Preparation, Characterization and Catalytic Behavior of 12-Molybdophosphoric Acid Encapsulated in the Supercage of Cs+-exchanged Y Zeolite*
WEI Ruiping (魏瑞平), GUO Maiping (郭麥平) and WANG Jun (王軍),**
?
Preparation, Characterization and Catalytic Behavior of 12-Molybdophosphoric Acid Encapsulated in the Supercage of Cs+-exchanged Y Zeolite*
WEI Ruiping (魏瑞平)1,2, GUO Maiping (郭麥平)1and WANG Jun (王軍)1,**
1State Key Laboratory of Materials-Oriented Chemical Engineering, College of Chemistry and Chemical Engineering, Nanjing University of Technology, Nanjing 210009, China2School of Chemistry and Chemical Engineering, Southeast University, Nanjing 211189, China
In order to solve the serious leaching problem of supported heteropoly acid catalysts in polar reaction media, 12-molybdophosphoric acid encapsulated in the supercage of Cs+-exchanged Y zeolite was prepared by the “ship in the bottle” synthesis. The influence of ion-exchange conditions and the synthesis parameters on the encapsulation of PMo12were investigated. The obtained solid sample was characterized by X-ray diffraction (XRD),31P magic angle spin nuclear magnetic resonance (MAS NMR) and Fourier Transform Infrared Spectroscopy (FT-IR), and its catalytic activity in the esterification of acetic acid and-butanol was tested. The ion-exchange time, concentration of aqueous Cs+solution, pH value, and amount of Mo added in the synthesis mixture were revealed to influence the encapsulation very remarkably. Under the optimal conditions, 12-molybdophosphoric acid could be successfully encapsulated in the supercage of CsY zeolite, and the samples showed considerable catalytic activity and excellent reusability in the esterification reaction.
Y zeolite, 12-molybdophosphoric acid, ship in the bottle, esterification
1 INTRODUCTION
Esterification is usually performed with various mineral acids as catalysts, such as H2SO4, HF, H3PO4,. The growing awareness of the unacceptability of these homogeneous catalysts gives a major impetus to the studies of environment friendly solid acids. However, ion-exchanged resin is presently the only solid acid commercially available for this purpose. Because these resins lack mechanical strength and thermal stability, problems like deactivation always occur due to swelling,and their usage is quite limited. Heteropolyacids (HPAs) of Keggin type possess a strong Br?nsted acidity and catalyze a wide variety of acid-catalyzed reactions [1-3]. Pure HPAs as heterogeneous catalysts are hindered by their low specific surface area (<10 m2·g-1), low thermal stability and high solubility in polar reaction media [4]. Immobilization of HPAs on a number of porous supports with high surface areas, such as silica [5], silica-alumina [6], active carbon [7], MCM-41 [8], SBA-15 [9], zeolite [10],., was therefore extensively studied. However, for polar reaction systems, the continuous leaching of HPAs from support into the reaction medium is the most serious problem for these supported catalysts [11].
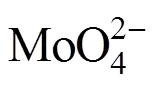
In the present work, we use the soluble Na2MoO4as Mo source and attempt to synthesize PMo12in the supercage of Cs exchanged Y zeolite by the “ship in the bottle” method. The detailed preparation conditions are investigated. Characterization data and good catalytic reusability in esterification convince the successful formation of PMo12in the supercage of Y zeolite.
2 EXPERIMENTAL
2.1 Catalyst preparation
2.00 g CsY--and 1.36 g Na2MoO4was added into 20.00 g deionized water with stirring, and then 85% aqueous solution of H3PO4in stoichiometric volume was added dropwise to the slurry, with pH being adjusted to 2 or 3 by the addition of 23.4% aqueous solution of HCl. After long time stirring, the solid was separated from the slurry by centrifuge and was washed by hot water for three times, followed by drying at 383 K for 12 h. The resulting catalysts were denoted asPMo12-CsY--, wherestands for the mass percentage of PMo12in the sample, assuming that the added Mo source was totally converted into PMo12.
2.2 Catalytic tests
The catalytic activities of the obtained samples were tested in liquid-phase esterification of acetic acid with-butanol in a 500 ml three-necked flask equipped with a magnetic stirrer and a reflux condenser.-Butanol, acetic acid and catalysts were put into the three-necked flask with the preset amount and then the mixture was heated. The typical reaction conditions were as follows: 11.9 ml-butanol, 7.4 ml acetic acid, 0.6 g catalyst (0.075 mm,.. 200 mesh), reaction at 395 K for 4 h. The reaction mixture was sampled periodically and filtered to remove catalyst particles, followed by a gas chromatography (GC) analysis using a 15 m×0.25 mm×0.25mm FFAP capillary column with Flame Ionization Detector (FID) as the detector. The conversion of-butanol was calculated based on the external standard technique. For selected catalysts, the reusability was measured. After simply separating the used catalyst from the reaction medium by centrifuge, it was transferred and charged into the next reaction medium for a new reaction cycle without regeneration.
2.3 Catalyst characterization
The powder X-ray diffraction (XRD) patterns for catalysts were collected on a Bruker D8 Advance X-ray powder diffractometer using Cu Kαradiation at 40 kV and 30 mA. The scanning range was 3°-40° with a scan rate of 2(°)·min-1. FTIR spectra of powdered samples pressed with dried KBr into discs were recorded in the range of wave numbers 400 to 1500 cm-1using a Thermo Nicolet NEXUS spectrometer. Solid state31P MAS NMR spectra were recorded using a Bruker Advance 400 D spectrometer with a spinning frequency of 9 kHz. The content of elements P and Mo in the catalyst were analyzed by Jarrell-Ash 1100 Inductively Coupled Plasma (ICP) Spectrometer.
3 RESULTS AND DISCUSSION
3.1 Influence of ion-exchange conditions on the encapsulation of PMo12
It is known that Cs+in the synthesis solution would facilitate the formation of PMo12anions possibly due to the insolubility of its cesium salt [16]. We tested this promotion effect by using Na2MoO4as Mo source and H3PO4as P source with or without Cs2CO3in the synthesis solution, whose pH was adjusted to between 1.0 and 5.0 by aqueous solution of HCl. It was found that the insoluble Cs salt of PMo12appeared with the addition of HCl and the turbidity was not obviously changed when the pH value was as high as 4.0. This phenomenon means that in the presence of Cs+the desired PMo12anions can be able to form at comparatively higher pH value. In contrast, PMo12usually formed under a low pH value less than 2.0. The observation that Cs+is beneficial to the formation of PMo12under a mild condition is considered very important when using Y zeolite without any dealumination to encapsulate PMo12anions, because the framework of Y zeolite tends to collapse and the porosity is destroyed in a strong acid media. Therefore, in this work, Cs+ions were exchanged into the Y zeolite as the counter cations, and part of them are distributed in the supercages, so as to induce the formation of the PMo12anions inside supercages.
Figure 1 shows the XRD patterns of CsY zeolite with different Cs+ion-exchange times (1, 2 and 3 times) and with different concentrations of Cs2CO3aqueous solutions (1.0% and 2.5%), respectively. It can be seen that XRD patterns for all CsY zeolites in Fig. 1 were more or less similar to that for the parent Y zeolite illustrated in Fig. 2, indicating that the CsY samples retained the porosity of Y zeolite. However, the intensities of XRD peaks decreased when the HY was subjected to the Cs+ion-exchange, and this was more obvious with increasing the ion-exchange time or the concentration of Cs2CO3aqueous solution. It can be deduced that the framework structure of the Y zeolite would be destroyed with high exchange degree. These results are in agreement with the previous report [17]
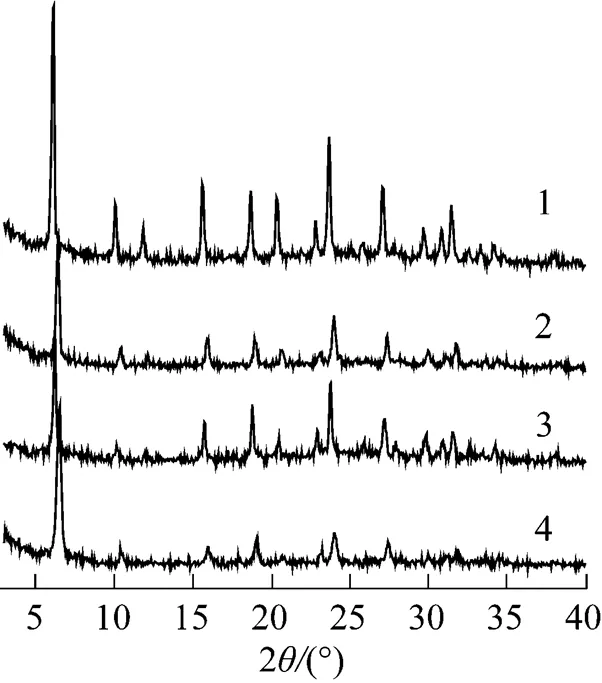
Figure 1 XRD patterns of CsY zeolite
1—CsY-1-1.0%; 2—CsY-2-1.0%; 3—CsY-3-1.0%;4—CsY-1-2.5%
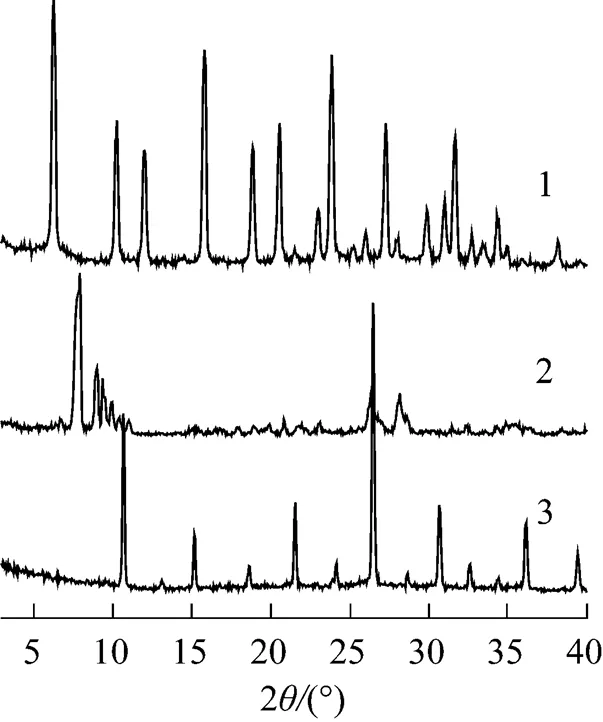
Figure 2 XRD patterns of Y, PMo12, and Cs3PMo12
1—Y; 2—PMo12; 3—Cs3PMo12
XRD patterns for 30%PMo12-CsY-1-samples prepared using different Cs2CO3concentrations under pH of 3 are displayed in Fig. 3. The framework structures of both CsY supports of the two samples was well maintained during the encapsulation procedure, as can be seen from the well shown peaks assigned to Y zeolite in Fig. 3. However, a sharp diffraction peak at around 2of 26° occurred for 30%PMo12-CsY-1-2.5%. With comparison to the XRD curves shown in Fig. 1, the sharp peak at around 26° should be assigned to the bulk crystal of the Cs salt of PMo12. In this case, Cs salt of PMo12inevitably deposited on the surface of CsY-1-2.5% support in form of crystal particles, whose size is large enough to be detected by XRD.
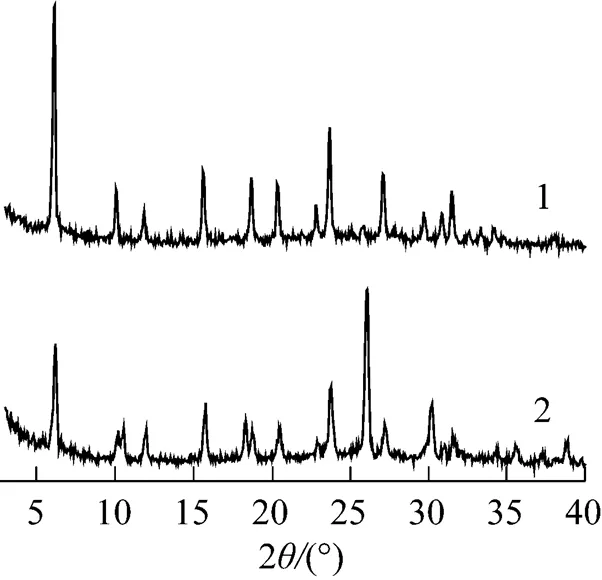
Figure 3 XRD patterns of 30%PMo12-CsY-1-1.0%, and 30%PMo12-CsY-1-2.5%
1—30%PMo12-CsY-1-1.0%; 2—30%PMo12-CsY-1-2.5%

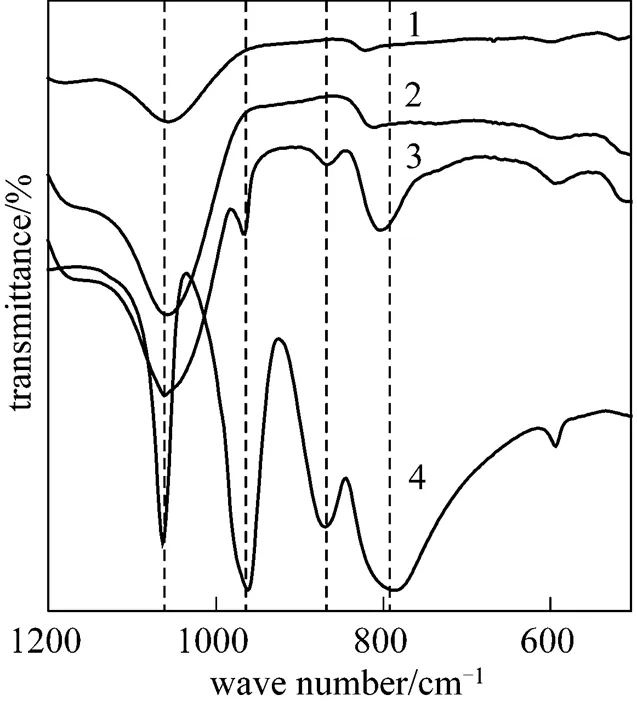
Figure 4 IR spectra of HY, 30%PMo12-CsY-1-1.0%, 30%PMo12-CsY-1-2.5%, and PMo12
1—HY; 2—30%PMo12-CsY-1-1.0%; 3—30%PMo12-CsY- 1-2.5%; 4—PMo12
The influence of ion-exchange time on the synthesis of CsY encapsulating PMo12under pH of 3 was also investigated. XRD and IR results for 30%PMo12-CsY--1.0% samples are shown in Figs. 5 and 6, respectively. It can be seen from Fig. 5 that the three samples were able to give the diffraction peaks assigned to Y zeolite, but the 30%PMo12-CsY-3-1.0% sample showed a severe distortion of the pore structure of Y zeolite by the sharply decreased peak intensities. Furthermore, there was no any peak assigned to the crystal of PMo12in all the XRD patterns, which means a high dispersion of PMo12species in samples if there was any PMo12species formed. Fig. 6 shows that the peaks around 880 cm-1and 975 cm-1assigned to the Keggin unit of PMo12were observed for the sample with an exchange time of 3 (30%PMo12- CsY-3-1.0%). This is an indication that 30%PMo12- CsY-3-1.0% possesses the encapsulated PMo12anions in the supercage of Y zeolite.
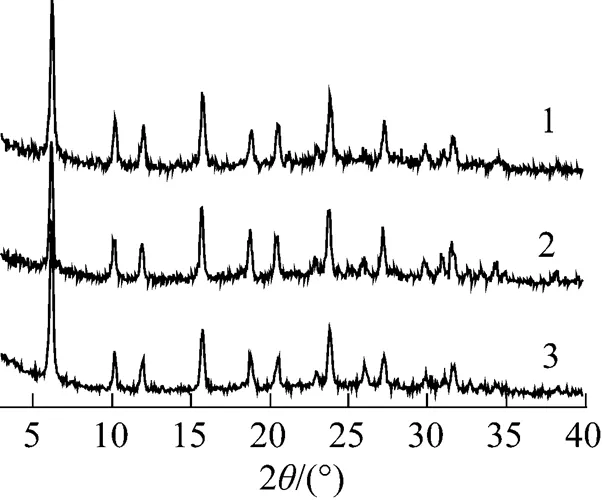
Figure 5 XRD patterns of catalysts with different ion- exchange times
1—30%PMo12-CsY-1-1.0%; 2—30%PMo12-CsY-2-1.0%;3—30%PMo12-CsY-3-1.0%
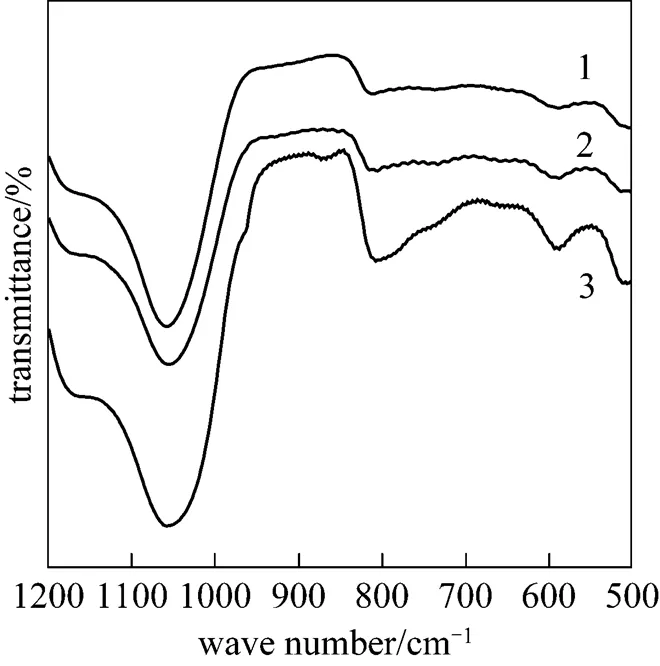
Figure 6 IR spectra of catalysts with different ion- exchange times
1—30%PMo12-CsY-1-1.0%; 2—30%PMo12-CsY-2-1.0%;3—30%PMo12-CsY-3-1.0%
3.2 Influence of the synthesis parameters on encapsulation of PMo12
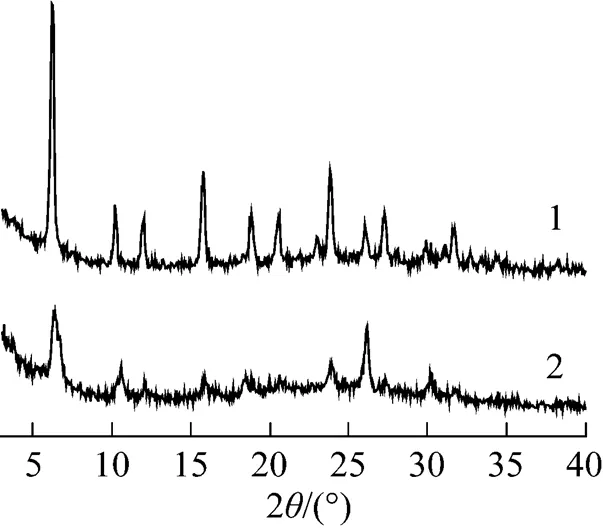
Figure 7 XRD patterns of 30%PMo12-CsY-3-1.0% synthesized in the solution with different pH
pH: 1—3.0; 2—2.0
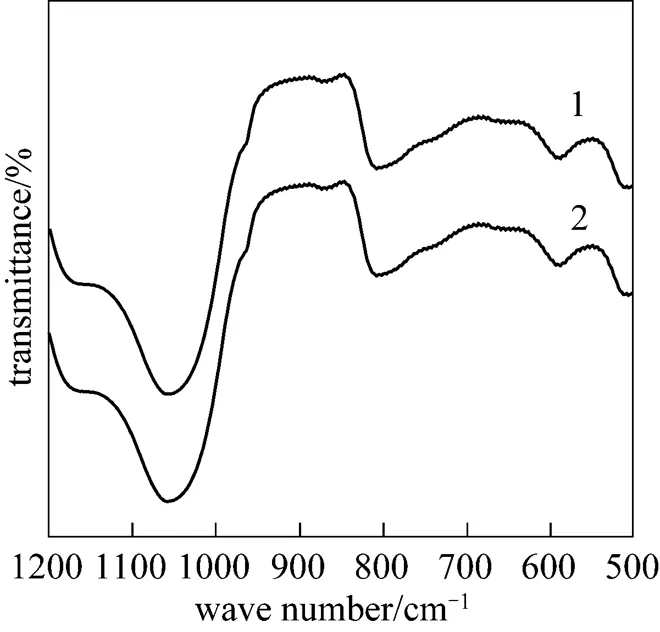
Figure 8 IR spectra of 30%PMo12-CsY-3-1.0% synthesized in the solution with different pH
pH: 1—3.0; 2—2.0
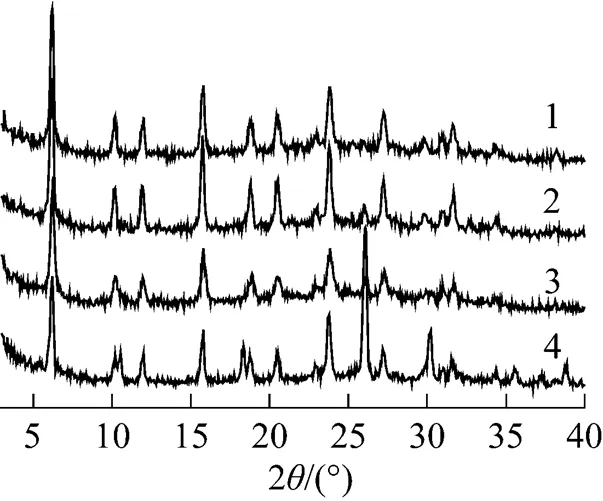
Figure 9 XRD patterns ofPMo12-CsY-3-1.0%
: 1—10%; 2—20%; 3—30%; 4—40%
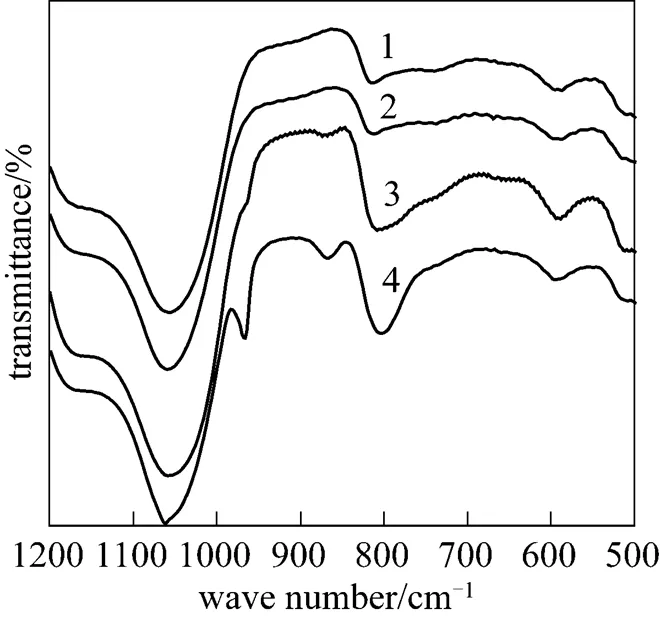
Figure 10 IR spectra ofPMo12-CsY-3-1.0%
: 1—10%; 2—20%; 3—30%; 4—40%
3.3 31P MAS NMR for the 30%PMo12-CsY-3-1.0% sample
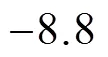
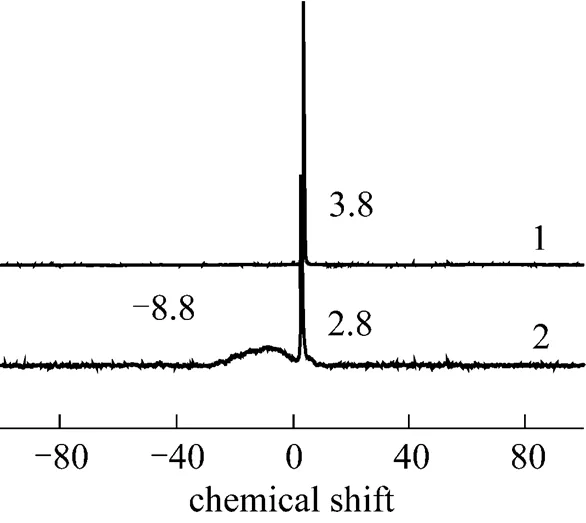
Figure 1131P MAS NMR of PMo12and 30%PMo12- CsY-3-1.0%
1—PMo12; 2—30% PMo12-CsY-3-1.0%
3.4 Catalytic activities in the esterification of acetic acid with n-butanol
From the XRD patterns in Figs. 2, 3 and 5, it can be seen that crystals of the Cs salt of PMo12were detected on the 30%PMo12-CsY-1-2.5% sample, which are proposed to locate on the external surface of Y zeolite; however, no peaks assigned to crystals of CsPMo12were observed on the samples with CsY--1.0% as supports. To exclude the influence of the external surface supported CsPMo12, 30%PMo12-CsY-3-1.0% is selected as the catalyst for the reaction of liquid-phase esterification so as to investigate the catalytic activity of the PMo12species encapsulated in the supercage of the Y zeolite.
Table 1 compares the catalytic activities of HY, PMo12, and 30%PMo12-CsY-3-1.0% catalysts in liquid- phase esterification of acetic acid with-butanol. As can be seen in Table 1, the selectivity of butyl acetate was 100% over all the samples. HY zeolite exhibited a low conversion of-butane of 37.8%, while the pure PMo12catalyst showed a high conversion of 76.7%, which implies that the Keggin-structured PMo12acts as efficient active sites for esterification. However, PMo12causes a homogeneous reaction system, and it is difficult to separate and reuse it. It is noteworthy of that 30%PMo12-CsY-3-1.0% gave a considerable conversion of 51.5%, which is mostly due to the introducing of additional active acidic sites of PMo12into the Y support.
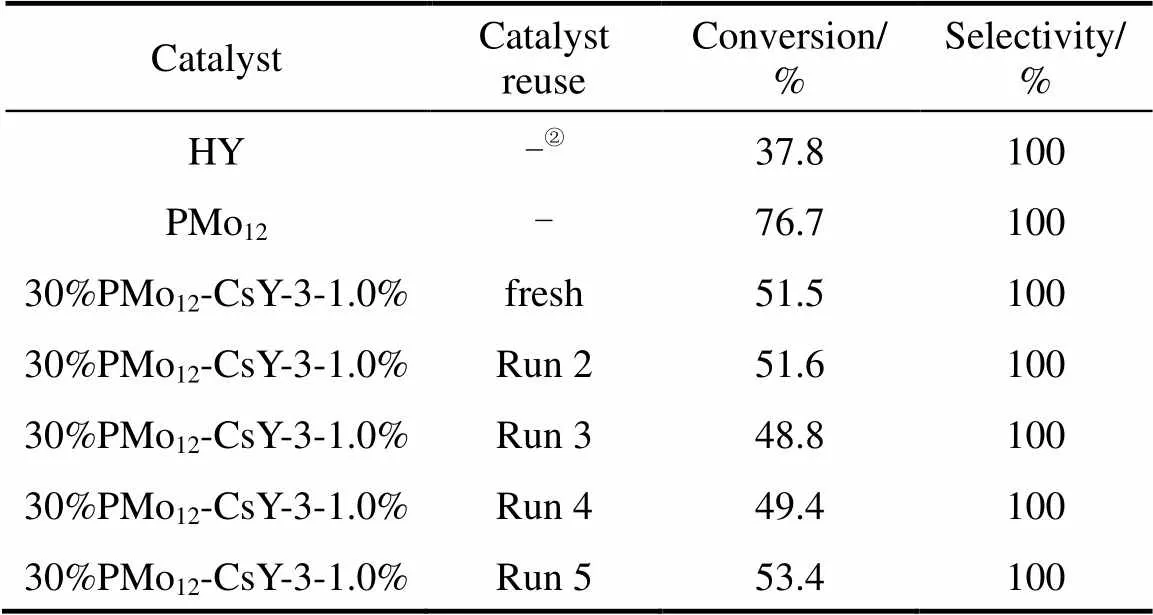
Table 1 Catalytic activities of various catalysts in the esterification of acetic acid with n-butanol①
① Reaction conditions: 11.9 ml-butanol, 7.4 ml acetic acid, 0.6 g catalyst, 395 K reaction temperature, 4 h reaction time.
②Not measured.
In order to investigate the catalytic stability of the encapsulated catalyst 30%PMo12-CsY-3-1.0%, it was separated from the reaction system by filter and drying, followed by transfer to the next reaction recycle. It can be seen in Table 1 that a rather stable conversion of around 50% could be achieved after five reaction runs. The high reusability should be attributed to the successful encapsulation of PMo12into the supercage of CsY zeolite, and it is impossible for the encapsulated PMo12anions to be leached into the polar solvent.
3.5 Elemental analysis of 30%PMo12-CsY-3-1.0% sample
Loadings of P and Mo for the fresh 30%PMo12-CsY-3-1.0% catalyst and the catalyst after 5 reaction recycles were measured by inductively coupled plasma (ICP). From the loading of Mo in the fresh catalyst (14.6%) and the preset loading of Mo (18.7%) for sample preparation, one can know that the yield of Mo in the final solid catalyst is about 78.1%. The molar ratio of Mo/P in fresh catalyst is 11.2︰1, a value very close to the theoretical ratio of Mo/P (12︰1) in the Keggin structure of the 12-molybdophosphoric acid. This data once again confirms that Keggin- structured 12-molybdophosphoric acid has been successfully encapsulated into the super cage of Y zeolite. The loadings of P and Mo in the 30%PMo12-CsY-3-1.0% catalyst after 5 reaction cycles are 0.4% and 13.9% respectively, only very slightly lower than those in fresh one. That means the 12-molybdophosphoric acid encapsulated into the super cage of Y zeolite is almost insoluble during the esterification reaction.
4 CONCLUSIONS
By carefully altering the Cs+ion-exchange conditions and synthesis parameters, PMo12is successfully encapsulated into the super cage of Y zeolite promoted by Cs counter cation in zeolite matrix, using 2.5% aqueous solution of Cs2CO3to do the ion-exchange for three times and with a high pH of 3.0 and 30% PMo12in the synthesis mixture. The assured encapsulation is jointly characterized by XRD, FT-IR, ICP, and31P MAS NMR spectra. By this method, 78.1% of the Mo in the synthesis mixture can be encapsulated in the final catalyst sample. The 30%PMo12-CsY-3-1.0% catalyst exhibits considerable catalytic activity (about 50% conversion with 100% selectivity) in liquid- phase esterification of acetic acid with-butanol under the optimal reaction conditions. Furthermore, the catalytic activity keeps rather stable during 5 reaction cycles, showing an outstanding reusability of the encapsulated PMo12catalyst.
1 Misono, M., “Heterogeneous catalysis by heteropoly compounds of molybdenum and tungsten”,...., 29, 269-321 (1987).
2 Izumi, Y., Ogawa, M., Nohara, W., Urabe, K., “Acidic alkali-metal salts and ammonium-salts of Keggin type heteropolyacids as efficient solid acid catalysts for liquid-phase Friedel-Crafts reactions”,.., 21, 1987-1990 (1992).
3 Kaur, J., Kozhevnikov, I.V., “Efficient acylation of toluene and anisole with aliphatic carboxylic acids catalysed by heteropoly salt Cs2.5H0.5PW12O40”,.., 21, 2508-2509 (2002).
4 Kozhevnikov, I.V., Kloetstra, K.R., Sinnema, A., Zandbergen, H.W., van Bekkum, H., “Study of catalysts comprising heteropoly acid H3PW12O40supported on MCM-41 molecular sieve and amorphous silica”,..., 114, 287-298 (1996).
5 Marme, F., Coudurier, G., Védrine, J.C., “Acid-type catalytic properties of heteropolyacid H3PW12O40supported on various porous silica-based materials”,..., 22, 151-163 (1998).
6 Nowinska, N., Fiedorov, R., Adamiec, J., “Catalytic activity of supported heteropoly acids for reactions requiring strong acid centers”,...., 87, 749-753 (1991).
7 Chimienti, M.E., Pizzio, L.R., Cáceres, C.V., Blanco M.N., “Tungstophosphoric and tungstosilicic acids on carbon as acidic catalysts”,.., 208, 7-19 (2001).
8 Kozhevnikov, I.V., Sinnema, A., Janse, R.J.J., Pamin, K., Bekkum, H., “New acid catalyst comprising heteropoly acid on a mesoporous molecular-sieve MCM-41”,.., 30, 241-252 (1995).
9 Liu, Q.Y., Wu, W.L., Wang, J., Ren, X.Q., Wang, Y.R., “Characterization of 12-tungstophosphoric acid impregnated on mesoporous silica SBA-15 and its catalytic performance in isopropylation of naphthalene with isopropanol”,..., 76, 51-60 (2004).
10 Zhang, F.M., Yuan, C.S., Wang, J., Kong, Y., Zhu, H.Y., Wang, C.Y., “Synthesis of fructone over dealmuinated USY supported heteropoly acid and its salt catalysts”,.., 247, 130-137 (2006).
11 Mizuno, N., Misono, M., “Heterogenous catalysis”,.., 98, 199-217 (1998).
12 Sulikowski, B., Haber, J., Kubacka, A., Olejniczak, Z., Ptaszyński, J., “Novel ‘ship-in-the-bottle’ type catalyst: evidence for encapsulation of 12-tungsto-phosphoric acid in the supercage of synthetic faujasite”,.., 39, 27-31 (1996).
13 Mukai, S.R., Masuda, T., Ogino, I., Hashimoto K., “Preparation of encaged heteropoly acid catalyst by synthesizing 12-molybdophosphoric acid in the supercages of Y-type zeolite”,.., 165, 219-226 (1997).
14 Mukai, S.R., Lin, L., Masuda, T., “Key factors for the encapsulation of Keggin-type heteropoly acids in the supercages of Y-type zeolite”,..., 56, 779-804 (2001).
15 Mukai, S.R., Shimoda, M., Lin, L., Tamon, H., Masuda, T., “Improvement of the preparation method of ‘ship-in-the-bottle’ type 12-molybdophosphoric acid encaged Y-type zeolite catalysts”,.., 256, 107-113 (2003).
16 Carma, A., Garcia, H., “Superamolecular host-guest systems in zeolites prepared by ship-in-a-bottle synthesis”,...., (6), 1143-1164 (2004).
17 Simon, A., K?hler, J., Keller, P., Buchholz, A., Hunger, M., “Phase transformation of zeolites Cs, Na-Y and Cs, Na-X impregnated with cesium hydroxide”,..., 68, 143-150 (2004).
18 Misono, M., “Heterogeneous catalysis by heteropoly compounds of molybdenum and tungsten”,...., 29 (2/3), 269-321 (1987).
19 Pizzio, L.R., Cáceres, C.V., Blanco, M.N., “Acid catalysts prepared by impregnation of tungstophosphoric acid solutions on different supports”,.., 167, 283-294 (1998).
20 Olejniczak, Z., Sulikowski, B., Kubacka, A., Gasior, M., “Heterogenization of 12-tungstophosphoroc acid on stabilized zeolite Y”,., 11/12, 391-400 (2000).
2008-01-16,
2008-10-28.
the National Natural Science Foundation of China (20476046) and the “Qinglan” Project of Jiangsu Province for Young Researchers.
** To whom correspondence should be addressed. E-mail: junwang@njut.edu.cn
 Chinese Journal of Chemical Engineering2009年1期
Chinese Journal of Chemical Engineering2009年1期
- Chinese Journal of Chemical Engineering的其它文章
- An Improved Fuzzy Predictive Control Algorithm and Its Application to an Industrial CSTR Process*
- Purification of Sulfuric and Hydriodic Acids Phases in the Iodine-sulfur Process*
- Synthesis and Characterization of Novel Temperature and pH Responsive Hydroxylpropyl Cellulose-based Graft Copolymers
- The Separation of Catechol from Carbofuran Phenol by Extractive Distillation*
- Electrochemical Performance of Nickel Hydroxide/Activated Carbon Supercapacitors Using a Modified Polyvinyl Alcohol Based Alkaline Polymer Electrolyte*
- Liquid Film Characteristics on Surface of Structured Packing*
