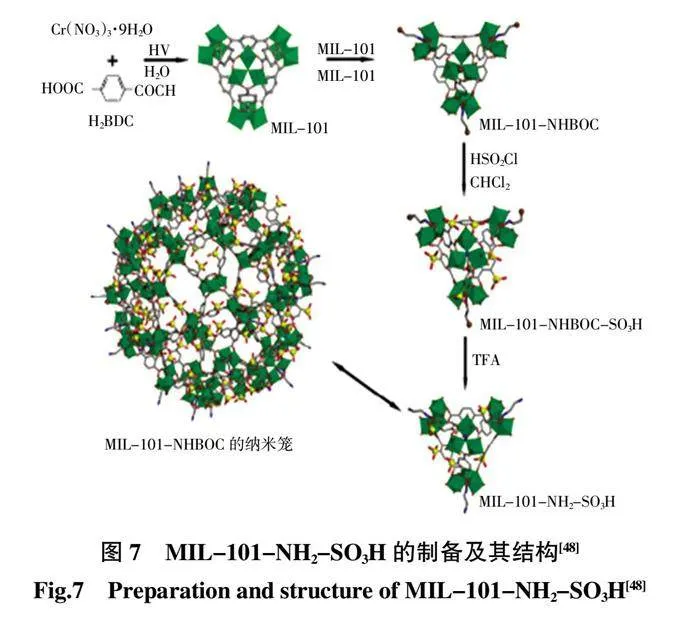
Wang 等[47]首次提出了一種酸堿對協同策略,即在混合連接劑MOFs 中同時引入酸性和堿性基團以獲得酸堿共存材料,以改善相對濕度75%下的質子傳導性能。Ru 等[48]通過水熱技術和后改性合成了新的氨基-磺酸基雙功能MOFs(MIL-101-NH2-SO3H),然后將其作為無機納米填料摻入SNF-PAEK 基質中,質子電導率達到0.192 S/cm(圖7)。
Moi 等[49]在MOF(Cu-SAT)中加入了胺鹽和磺酸鹽,它在水和稀鹽酸中具有很高的穩定性,進一步制備了Cu-SAT 和聚偏氟乙烯(PVDF)-聚乙烯基吡咯烷酮(PVP)基質的復合膜,該膜在80 ℃和98%相對濕度下質子電導率高達0.80×10-3S/cm,這也是該材料在PEMFC 中實際應用的一個進步。
為了進一步探索高質子傳導MOFs,Wang 等[50]制備MOF-azo,利用配體交換獲得MOF-bpy 和MOF-bpe。在配體的交換過程中,MOFs 空腔的功能化為質子轉移產生了有利的缺陷,與Nafion/MOF-azo 相比,Nafion/MOF-bpy 和Nafion/MOF-bpe 的質子電導率分別達到2.6×10-2S/cm 和1.95×10-2S/cm,為研究質子傳導中的客體交換提供了重要參考。MOF-801 中含有大量親水性官能團,可有效促進MOF-801 與聚合物基體之間的界面相容性。Zhang 等[51]通過溶劑熱法,添加過量甲酸作為調節劑,合成了具有結構缺陷的MOF-801,與C-SPAEK 基質形成復合膜。由于結構缺陷賦予MOF-801 更大的比表面積,提高了吸水能力。此外,MOF-801 中的大量—COOH 和—OH 基團促進了MOF-801和C-SPAEK 的兼容性,構建氫鍵網絡,可以大大提高復合膜的質子電導率和穩定性。
隨著研究發展,出現了=來=多結構和組分的MOFs,將2 種不同的金屬整合到框架中,即制備雙金屬MOFs 成為人們研究的新趨勢。目前已有研究者探索雙金屬MOFs 在催化及氣體儲存和分離領域的應用[52-57]。 在PEM 方面,Neelakandan 等[58]利用MOFs 的高質子傳導性和支化聚合物的良好化學穩定性,合成了一種新的異質金屬傳導的MOFs(Zr-Cr-SO3H),并用作高度支化磺化聚合物的填料。這項工作為異質雙金屬MOFs作為填料在質子交換膜的應用提供了新的策略。
2.2 第三相增強MOFs/聚合物復合質子交換膜
由于直接引入MOFs 到基質中可能會導致MOFs聚集,無法在所得的復合膜中建立連續的質子傳輸通道。將碳納米材料(如碳納米管(CNT)和氧化石墨烯(GO))或聚合物材料(如殼聚糖(CS))作為第三相增強體引入MOFs 中形成復合材料[59],并進一步將復合材料納入Nafion 或其他基質中,是克服上述問題并獲得具有超高質子傳導率復合膜的有效策略。
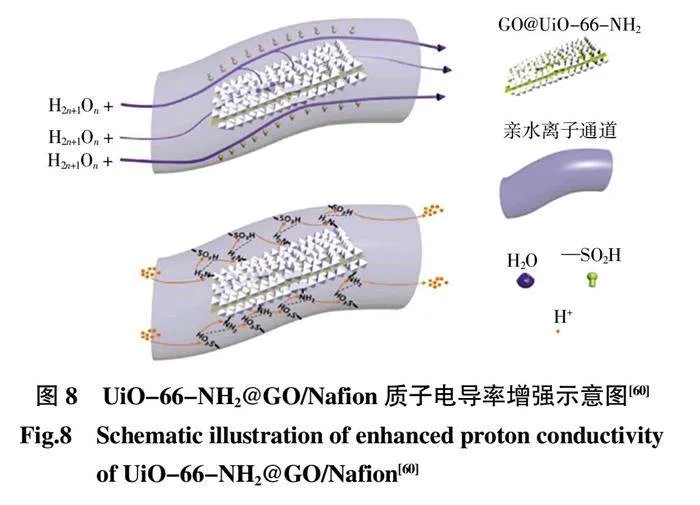
GO 是一種二維材料,表面具有豐富的含氧官能團(例如環氧化物、羥基和羧基)和高的比表面積,并且具有良好的質子傳導性。Rao 等[60]將UiO-66-NH2 連接到GO 表面上,然后摻入Nafion 基質中。由于GO 表面的束縛效應和MOF 晶粒之間的相互連接,構建良好的金屬有機骨架(MOF)結構(GO@UiO-66-NH2)。然而,即使MOFs分散體通過超聲預處理,但由于疏水性強的粒子間相互作用,粒子間的聚集仍然是不可避免的(圖8)。
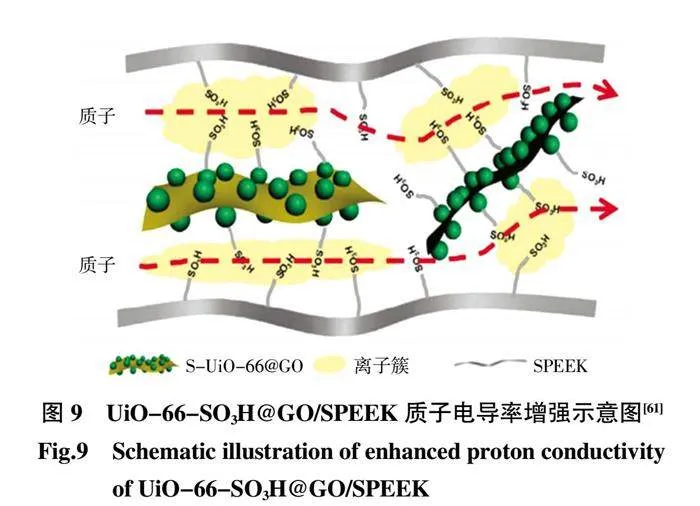
Sun 等[61]選擇具有—SO3H 官能團的S-UiO-66 作為穩定的MOFs,通過在GO 上的-位生長制備S-UiO-66@GO 復合納米片,然后將S-UiO-66@GO 摻入到SPEEK 制備復合膜(圖9)。
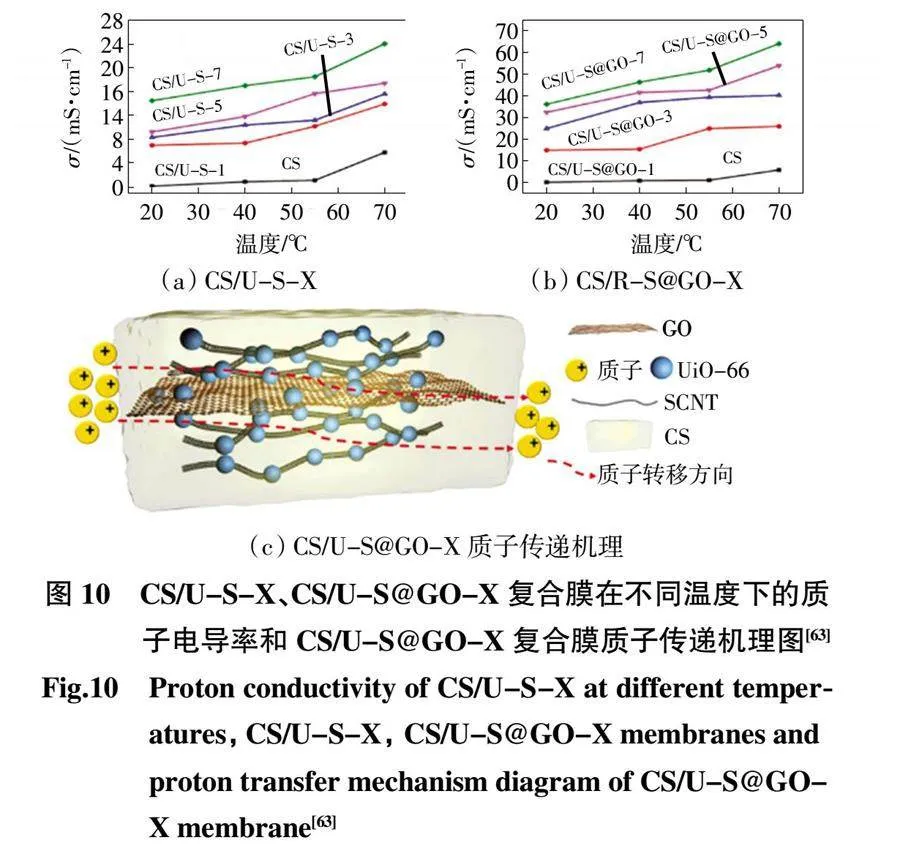
Zheng 等[62]成功設計并合成了具有串珠狀納米結構的UiO-66-NH2/CNT,UiO-66-NH2/CNT 中豐富的氨基和羧基提供了額外的質子受體和供體,極大地促進了復合膜的質子導電性。Cui 等[63]將一維木質素胰島素酸鈉功能化碳納米管(SCNT)、GO 和UiO-66 自組裝作為PEM 的填充材料,通過溶液澆鑄法制備了UiO-66-SCNT和UiO-66-SCNT@GO 納米雜化CS 質子交換膜(圖10),復合質子交換膜具有比純CS 膜更高的離子交換容量,顯示出更高的質子電導率和選擇性,為直接甲醇燃料電池的實際應用提供了理論基礎和數據支持。
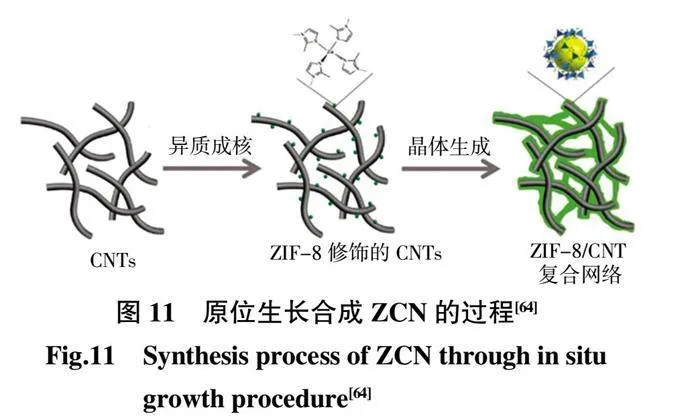
為了進一步提高MOFs 在聚合物基體中質子傳導的連續性,Sun 等[64]通過合理設計ZIF-8 的物理形式,合成了二維沸石咪唑酯骨架(ZIF-8)/CNT 雜化交聯網絡(ZCN),然后將ZCN 與SPEEK 基質通過一系列雜交來制備復合質子交換膜(圖11)。
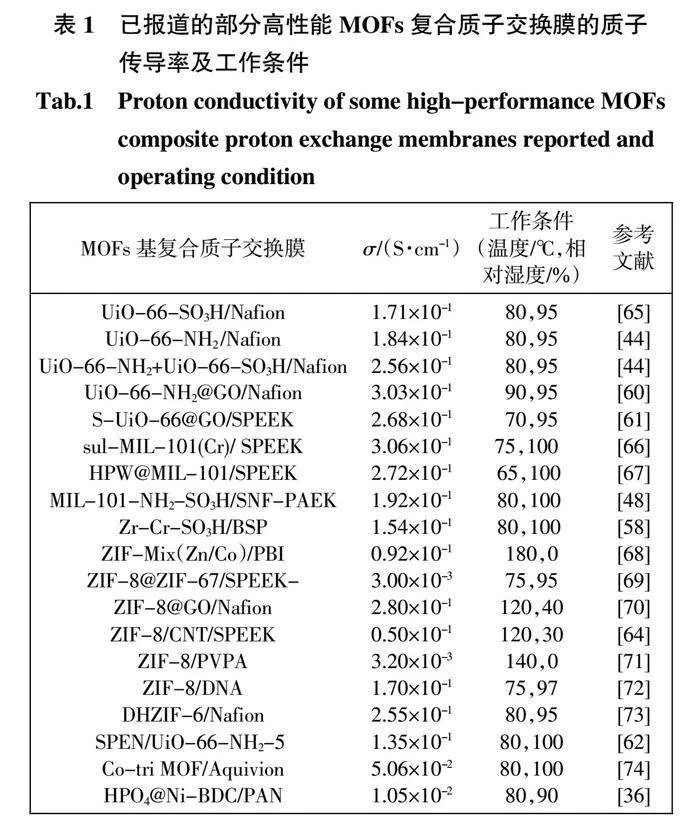
受℃于ZCN 的形態和組成優勢,SPEEK/ZCN 復合膜在各種條件下的質子傳導性顯著增強。特別是,SPEEK/ZCN-2.5 在120℃、相對濕度30%下的質子傳導率高達50.24 mS/cm。同時,SPEEK/ZCN 復合膜的甲醇滲透率大大降低。基于MOFs 骨架結構的第三相增強材料的設計提供了一種新的策略來提高復合膜的性能(表1)。
2.3 MOFs 納米纖維/聚合物復合質子交換膜
為了進一步構建長程連續的質子傳輸通道,制備MOFs 納米纖維/聚合物復合質子交換膜,已成為目前的研究熱點。靜電紡絲由于其具備制備工藝簡單、-材料廣泛、成本低、制備工藝連續等特點,被認為是一種成熟高效的納米纖維的制備方法。納米纖維具有高縱橫比、大比表面積、高孔隙率和高柔韌性。目前納米纖維已成為將各種MOFs 顆粒構造成具有多尺度孔隙率和附加功能的復合材料的理想框架,從而將MOFs顆粒轉化為自支撐的MOFs 納米纖維膜。目前MOFs納米纖維的制備途徑有直接靜電紡絲和表面修飾2種[75-80]。直接靜電紡絲是將MOFs 顆粒直接引入到靜電紡絲液中進行共混紡絲,得到MOFs 和納米纖維的復合體。表面修飾是在納米纖維表面通過物理或者化學加工實現MOFs 與納米纖維的復合。得℃于靜電紡絲納米纖維良好的網絡結構,MOFs 納米纖維能夠有效地避免MOFs 顆粒聚集造成性能的下降,提高MOFs的有效利用率。
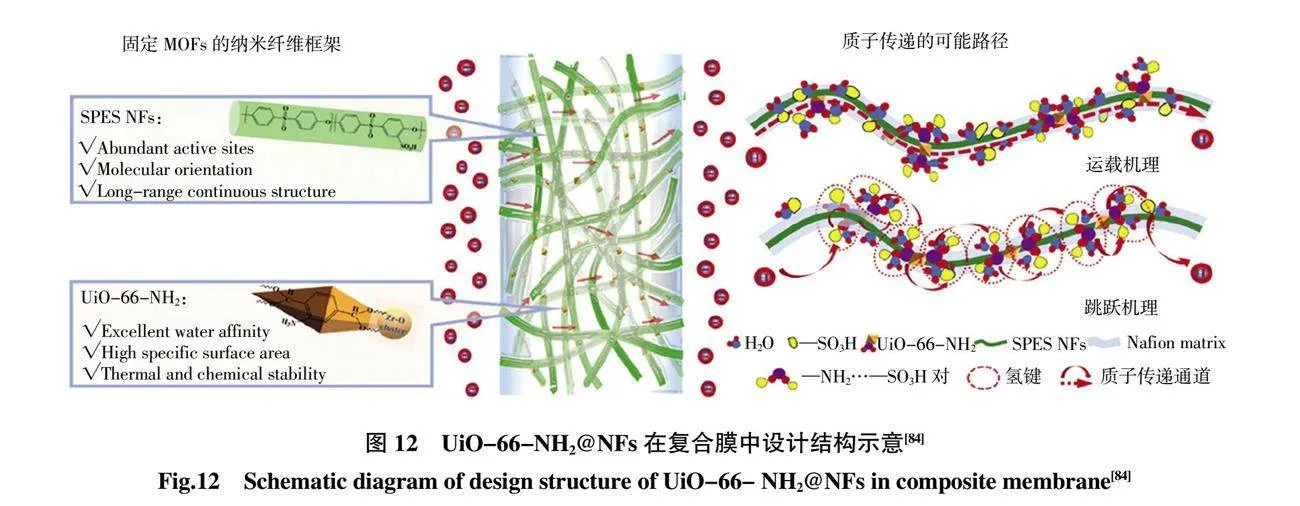
在質子交換膜領域,Wu 等[81]首次使用與磺酰氯化聚醚砜酮(SPPESK-Cl)和Zn2(C2O4)(C2N4H3)2(H2O)0.5(ZCCH)組成的定向電紡納米纖維膜,在高溫無水條件下實現高質子傳導性,低甲醇滲透性和良好的化學和熱穩定性。Sun 等[82]將氨基磺酸(SA)摻雜的MIL-101 納入PVP-PVDF 共混納米纖維膜中。其中,SA 是一種強質子供體,熔點高(超過200 ℃),與磷酸親和力好,有利于無水質子傳導,通過靜電紡絲和熱壓相結合的方法構建長程導電網絡,氨基磺酸MIL-101(SA/MIL-101)作為填料以提高PVP-PVDF 膜在高溫下的導電性。SA/MIL101@PVP-PVDF 復合膜在160 ℃時質子傳導達到0.237 S/cm。UiO-66 由于其高工作能力,優異的熱穩定性和低成本的可再生性而在MOFs 納米纖維/聚合物復合PEM 的研究引起了相當大的興趣。采用靜電紡絲將UiO-66-NH2 嵌入納米纖維中,納米纖維可以與MOFs 晶體很好地配位,形成連續的質子傳輸路徑,同時,具有—NH2 基團作為共增強單元的MOF 與通過酸堿靜電吸引力束縛在納米纖維中,提高了親水復合膜的質子導電性和尺寸穩定性,進一步促進質子傳導[83-84]。Wang 等[83]通過靜電紡絲工藝獲得的纖維素和UiO-66-NH2(Celle/UiO-66-NH2)的共混納米纖維嵌入到SPSF 基體中,以獲得UiO-66-NH2 有序排列的高性能復合質子交換膜。在納米纖維中引入功能性MOFs 可能是構建具有酸堿對的互連納米纖維網絡的有效方法。Wang 等[84]通過共混靜電紡絲構建一種新型MOFs 錨定納米纖維框架,即UiO-66-NH2 錨定的SPES 納米纖維(UiO-66-NH2@NFs)框架,SPES 納米纖維以—SO3H 基團為鍵合可以很好地與MOFs 晶體協調,形成連續的質子傳輸路徑(圖12)。
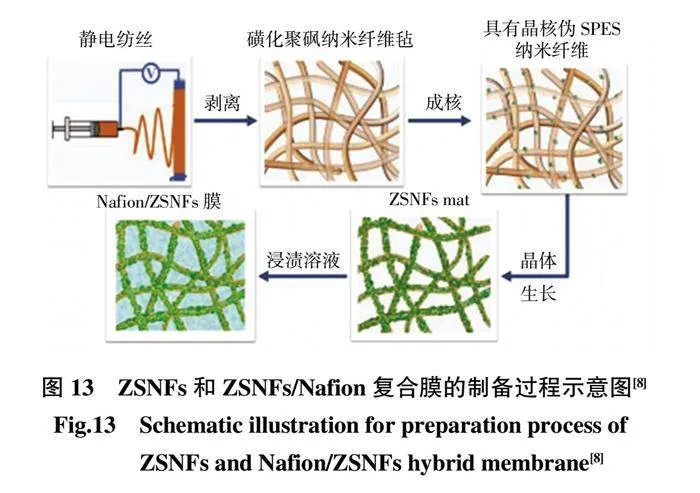
由于共混靜電紡時MOFs 顆粒大都包覆在纖維內部,只有少量MOFs 顆粒嵌在纖維外部,限制了界面酸堿對的形成。Wang 等[85]通過在電紡SPES 納米纖維表面上-位組裝ZIF-8 構建ZSNF。SPES@ZIF-8 納米纖維可能促進形成具有良好互連性的質子傳導通道,導致相互穿透的傳輸通道,從而進一步加快質子的擴散和傳導(圖13)。在Nafion/SPES 對比樣品中,Nafion/ZSNFs 復合膜的有水和無水質子電導率最高,分別為0.265 S/cm(80 ℃)和4.78×10-3S/cm(120 ℃)。
Zhao 等[86]通過將ZIF-67 自組裝到聚丙烯腈(PAN)上,制備了一種新型MOFs 納米纖維膜(NFM)。隨后,將ZIF-67NFM 引入Nafion 基質以制備ZIF-67@Nafion構建連續質子傳導通道的復合膜。質子傳導率達到0.288 S/cm(80 ℃,相對濕度100%),甲醇滲透為7.4×10-7cm2/s。類似的還有Ju 等[87]通過在具有多尺度納米纖維和高親水性的多級PMIA 納米纖維(HNFs)表面-位生長ZIF-67,將PMIA@ZIF-8 引入到Nafion 溶液中成功制備了高性能質子交換膜。該復合膜在80 ℃、相對濕度100%下的質子傳導率為0.277 S/cm(甲醇滲透率降低至1.415×10-7cm2/s,表現出優異的性能,有望應用于直接甲醇燃料電池。
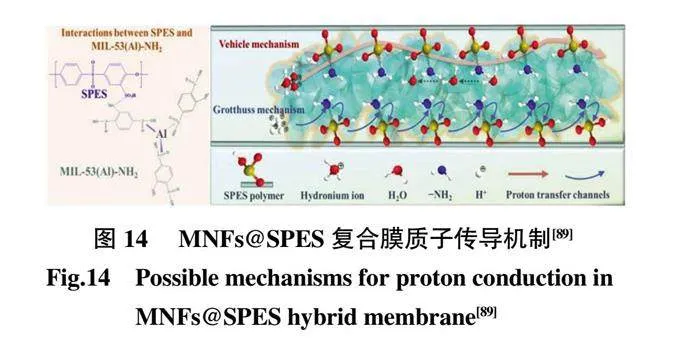
相變是在納米纖維結構表面上生長MOFs 的另一種方法,從將MOFs 的前驅體摻入聚合物納米纖維開始,在第2 步中-位生長期間前驅體轉化為MOFs 結構。Liang 等[88]提出了一種通過優化的水/溶劑熱使金屬氧化物納米纖維發生相轉變來構建各種柔性自支撐MOF 納米纖維墊的方法。Wang 等[89]成功構建了新型花狀MIL-53(Al)-NH2 納米纖維(MNF),其中靜電溶液噴射Al2O3 納米纖維提高金屬前驅體(Al),在水熱反應中與配體配位形成MOFs 納米纖維,然后將功能性和連續性MNF 摻入到SPES 基質中獲得高性能MNFs@SPES 復合膜(圖14)。隨著MNF 含量增加至5%,質子傳導率顯著提高至0.201 S/cm,實現高質子傳導率。MOFs 納米纖維兼具MOFs 的優勢及纖維結構優勢,它的出現為構筑結構穩定的纖維狀MOFs 以及擴大MOFs 在復合膜中的作用提供了新的機遇。
3 結束語
近年來,由于MOFs 材料具有高孔隙率、高比表面積和功能易于調節的特性,使其在環境保護、新能源等領域具有廣泛的應用。研究者們采用對MOFs 進行基團修飾、加入第三相增強體及制備MOFs 纖維的策略,有效提升MOFs 基復合質子交換膜的質子傳導率等各項性能,使其在質子交換膜燃料電池領域具有廣闊的應用前景。盡管MOFs 基復合質子交換膜的研究取得了較大進展,然而,在實際開發MOFs 基復合基質子交換膜的應用方面仍存在諸多挑戰:
(1)由于MOFs 和聚合物之間的界面效應可能形成缺陷并破壞質子傳輸通道,且復合膜中MOFs 的摻雜含量不宜過高,否則會發生顆粒聚集,導致質子傳導率降低。選擇合適的MOFs 和聚合物基質,優化MOFs 與聚合物基質間的界面效應并提升MOFs 在聚合物基質中的均勻分散度是全面提高復合膜性能的重要研究方向。
(2)關于MOF 基復合質子交換膜中的質子傳導機制尚不明確。目前,仍然使用MOFs 傳導機制來解釋MOFs 基復合質子交換膜質子傳導機制,然而,MOFs 和MOFs 基復合質子交換膜的工作條件不同,復合膜中MOFs 和聚合物之間的相互作用仍處于探索階段。正確認識MOFs 基復合質子交換膜的質子傳導機理,將為優化MOFs 基復合質子交換膜的性能提供理論指導。
(3)由于MOFs 材料合成成本較高,規模較小,將其放大、工業化也是現階段面臨的另一大挑戰。如何快速、低成本的大規模制備MOFs 材料是MOFs 基復合質子交換膜當前產業化的一大難題。
參考文獻:
[1] ROSLI R E,SULONG A B,DAUD W R W,et al. A review ofhigh-temperature proton exchange membrane fuel cell(HT-PEMFC) system[J]. International Journal of Hydrogen Energy,2017,42(14): 9293-9314.
[2] LIM J W,LEE D,KIM M,et al. Composite structures for pro-ton exchange membrane fuel cells(PEMFC) and energy storagesystems(ESS): Review[J]. Composite Structures,2015,134:927-949.
[3] CHIEN H C,TSAI L D,HUANG C P,et al. Sulfonated gra-phene oxide/Nafion composite membranes for high -perfor-mance direct methanol fuel cells [J]. International Journal ofHydrogen Energy,2013,38(31): 13792-13801.
[4] JIANG Z Q,JIANG Z J. Plasma techniques for the fabricationof polymer electrolyte membranes for fuel cells [J]. Journal ofMembrane Science,2014,456: 85-106.
[5] TEIXEIRA F C,DE Sá A I,TEIXEIRA A P S,et al. Nafionphosphonic acid composite membranes for proton exchangemembranes fuel cells[J]. Applied Surface Science,2019,487:889-897.
[6] DEKEL D R. Review of cell performance in anion exchangemembrane fuel cells[J]. Journal of Power Sources,2018,375:158-169.
[7] WEI Y C,SHANG Y B,NI C J,et al. Modified nanocrystalcellulose/fluorene-containing sulfonated poly(ether ether ke-tone ketone) composites for proton exchange membranes [J].Applied Surface Science,2017,416: 996-1006.
[8] FENG M N,HUANG Y M,CHENG T,et al. Synergistic ef-fect of graphene oxide and carbon nanotubes on sulfonated poly(arylene ether nitrile)-based proton conducting membranes[J].International Journal of Hydrogen Energy,2017,42(12):8224-8232.
[9] BOARETTI C,PASQUINI L,SOOD R,et al. Mechanicallystable nanofibrous sPEEK/Aquivion R composite membranesfor fuel cell applications[J]. Journal ofMembrane Science,2018,545: 66-74.
[10] ZHANG Y X,ZHENG L Y,LIU B,et al. Sulfonated polysul-fone proton exchange membrane influenced by a varied sul-fonation degree for vanadium redox flow battery [J]. Journal ofMembrane Science,2019,584: 173-180.
[11] ZHU M,SONG Y,HU W,et al. SPAEK-based binary blendsand ternary composites as proton exchange membranes forDMFCs[J]. Journal of Membrane Science,2012,415/416:520-526.
[12] MURMU R. A novel SPEEK -PVA -TiO2 proton conductingcomposite membrane for PEMFC operations at elevated tem-perature[J]. Journal of Polymer Materials,2019,35(4): 409-431.
[13] XU J M,WANG Z,NI H Z,et al. A facile functionalized rou-tine for the synthesis of side -chain sulfonated poly(aryleneether ketone sulfone) as proton exchange membranes[J]. Inter-national Journal of Hydrogen Energy,2017,42(8): 5295-5305.
[14] FENG S N,SHEN K Z,WANG Y,et al. Concentrated sul-fonated poly(ether sulfone)s as proton exchange membranes[J].Journal of Power Sources,2013,224: 42-49.
[15] SUN F C,LI Q,XUE H G,et al. Pristine transition-metal-based metal-organic frameworks for electrocatalysis[J]. Chem-ElectroChem,2019,6(5): 1273-1299.
[16] TSUMORI N,CHEN L Y,WANG Q J,et al. Quasi-MOF:Exposing inorganic nodes to guest metal nanoparticles for dras-tically enhanced catalytic activity[J]. Chem,2018,4(4): 845-856.
[17] CAI M,LOAGUE Q,MORRIS A J. Design rules for efficientcharge transfer in metal -organic framework films: The poresize effect[J]. The Journal of Physical Chemistry Letters,2020,11(3): 702-709.
[18] CHEN D M,ZHANG N N,TIAN J Y,et al. Pore modulationof metal-organic frameworks towards enhanced hydrothermalstability and acetylene uptake via incorporation of differentfunctional brackets[J]. Journal ofMaterials ChemistryA,2017,5(10): 4861-4867.
[19] XUE D X,WANG Q,BAI J F. Amide-functionalized metal-organic frameworks: Syntheses,structures and improved gasstorage and separation properties [J]. Coordination ChemistryReviews,2019,378: 2-16.
[20] LIU C S,LI J J,PANG H. Metal-organic framework-basedmaterials as an emerging platform for advanced electrochemi-cal sensing[J]. Coordination Chemistry Reviews,2020,410:213222.
[21] AMIRILARGANI M,MERLET R B,HEDAYATI P,et al.MIL-53(Al) and NH2-MIL-53(Al) modified 琢-alumina me-mbranes for efficient adsorption of dyes from organic solvents[J]. Chemical Communications,2019,55(28):4119-4122.
[22] SEOANE B,T魪LLEZ C,CORONAS J,et al. NH2 -MIL-53(Al) and NH2-MIL-101(Al) in sulfur-containing copolyimidemixed matrix membranes for gas separation[J]. Separation andPurification Technology,2013,111: 72-81.
[23] BIAN Y,WANG S J,JIN D D,et al. A general anion ex-change strategy to transform metal-organic framework embed-ded nanofibers into high -performance lithium-ion capacitors[J]. Nano Energy,2020,75: 104935.
[24] YANG C,ZHU Y M,WANG J,et al. A novel granular MOFcomposite with dense and ordered MIL-100(Fe) nanoparticlesgrown on porous alumina: Green synthesis and enhanced ad-sorption of tetracycline hydrochloride[J]. Chemical EngineeringJournal,2021,426: 131724.
[25] LIM D W,KITAGAWA H. Rational strategies for proton-con-ductive metal-organic frameworks[J]. Chemical Society Revi-ews,2021,50(11): 6349-6368.
[26] SADAKIYO M,YAMADA T,KITAGAWA H. Rational de-signs for highly proton-conductive metal-organic frameworks[J].Journal of the American Chemical Society,2009,131(29):9906-9907.
[27] LIU R L,ZHAO L L,YU S H,et al. Enhancing proton con-ductivity of a 3D metal-organic framework by attaching guestNH3 molecules[J]. Inorganic Chemistry,2018,57(18): 11560-11568.
[28] ZHAO S-N,SONG X-Z,ZHU M,et al. Assembly of threecoordination polymers based on a sulfonic -carboxylic ligandshowing high proton conductivity[J]. Dalton Transactions,2015,44(3): 948-954.
[29] KREUER K D. Proton conductivity:Materials and applications[J]. Chemistry of Materials,1996,8(3): 610-641.
[30] KREUER K D,RABENAU A,WEPPNER W. Vehicle mech-anism: A new model for the interpretation of the conductivity offast proton conductors [J] . Angewandte Chemie InternationalEdition in English,1982,21(3): 208-209.
[31] LI R Y,LIU H T,CHU Z T,et al. Two nonporous MOFs withuncoordinated carboxylate groups: Fillers for enhancing theproton conductivities of nafion membrane[J]. Journal of SolidState Chemistry,2020,281: 121020.
[32] XIE X X,YANG Y C,DOU B H,et al. Proton conductivecarboxylate-based metal-organic frameworks[J]. CoordinationChemistry Reviews,2020,403: 213100.
[33] BAI Z X,LIU S C,CHENG G J,et al. High proton conduc-tivity of MOFs-polymer composite membranes by phosphoricacid impregnation[J]. Microporous and Mesoporous Materials,2020,292: 109763.
[34] PHANG W J,JO H,LEE W R,et al. Superprotonic conduc-tivity of a UiO-66 framework functionalized with sulfonic acidgroups by facile postsynthetic oxidation[J]. Angewandte ChemieInternational Edition,2015,54(17): 5142-5146.
[35] YE Y X,GUO W G,WANG L H,et al. Straightforward load-ing of imidazole molecules into metal -organic framework forhigh proton conduction[J]. Journal of the American ChemicalSociety,2017,139(44): 15604-15607.
[36] LIU L Z,YAO Z Z,YE Y X,et al. Enhancement of intrinsicproton conductivity and aniline sensitivity by introducing dyemolecules into the MOF channel[J]. ACS Applied Materials amp;Interfaces,2019,11(18): 16490-16495.
[37] PATEL H A,MANSOR N,GADIPELLI S,et al. Superacidityin nafion/MOF hybrid membranes retains water at low humidityto enhance proton conduction for fuel cells[J]. ACS AppliedMaterials amp; Interfaces,2016,8(45): 30687-30691.
[38] GUO Y,JIANG Z Q,WANG X B,et al. Zwitterion threadedmetal-organic framework membranes for direct methanol fuelcells[J]. Journal of Materials Chemistry A,2018,6(40):19547-19554.
[39] TADDEI M,COSTANTINO F,IENCO A,et al. Synthesis,breathing,and gas sorption study of the first isoreticularmixed-linker phosphonate based metal-organic frameworks[J].Chemical Communications,2013,49(13): 1315-1317.
[40] GAGNON K J,PERRY H P,CLEARFIELD A. Conventionaland unconventional metal-organic frameworks based on phos-phonate ligands: MOFs and UMOFs [J]. Chemical Reviews,2012,112(2):1034-1054.
[41] ZHANG Z G,REN J H,XU J M,et al. Enhanced proton con-ductivity of sulfonated poly(arylene ether ketone sulfone)polymers by incorporating phosphotungstic acid-ionic-liquid-functionalized metal-organic framework[J]. Journal of Mem-brane Science,2021,630: 119304.
[42] HU F Q,ZHONG F,WANG J E,et al. The preparation ofmetal-organic-framework/boron phosphate hybrid materials forimproved performances in proton exchange membranes[J]. Ma-cromolecular Materials and Engineering,2021,306(6): 1-10.
[43] CAI K,SUN F X,LIANG X Q,et al. An acid-stable hexaph-osphate ester based metal-organic framework and its polymercomposite as proton exchange membrane[J]. Journal of Materi-als Chemistry A,2017,5(25): 12943-12950.
[44] RAO Z A,TANG B B,WU P Y. Proton conductivity of protonexchange membrane synergistically promoted by differentfunctionalized metal-organic frameworks[J]. ACS Applied Ma-terials amp; Interfaces,2017,9(27): 22597-22603.
[45] DONG X Y,WANG J H,LIU S S,et al. Synergy between iso-morphous acid and basic metal-organic frameworks for anhy-drous proton conduction of low-cost hybrid membranes at hightemperatures[J]. ACS Applied Materials amp; Interfaces,2018,10(44): 38209-38216.
[46] ZHENG P L,LIU Q Y,WANG D H,et al. Preparation of co-valent -ionically cross -linked UiO -66 -NH2 /sulfonated aro-matic composite proton exchange membranes with excellentperformance[J]. Frontiers in Chemistry,2020,8: 56.
[47] WANG Q H,ZHENG X F,CHEN H X,et al. Synergistic ef-fect of MOF -Directed acid -base pairs for enhanced protonconduction[J]. Microporous and Mesoporous Materials,2021,323: 111199.
[48] RU C Y,LI Z H,ZHAO C J,et al. Enhanced proton conduc-tivity of sulfonated hybrid poly(arylene ether ketone) mem-branes by incorporating an amino-sulfo bifunctionalized met-al-organic framework for direct methanol fuel cells[J]. ACS Ap-plied Materials amp; Interfaces,2018,10(9): 7963-7973.
[49] MOI R,GHORAI A,BANERJEE S,et al. Amino- and sul-fonate-functionalized metal-organic framework for fabricationof proton exchange membranes with improved proton conduc-tivity[J]. Crystal Growth amp; Design,2020,20(8): 5557-5563.
[50] WANG H F,WEN T Y,SHAO Z C,et al. High proton con-ductivity in nafion/Ni-MOF composite membranes promoted byligand exchange under ambient conditions[J]. Inorganic Chem-istry,2021,60(14): 10492-10501.
[51] ZHANG Z G,REN J H,JU M C,et al. Construction of newalternative transmission sites by incorporating structure-defectmetal -organic framework into sulfonated poly(arylene etherketone sulfone)s[J]. International Journal of Hydrogen Energy,2021,46(53): 27193-27206.
[52] LIANG T Y,SENTHIL RAJA D,CHIN K C,et al. Bimetallicmetal -organic framework -derived hybrid nanostructures ashigh-performance catalysts for methane dry reforming[J]. ACSApplied Materials amp; Interfaces,2020,12(13): 15183-15193.
[53] XIA X N,XU Y Z,CHEN Y,et al. Fabrication of MIL-101(Cr/Al) with flower-like morphology and its catalytic perfor-mance[J]. Applied Catalysis A: General,2018,559:138-145.
[54] YANG X C,XU Q A. Bimetallic metal-organic frameworks forgas storage and separation[J]. Crystal Growth amp; Design,2017,17(4): 1450-1455.
[55] SUN F Z,WANG G,DING Y Q,et al. NiFe-based metal-or-ganic framework nanosheets directly supported on nickel foamacting as robust electrodes for electrochemical oxygen evolu-tion reaction[J]. Advanced Energy Materials,2018,8(21):1800584-1800594.
[56] LI S S,GAO Y Q,LI N,et al. Transition metal-based bime-tallic MOFs and MOF -derived catalysts for electrochemicaloxygen evolution reaction[J]. Energy amp; Environmental Science,2021,14(4): 1897-1927.
[57] WANG Z,WU C W,ZHANG Z,et al. Bimetallic Fe/co-MOFsfor tetracycline elimination[J]. Journal of Materials Science,2021,56(28): 15684-15697.
[58] NEELAKANDAN S,RAMACHANDRAN R,FANG M L,etal. Improving the performance of sulfonated polymer membraneby using sulfonic acid functionalized hetero -metallic metal -organic framework for DMFC applications[J]. International Jour-nal of Energy Research,2020,44(3): 1673-1684.
[59] LIN R J,GE L,LIU S M,et al. Mixed -matrix membraneswith metal-organic framework-decorated CNT fillers for effi-cient CO2 separation[J]. ACS Applied Materials amp; Interfaces,2015,7(27): 14750-14757.
[60] RAO Z,FENG K,TANG B B,et al. Construction of well in-terconnected metal-organic framework structure for effectivelypromoting proton conductivity of proton exchange membrane[J].Journal of Membrane Science,2017,533: 160-170.
[61] SUN H Z,TANG B B,WU P Y. Rational design of S-UiO-66@GO hybrid nanosheets for proton exchange membraneswith significantly enhanced transport performance[J]. ACS Ap-plied Materials amp; Interfaces,2017,9(31): 26077-26087.
[62] ZHENG P L,WANG R,LI Z K,et al. Enhanced protontransport properties of sulfonated polyarylene ether nitrile(SPEN) with moniliform nanostructure UiO-66-NH2 /CNT[J].High Performance Polymers,2021,33(9): 1035-1046.
[63] CUI F Y,WANG W Y,LIU C N,et al. Carbon nanocompos-ites self -assembly UiO-66 -doped chitosan proton exchangemembrane with enhanced proton conductivity[J]. InternationalJournal of Energy Research,2020,44(6): 4426-4437.
[64] SUN H Z,TANG B B,WU P Y. Two-dimensional zeolitic im-idazolate framework/carbon nanotube hybrid networks modifiedproton exchange membranes for improving transport properties[J]. ACS Applied Materials amp; Interfaces,2017,9(40): 35075-35085.
[65] DONNADIO A,NARDUCCI R,CASCIOLA M,et al. Mixedmembrane matrices based on nafion/UiO -66/SO3H -UiO -66nano-MOFs: Revealing the effect of crystal size,sulfonation,and filler loading on the mechanical and conductivity proper-ties[J]. ACS Applied Materials amp; Interfaces,2017,9(48):42239-42246.
[66] LI Z,HE G W,ZHAO Y N,et al. Enhanced proton condu-ctivity of proton exchange membranes by incorporating sulfo-nated metal-organic frameworks[J]. Journal of Power Sources,2014,262: 372-379.
[67] ZHANG B,CAO Y,LI Z,et al. Proton exchange nanohybridmembranes with high phosphotungstic acid loading within met-al-organic frameworks for PEMFC applications [J]. Electro -chimica Acta,2017,240: 186-194.
[68] ESCORIHUELA J, SAHUQUILLO ó, GARCíA-BERNABéA, et al. Phosphoric acid doped polybenzimidazole (PBI)/zeolitic imidazolate framework composite membranes with significantly enhanced proton conductivity under low humidity conditions[J]. Nanomaterials, 2018, 8(10): 775–787.
[69] VEGA J,ANDRIO A,LEMUS A A,et al. Conductivity studyof zeolitic imidazolate frameworks,tetrabutylammonium hy-droxide doped with zeolitic imidazolate frameworks,and mixedmatrix membranes of polyetherimide/tetrabutylammonium hy-droxide doped with zeolitic imidazolate frameworks for protonconducting applications[J]. Electrochimica Acta,2017,258:153-166.
[70] YANG L J,TANG B B,WU P Y. Metal-organic framework-graphene oxide composites: A facile method to highly improvethe proton conductivity of PEMs operated under low humidity[J]. Journal of Materials Chemistry A,2015,3(31): 15838-15842.
[71] SEN U,ERKARTAL M,KUNG C W,et al. Proton conductingself -assembled metal -organic framework/polyelectrolyte hol-low hybrid nanostructures[J]. ACS Applied Materials amp; Inter-faces,2016,8(35): 23015-23021.
[72] GUO Y,JIANG Z Q,YING W,et al. A DNA-threaded ZIF-8membrane with high proton conductivity and low methanolpermeability[J]. Advanced Materials,2018,30(2): 1705155-1705162.
[73] RAO Z,LAN M Q,WANG Z Y,et al. Effectively facilitatingthe proton conduction of proton exchange membrane by poly-dopamine modified hollow metal-organic framework[J]. Journalof Membrane Science,2022,644: 120098.
[74] PAUL S,CHOI S J,KIM H J. Co-tri MOF-impregnated Aqui-vion R composites as proton exchange membranes for fuel cellapplications[J]. Ionics,2021,27(4): 1653-1666.
[75] DOU Y B,ZHANG W J,KAISER A. Electrospinning of met-al-organic frameworks for energy and environmental applica-tions[J]. Advanced Science,2020,7(3): 1902590-1902610.
[76] BECHELANY M,DROBEK M,VALLICARI C,et al. Highlycrystalline MOF-based materials grown on electrospun nano-fibers[J]. Nanoscale,2015,7(13): 5794-5802.
[77] LI T T,ZHANG Z M,HAN Z B. Research progress in poly-mer-based metal -organic framework nanofibrous membranesbased on electrospinning[J]. Journal of Inorganic Materials,2021,36(6): 592-600.
[78] CHEN C C,ZHANG W D,ZHU H,et al. Fabrication of met-al-organic framework-based nanofibrous separator via one-potelectrospinning strategy [ J ] . Nano Research,2021,14(5):1465-1470.
[79] LIU C,WU Y N,MORLAY C,et al. General deposition ofmetal-organic frameworks on highly adaptive organic-inorgan-ic hybrid electrospun fibrous substrates[J]. ACS Applied Mate-rials amp; Interfaces,2016,8(4): 2552-2561.
[80] SONG J L,HUANG M H,LIN X H,et al. Novel Fe-basedmetal-organic framework(MOF) modified carbon nanofiber asa highly selective and sensitive electrochemical sensor for te-tracycline detection[J]. Chemical Engineering Journal,2022,427: 130913.
[81] WU B,PAN J F,GE L,et al. Oriented MOF-polymer com-posite nanofiber membranes for high proton conductivity athigh temperature and anhydrous condition[J]. Scientific Re-ports,2014,4(1): 4334-4340.
[82] SUN L A,GU Q C,WANG H L,et al. Anhydrous proton con-ductivity of electrospun phosphoric acid -doped PVP -PVDFnanofibers and composite membranes containing MOF fillers[J]. RSC Advances,2021,11(47): 29527-29536.
[83] WANG S B,LIN Y,YANG J,et al. UiO-66-NH2 functional-ized cellulose nanofibers embedded in sulfonated polysulfoneas proton exchange membrane[J]. International Journal of Hy-drogen Energy,2021,46(36): 19106-19115.
[84] WANG L Y,DENG N P,LIANG Y Y,et al. Metal-organicframework anchored sulfonated poly(ether sulfone) nanofibersas highly conductive channels for hybrid proton exchangemembranes[J]. Journal of Power Sources,2020,450: 227592.
[85] WANG L Y,DENG N P,WANG G,et al. Construction of in-terpenetrating transport channels and compatible interfaces viaa zeolitic imidazolate framework \"bridge\" for nanofibrous hy-brid PEMs with enhanced proton conduction and methanol re-sistance[J]. ACS Sustainable Chemistry amp; Engineering,2020,8(34): 12976-12989.
[86] ZHAO G D,SHI L,ZHANG M L,et al. Self-assembly of met-al-organic framework onto nanofibrous mats to enhance protonconductivity for proton exchange membrane[J] . InternationalJournal of Hydrogen Energy,2021,46(73): 36415-36423.
[87] JU J G,SHI J L,WANG M,et al. Constructing interconnect-ed nanofibers@ZIF-67 superstructure in nafion membranes foraccelerating proton transport and confining methanol perme-ation[J]. International Journal of Hydrogen Energy,2021,46(78): 38782-38794.
[88] LIANG H X,JIAO X L,LI C,et al. Flexible self-supportedmetal-organic framework mats with exceptionally high porosityfor enhanced separation and catalysis[J]. Journal of MaterialsChemistry A,2018,6(2): 334-341.
[89] WANG L Y,DENG N P,WANG G,et al. Constructing amino-functionalized flower-like metal-organic framework nanofibersin sulfonated poly(ether sulfone) proton exchange membranefor simultaneously enhancing interface compatibility and pro-ton conduction[J]. ACS Applied Materials amp; Interfaces,2019,11(43): 39979-39990.
本文引文格式:
李磊,王00,魏國蘭. 金屬有機骨架(MOFs)基復合質子交換膜的研究進展[J]. 天津工業大學學報,2024,43(1):17-27.
LI L,WANG Y Y,WEI G L. Research progress of metalorgan-ic frameworks(MOFs)based composite proton exchange mem-branes[J]. Journal of Tiangong University,2024,43(1):17-27(in Chinese).

