CB2受體激活對慢性PD模型小鼠運動功能和黑質(zhì)膠質(zhì)細胞活化影響
[摘要]目的通過行為學、免疫印跡技術及免疫組織化學技術探討大麻素Ⅱ型(CB2)受體對1-甲基-4-苯基吡啶(MPTP)誘導的慢性帕金森病(PD)模型小鼠運動功能、黑質(zhì)(SN)區(qū)酪氨酸羥化酶(TH)蛋白表達及小膠質(zhì)細胞和星形膠質(zhì)細胞活化的影響。
方法將30只8周齡雄性C57BL/6野生型(WT)小鼠隨機分為WT對照組(A組)、WT MPTP組(B組)、WT CB2受體激動劑(JWH133)組(C組)、WT MPTP+JWH133組(D組)和WT MPTP+JWH133+CB2受體拮抗劑(AM630)組(E組),12只8周齡雄性CB2受體敲除(CB2-KO)C57BL/6小鼠隨機分為CB2-KO對照組(F組)和CB2-KO MPTP組(G組)。模型組小鼠首先腹腔注射20 μg/(kg·d)AM630和(或)10 μg/(kg·d)JWH133,每天1次,連續(xù)注射30 d;然后腹腔注射30 mg/(kg·d)的MPTP,每周2次,持續(xù)4周。對照組小鼠腹腔注射等量的生理鹽水。應用行為學實驗檢測各組小鼠爬桿與轉棒時間,免疫印跡技術檢測SN區(qū)TH蛋白的表達,免疫組織化學染色檢測SN區(qū)小膠質(zhì)細胞和星形膠質(zhì)細胞數(shù)量和形態(tài)變化。
結果與A組相比,B組小鼠爬桿時間增加,轉棒時間減少;與B組相比,D組小鼠爬桿時間減少,轉棒時間增加;與D組相比,E組小鼠爬桿時間增加,轉棒時間減少;與F組相比,G組小鼠爬桿時間增加,轉棒時間減少。上述差異具有統(tǒng)計學意義(F=29.70、45.45,q=4.87~18.09,P<0.05)。與A組相比,B組小鼠SN區(qū)TH蛋白表達水平下降;與B組相比,D組小鼠SN區(qū)TH蛋白表達水平上調(diào);與D組相比,E組小鼠SN區(qū)TH蛋白表達水平235a007ab0da0788f67618cbce1e13718f1515b1efc38d204e8c11bfeb149e8f下降;與F組相比,G組小鼠SN區(qū)TH蛋白表達水平下降。上述差異具有統(tǒng)計學意義(F=24.88,q=5.09~8.88,P<0.001)。小鼠SN區(qū)活化的小膠質(zhì)細胞和星形膠質(zhì)細胞計數(shù)顯示,與A組相比,B組明顯增加;與B組相比,D組明顯減少;與D組相比,E組明顯增加;與F組相比,G組明顯增加。上述差異具有統(tǒng)計學意義(F=269.80、708.50,q=13.29~54.78,P<0.01)。
結論激活CB2受體能夠改善MPTP誘導的慢性PD模型小鼠的運動功能障礙,抑制小鼠SN區(qū)小膠質(zhì)細胞和星形膠質(zhì)細胞的活化。
[關鍵詞]受體,大麻酚,CB2;帕金森病;1-甲基-4-苯基吡啶;旋轉棒性能試驗;黑質(zhì);小神經(jīng)膠質(zhì)細胞;星形細胞;小鼠,近交C57BL
[中圖分類號]R392.1;R742.5
[文獻標志碼]A
[文章編號]2096-5532(2024)04-0478-05doi:10.11712/jms.2096-5532.2024.60.093
[開放科學(資源服務)標識碼(OSID)]
[網(wǎng)絡出版]https://link.cnki.net/urlid/37.1517.R.20240726.0931.005;2024-07-2616:43:35
Effect of cannabinoid type 2 receptor activation on motor function and glial cell activation in the substantia nigra in a mouse model of chronic Parkinson’s disease
LIU Xinyu, ZHANG Li, MA Zegang(Department of Physiology, School of Basic Medicine, Qingdao University, Qingdao 266071, China); [Abstract]ObjectiveTo investigate the effect of cannabinoid type 2 (CB2) receptor on motor function, the protein expression of tyrosine hydroxylase (TH), and the activation of microglial cells and astrocytes in the substantia nigra (SN) in a mouse model of chronic Parkinson’s disease (PD) induced by 1-methyl-4-phenyl-1,2, 3, 6-tetrahydropyridine (MPTP) based on beha-
vioristics, Western blotting, and immunohistochemistry. MethodsA total of 30 wild-type (WT) male C57BL/6 mice, aged 8 weeks, were randomly divided into WT control group (group A), WT MPTP group (group B), WT CB2 receptor agonist JWH133 group (group C), WT MPTP+JWH133 group (group D), and WT MPTP+JWH133+CB2 receptor antagonist AM630 group (group E), and 12 CB2 receptor-knockout (CB2-KO) male C57BL/6 mice were randomly divided into CB2-KO control group (group F) and CB2-KO MPTP group (group G). The mice in the model group were given intraperitoneal injection of 20 μg/(kg·d) AM630 and/or 10 μg/(kg·d) JWH133 once a day for 30 consecutive days, followed by intraperitoneal injection of 30 mg/(kg·d) MPTP twice a week for four weeks, while those in the control group were given intraperitoneal injection ofan equal volume of normal saline. Behavioral experiments were used to measure the time of poleclimbing and the time spent on the rotating rod; Westernblotting was used to measure the protein expression of TH in the
SN; immunohistochemical staining was used to observe changes in the number and morphology of microglial cells and astrocytes in the SN.
ResultsCompared with group A, group B had a significant increase in the time of poleclimbing and a significant reduction in the time spent on the rotating rod; compared with group B, group D had a significant reduction in the time of poleclimbing and a significant increase in the time spent on the rotating rod; compared with group D, group E had a significant increase in the time of poleclimbing and a significant reduction in the time spent on the rotating rod; compared with group F, group D had a signi-
ficant increase in the time of poleclimbing and a significant reduction in the time spent on the rotating rod (F=29.70,45.45;q=4.87-18.09;P<0.05). Compared with group A, group B had a significant reduction in the protein expression level of TH in the SN of mice; compared with group B, group D had a significant increase in the protein expression level of TH; compared with group D, group E had a significant reduction in the protein expression level of TH; compared with group F, group G had a significant reduction in the protein expression level of TH (F=24.88,q=5.09-8.88,P<0.001). Compared with group A, group B had significant increases in the numbers of activated microglial cells and astrocytes in the SN of mice; compared with B, group D had significant reductions in these numbers; compared with group D, group E had significant increases in these numbers; compared with group F, group G had significant increases in these numbers (F=269.80,708.50;q=13.29-54.78;P<0.01).
ConclusionActivation of CB2 receptor can improve dyskinesia and inhibit the activation of microglial cells and astrocytes in the SN of mice with MPTP-induced chronic PD.
[Key words]receptor, cannabinoid, CB2; Parkinson disease; 1-methyl-4-phenylpyridinium; rotarod performance test; substantia nigra; microglia; astrocytes; mice, inbred C57BL
帕金森病(PD)是一種常見的神經(jīng)退行性疾病,其臨床表現(xiàn)為運動緩慢、靜止性震顫、強直和姿勢不穩(wěn)以及其他一些非運動癥狀[1-2]。PD病理特征是黑質(zhì)(SN)多巴胺能神經(jīng)元丟失、α-突觸核蛋白聚集以及鐵沉積。大麻素Ⅱ型(CB2)受體主要分布在外周組織當中,包括脾臟、免疫細胞、扁桃體、胸腺和肝臟等[3]。近年來研究發(fā)現(xiàn)CB2受體在中樞神經(jīng)系統(tǒng)也有表達,包括SN、海馬、腹側被蓋區(qū)等[4]。免疫熒光染色證實,在大鼠和小鼠的多個腦區(qū)都有CB2受體表達[5]。大腦發(fā)生炎癥時,活化的膠質(zhì)細胞上CB2受體上調(diào),并在許多神經(jīng)系統(tǒng)疾病中起重要作用。有研究表明,在1-甲基-4-苯基吡啶(MPTP)誘導的急性PD模型小鼠中,CB2受體激活對小鼠SN多巴胺能神經(jīng)元具有保護作用[6],同時可抑制膠質(zhì)細胞神經(jīng)毒性遞質(zhì)的產(chǎn)生和外周免疫細胞的浸潤[7],從而保護多巴胺能神經(jīng)元。但是,CB2受體的激活對慢性PD模型小鼠的保護作用及具體機制尚不夠清楚,所以本研究通過使用MPTP建立慢性PD小鼠模型[8-9],觀察CB2受體激活對模型小鼠的運動功能及SN區(qū)小膠質(zhì)細胞和星形膠質(zhì)細胞活化的影響。
1材料與方法
1.1實驗材料
1.1.1實驗動物及飼養(yǎng)選用SPF級雄性健康C57BL/6J野生型(WT)和CB2受體敲除(CB2-KO)小鼠,8周齡,體質(zhì)量為18~22 g,其中WT小鼠購于北京維通利華實驗動物技術有限公司,CB2-KO小鼠由美國巴羅神經(jīng)研究所贈予。小鼠飼養(yǎng)環(huán)境:室溫23~26 ℃,濕度40%~60%,12 h-12 h晝夜循環(huán)光照,自由飲水進食。每籠3~4只。實驗開始前小鼠需要適應飼養(yǎng)環(huán)境1周。
1.1.2主要試劑來源CB2受體激動劑JWH133、CB2受體拮抗劑AM630均購于美國APE x BIO生物科技公司;神經(jīng)膠質(zhì)酸性蛋白(GFAP)抗體、離子鈣結合銜接分子-1(IBA-1)抗體均購于美國Cell Signaling Technology公司,酪氨酸羥化酶(TH)抗體、山羊抗兔熒光二抗均購于美國Thermo Fisher Scientific公司;MPTP購于Sigma公司。其他試劑均為國產(chǎn)分析純。
1.2實驗方法
1.2.1動物分組及處理將30只野生型小鼠隨機分為對照組(A組)、MPTP組(B組)、JWH133組(C組)、MPTP+JWH133組(D組)和MPTP+JWH133+AM630組(E組)共5組,12只CB2基因敲除小鼠隨機分為對照組(F組)和MPTP組(G組)兩組。根據(jù)小鼠體質(zhì)量,模型組小鼠首先腹腔注射20 μg/(kg·d) 的AM630和(或)10 μg/(kg·d) 的JWH133,每天1次,連續(xù)注射30 d;然后腹腔注射30 mg/(kg·d) 的MPTP,每周2次,持續(xù)4周。對照組小鼠腹腔注射等量的生理鹽水。
1.2.2運動行為學檢測①爬桿實驗:將一根直徑1 cm、高60 cm的木桿垂直放置在籠中,桿的頂部有一個直徑2 cm的球形突起,將小鼠頭朝上放置在球形突起的頂部,記錄小鼠從轉頭到頭朝下爬到桿底部時間。②轉棒實驗:首先將小鼠放在旋轉棒上
適應2 min,打開轉棒,在5 min內(nèi)轉速從4 r/min勻速增加到40 r/min。小鼠隨轉棒開始爬行,小鼠脫落轉棒,系統(tǒng)會自動停止計時,并記錄小鼠在轉棒上停留的時間。上述兩個實驗均為每只小鼠進行3次測試并取平均值進行統(tǒng)計分析。每只小鼠每次測量的時間間隔不少于1 h。
1.2.3免疫印跡檢測將含有10 μg蛋白質(zhì)的樣品通過100 g/L十二烷基硫酸鈉聚丙烯酰胺凝膠進行電泳分離,隨后轉移到聚偏二氟乙烯膜(PVDF)上。將PVDF置于50 g/L脫脂牛奶中室溫封閉2 h,加TH(1∶2 000)和β-actin (1∶10 000)一抗于4 ℃孵育過夜。然后使用TBST洗滌目的條帶3次,每次10 min,加山羊抗兔IgG(H+L)(1∶10 000)二抗在室溫下共同孵育1 h。用ECL檢測試劑盒顯現(xiàn)蛋白質(zhì)條帶,以Image J軟件分析TH蛋白的表達。
1.2.4免疫組織化學染色檢測將各組小鼠灌注取腦,先后用200和300 g/L的蔗糖溶液對其脫水沉糖,應用切片機對小鼠SN區(qū)進行連續(xù)切片。將腦片置于40 g/L甲醛溶液中固定10 min;用PBS漂洗 3次,每次10 min;用PBST稀釋的體積分數(shù)0.05驢血清溶液封閉腦片1 h后,將腦片置于一抗(IBA-1(1∶200),GFAP(1∶300))中4 ℃搖床孵育過夜;PBS漂洗3次,每次10 min;加入二抗室溫避光搖床孵育2 h后,每孔中加入DAPI染色液50 μL繼續(xù)孵育 5 min;PBS漂洗 3次,每次 10 min。將腦片平鋪至潔凈的病理防脫載玻片上,適當干燥后用體積分數(shù)0.70的甘油封片,使用Olympus數(shù)字病理切片掃描系統(tǒng)進行觀察并掃描。分別計數(shù)每個高倍視野(400倍)內(nèi)SN區(qū)IBA-1和GFAP陽性細胞總數(shù)并取平均值。
1.3統(tǒng)計學處理
應用Graph Pad Prism 8.0統(tǒng)計軟件進行分析。實驗所得計量資料數(shù)據(jù)以±s表示,多組均數(shù)比較采用單因素方差分析(One-way ANOVA),并應用Turkey法進行兩兩比較。以P<0.05表示差異有統(tǒng)計學意義。
2結果
2.1CB2受體激活對小鼠運動功能影響
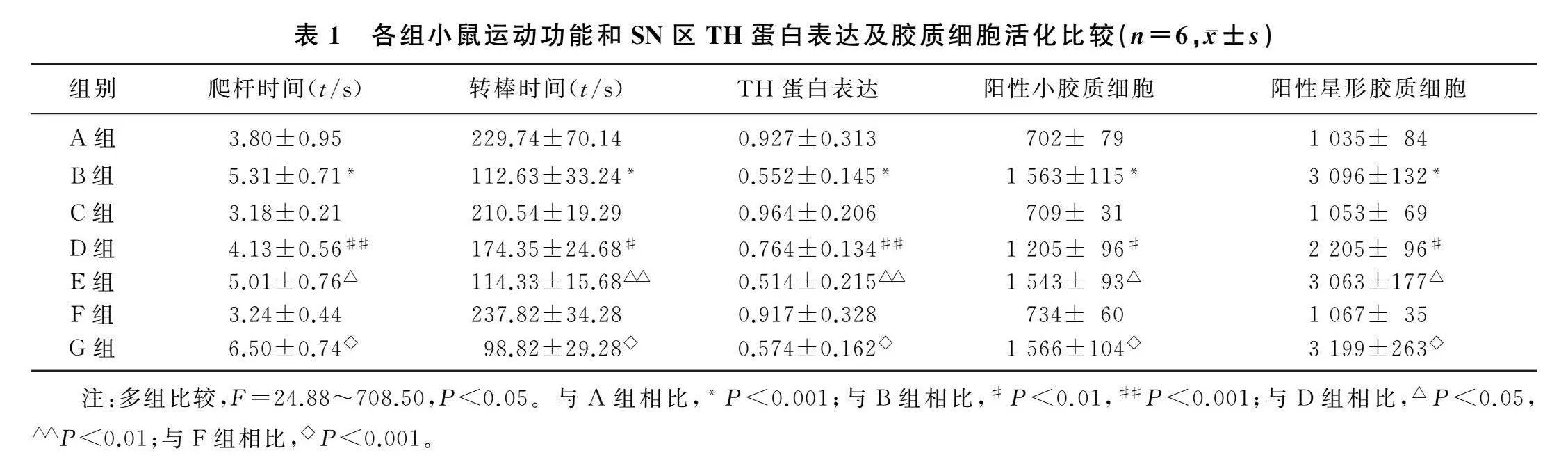
與A組相比,B組小鼠爬桿時間顯著增加(F=45.45,q=8.37,P<0.001),D組可抑制MPTP所引起小鼠爬桿時間顯著增加(q=6.55,P<0.001),E組可以阻斷JWH133的作用從而使小鼠爬桿時間顯著增加(q=4.87,P<0.05);與F組相比,G組小鼠爬桿時間顯著增加(q=18.09,P<0.001)。與A組相比,B組小鼠轉棒時間顯著減少(F=29.70,q=10.86,P<0.001),D組可以抑制MPTP所引起小鼠轉棒時間顯著減少(q=5.84,P<0.01),E組可以阻斷JWH133的作用從而使小鼠轉棒時間顯著減少(q=5.48,P<0.01);與F組相比,G組小鼠爬桿時間顯著增加(q=12.62,P<0.001)。見表1。
2.2CB2受體激活對小鼠SN區(qū)TH蛋白表達影響
免疫印跡檢測結果顯示,與A組相比,B組小鼠SN區(qū)TH蛋白表達顯著下降(F=24.88,q=8.88,P<0.001),D組抑制了MPTP誘導的小鼠SN區(qū)TH蛋白表達下降(q=5.09,P<0.01),E組阻斷了JWH133的作用從而使TH蛋白表達下降(q=5.96,P<0.01);與F組相比,G組小鼠SN
區(qū)TH蛋白表達下降(q=7.58,P<0.001)。見表1。
2.3CB2受體激活對小鼠SN區(qū)膠質(zhì)細胞活化影響
免疫熒光染色結果顯示,與A組小鼠SN區(qū)小膠質(zhì)細胞和星形膠質(zhì)細胞陽性細胞數(shù)相比,B組兩者陽性細胞數(shù)增加(F=269.80、708.50,q=33.46、52.96,P<0.01),D組抑制了MPTP誘導的兩種陽性細胞數(shù)的增加(q=13.90、22.90,P<0.01),E組阻斷了JWH133的作用從而使兩種陽性細胞數(shù)量增多(q=13.29、22.06,P<0.01);與F組相比,G組小鼠SN區(qū)活化的小膠質(zhì)細胞和星形膠質(zhì)細胞數(shù)量顯著增加(q=32.32、54.78,P<0.01)。見表1。
3討論
PD是繼阿爾茨海默病(AD)之后第二常見的慢性神經(jīng)退行性疾病,其病理學特征是中腦SN區(qū)多巴胺能神經(jīng)元的進行性丟失[10]。PD病人以運動和非運動癥狀為特征,通常表現(xiàn)出靜止性震顫、強直、運動遲緩和彎腰姿勢等。PD還可能與神經(jīng)行為障礙(抑郁、焦慮)、認知障礙(癡呆)和自主神經(jīng)功能障礙(例如多汗癥)等密切相關[11-13]。目前,左旋多巴(L-dopa)替代療法是臨床治療PD的重要手段,但卻不能抑制PD的進展。因此,探究導致多巴胺能神經(jīng)元死亡的確切機制,針對性地尋找治療靶點,是目前改善PD療效的關鍵。
近年越來越多的研究顯示,大麻素具有神經(jīng)保護和調(diào)節(jié)運動的作用[14-26]。CB2受體是內(nèi)源性大麻素系統(tǒng)(ECS)的組成部分之一,ECS由兩種內(nèi)源性大麻素和大麻素Ⅰ及Ⅱ型(CB1和CB2)受體以及合成和降解它們的酶組成[14-15]。不同于CB1受體的激活,CB2受體的激活沒有精神副作用,而且廣泛地分布在神經(jīng)膠質(zhì)細胞中[16]。因此,CB2受體及其特定配體近年來獲得更多的關注。JWH133是一種合成激動劑,對CB2受體具有高選擇性[17],能夠特異性地激活CB2受體,可以減少MPTP引起的小鼠SN多巴胺能神經(jīng)元的死亡[18],以及抑制MPP+誘導的星形膠質(zhì)細胞炎癥反應和影響鐵離子的轉運[19]。同時,JWH133還具有抗癌、心臟保護、抗炎和免疫調(diào)節(jié)活性的作用[20-23],其還被證實對缺血性卒中、抑郁癥、焦慮癥、AD、癲癇和神經(jīng)性疼痛等疾病具有神經(jīng)保護作用[24-26]。因此,這提示JWH133可能具有很好的藥用前景4892674c46cce019ca367a4f78057125ca4b8b343c00178f511bcbf8f7e98992。
神經(jīng)炎癥是促進PD進展的重要病理因素,是由中樞神經(jīng)系統(tǒng)中的免疫細胞活化引發(fā)的,神經(jīng)炎癥是中樞神經(jīng)系統(tǒng)損傷、感染、毒性或自身免疫的結果。有研究表明,神經(jīng)膠質(zhì)細胞的異常激活可能介導神經(jīng)炎癥,導致神經(jīng)退行性疾病。在PD病人尸體解剖的SN中,除了多巴胺能神經(jīng)元丟失之外,還檢測到活化的膠質(zhì)細胞和大量的炎癥因子[27],這表明神經(jīng)炎癥參與了PD的發(fā)病。因此,有效抑制膠質(zhì)細胞介導的炎癥反應,可能有助于PD的治療。研究表明,未活化M0小膠質(zhì)細胞中CB2受體的激活與細胞遷移密切相關,因為BV2細胞中CB2受體激活會觸發(fā)細胞遷移,這可能與小膠質(zhì)細胞板狀偽足前緣的CB2受體表達有關[28]。脂多糖/干擾素γ刺激的原代小膠質(zhì)細胞與CB2受體的配體AEA共同治療,可以劑量依賴性方式增加白細胞介素-10的表達,而白細胞介素-10是M2極化的標志物[29]。提示CB2受體的激活不僅能夠減少促炎型小膠質(zhì)細胞的激活,而且還能增加抗炎型小膠質(zhì)細胞的表達。以上研究提示,激活CB2受體與神經(jīng)炎癥的治療存在正向關系。
本文課題組前期研究已經(jīng)證實,激活CB2受體可抑制MPP+處理的BV2小膠質(zhì)細胞M1極化,并且促進BV2小膠質(zhì)細胞從M1型轉化為M2型[30]。所以,我們選擇IBA-1和GFAP特異性地標記小膠質(zhì)細胞和星形膠質(zhì)細胞,在小鼠SN區(qū)進行免疫組織化學分析,檢測了小膠質(zhì)細胞和星形膠質(zhì)細胞激活的數(shù)量變化,并且通過觀察小鼠的運動行為學實驗指標變化來進一步佐證我們的觀點。本文研究結果顯示,JWH133能夠抑制MPTP誘導的小鼠SN區(qū)膠質(zhì)細胞的激活并且改善模型鼠的運動行為障礙,而進一步使用CB2受體抑制劑AM630驗證的結果顯示JWH133作用被阻斷。
綜上所述,激活CB2受體能夠改善MPTP誘導的慢性PD模型小鼠的運動功能障礙,抑制小鼠SN區(qū)小膠質(zhì)細胞和星形膠質(zhì)細胞的活化。CB2受體激活在PD發(fā)病中發(fā)揮了抗炎作用,但其涉及到的機制仍未明確,有待進一步探究。由于CB2受體在神經(jīng)膠質(zhì)細胞中廣泛表達,而作為CB2受體特異性激動劑的JWH133,可能對治療PD等神經(jīng)退行性疾病的藥物開發(fā)具有很好前景,值得進一步研究。
[參考文獻]
[1]GONALVES E D, DUTRA R C. Cannabinoid receptors as therapeutic targets for autoimmune diseases: where do we stand[J]? Drug Discovery Today, 2019,24(9):1845-1853.
[2]GRECO R, DEMARTINI C, ZANABONI A M, et al. The endocannabinoid system and related lipids as potential targets for the treatment of migraine-related pain[J]. Headache, 2022,62(3):227-240.
[3]HOWLETT A C, ABOOD M E. CB1 and CB2 receptor pharmacology[J]. Advances in Pharmacology (San Diego, Calif), 2017,80:169-206.
[4]BOROWSKA M, CZARNYWOJTEK A, SAWICKA-GUTAJ N, et al. The effects of cannabinoids on the endocrine system[J]. Endokrynologia Polska, 2018,69(6):705-719.
[5]LEUNG K. 1-(2,4-Dichlorophenyl)-4-cyano-5-(4-[11C]methoxyphenyl)-N-(pyrrolidin-1-yl)-1H-pyrazole-3-carboxamide[M/OL]// Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda
(MD): National Center for Bio-
technology Information (US); 2004-2013. https://www.ncbi.nlm.nih.gov/books/NBK5923/.
[6]GARCA C, PALOMO-GARO C, GMEZ-GALVEZ Y, et al. Cannabinoid-dopamine interactions in the physiology and physiopathology of the basal Ganglia[J]. British Journal of Pharmacology, 2016,173(13):2069-2079.
[7]PRICE D J, KENNEDY H, DEHAY C, et al. The development of cortical connections[J]. The European Journal of Neuroscience, 2006,23(4):910-920.
[8]PETROSKE E, MEREDITH G E, CALLEN S, et al. Mouse model of Parkinsonism: a comparison between subacute MPTP and chronic MPTP/probenecid treatment[J]. Neuroscience, 2001,106(3):589-601.
[9]SELVAKUMAR G P, JANAKIRAMAN U, ESSA M M, et al. Escin attenuates behavioral impairments, oxidative stress and inflammation in a chronic MPTP/probenecid mouse model of Parkinson’s disease[J]. Brain Research, 2014,1585:23-36.
[10]PAKKENBERG B, MLLER A, GUNDERSEN H J, et al. The absolute number of nerve cells in substantia nigra in normal subjects and in patients with Parkinson’s disease estimated with an unbiased stereological method[J]. Journal of Neurology, Neurosurgery, and Psychiatry, 1991,54(1):30-33.
[11]RAY S, AGARWAL P. Depression and anxiety in parkinson disease[J]. Clinics in Geriatric Medicine, 2020,36(1):93-104.
[12]GOLDMAN J G, SIEG E. Cognitive impairment and dementia in parkinson disease[J]. Clinics in Geriatric Medicine, 2020,36(2):365-377.
[13]CHEN Z C, LI G L, LIU J. Autonomic dysfunction in Parkinson’s disease: implications for pathophysiology, diagnosis, and treatment[J]. Neurobiology of Disease, 2020,134:104700.
[14]DI MARZO V, PISCITELLI F. The endocannabinoid system and its modulation by phytocannabinoids[J]. Neurotherapeutics, 2015,12(4):692-698.
[15]BISOGNO T, MACCARRONE M. Endocannabinoid signaling and its regulation by nutrients[J]. BioFactors, 2014,40(4):373-380.
[16]NAGOOR MEERAN M F, SHARMA C, GOYAL S N, et al. CB2 receptor-selective agonists as candidates for targeting infection, inflammation, and immunity in SARS-CoV-2 infections[J]. Drug Development Research, 2021,82(1):7-11.
[17]CHEN M, YAN X T, YE L, et al. Dexmedetomidine ameliorates lung injury induced by intestinal ischemia/reperfusion by upregulating cannabinoid receptor 2, followed by the activation of the phosphatidylinositol 3-kinase/akt pathway[J]. Oxidative Medicine and Cellular Longevity, 2020, 2020:6120194.
[18]CHUNG Y C, SHIN W H, BAEK J Y, et al. CB2 receptor activation prevents glial-derived neurotoxic mediator production, BBB leakage and peripheral immune cell infiltration and rescues dopamine neurons in the MPTP model of Parkinson’s disease[J]. Experimental & Molecular Medicine, 2016,48(1):e205.
[19]JIA Y, DENG H, QIN Q Y, et al. JWH133 inhibits MPP+-induced inflammatory response and iron influx in astrocytes[J]. Neuroscience Letters, 2020,720:134779.
[20]QAMRI Z, PREET A, NASSER M W, et al. Synthetic cannabinoid receptor agonists inhibit tumor growth and metastasis of breast cancer[J]. Molecular Cancer Therapeutics, 2009,8(11):3117-3129.
[21]SERVETTAZ A, KAVIAN N, NICCO C, et al. Targeting the cannabinoid pathway limits the development of fibrosis and autoimmunity in a mouse model of systemic sclerosis[J]. The American Journal of Pathology, 2010,177(1):187-196.
[22]AKR M, TEKIN S, OKAN A, et al. The ameliorating effect of cannabinoid type 2 receptor activation on brain, lung, liver and heart damage in cecal ligation and puncture-induced sepsis model in rats[J]. International Immunopharmacology, 2020,78:105978.
[23]ZHU M, YU B Q, BAI J X, et al. Cannabinoid receptor 2 agonist prevents local and systemic inflammatory bone destruction in rheumatoid arthritis[J]. Journal of Bone and Mineral Research, 2019,34(4):739-751.
[24]KRUK-SLOMKA M, MICHALAK A, BIALA G. Antidepressant-like effects of the cannabinoid receptor ligands in the forced swimming test in mice: mechanism of action and possible interactions with cholinergic system[J]. Behavioural Brain Research, 2015,284:24-36.
[25]SHENG W S, CHAUHAN P, HU S X, et al. Antiallodynic effects of cannabinoid receptor 2 (CB2R) agonists on retrovirus infection-induced neuropathic pain[J]. Pain Research & Ma-
nagement, 2019,2019:1260353.
[26]CAO Q J, YANG F H, WANG H. CB2R induces a protective response against epileptic seizures through ERK and p38 signaling pathways[J]. The International Journal of Neuroscience, 2021,131(8):735-744.
[27]XU S B, LU J N, SHAO A W, et al. Glial cells: role of the immune response in ischemic stroke[J]. Frontiers in Immu-
nology, 2020,11:294.
[28]WALTER L, FRANKLIN A, WITTING A, et al. Nonpsy-
chotropic cannabinoid receptors regulate microglial cell migration[J]. The Journal of Neuroscience, 2003,23(4):1398-1405.
[29]CORREA F, HERNANGMEZ M, MESTRE L, et al. Anandamide enhances IL-10 production in activated microglia by targeting CB(2) receptors: roles of ERK1/2, JNK, and NF-kappaB[J]. Glia, 2010,58(2):135-147.
[30]王夢雅,劉曼,馬澤剛. 激活CB2受體對MPP+誘導BV2小膠質(zhì)細胞iNOS和Arg-1表達的影響[J]. 青島大學學報(醫(yī)學版), 2023,59(2):195-198.
(本文編輯于國藝)

