Hydrotalcite-like Materials as Catalyst for the Conjugate Addition of Amines to Electron Deficient Alkenes*
CHEN Lu (陳露), ZHU Juan (朱娟), WANG Jian (王箭), WU Haihong (吳海虹) and YANG Jianguo (楊建國),2,**
?
Hydrotalcite-like Materials as Catalyst for the Conjugate Addition of Amines to Electron Deficient Alkenes*
CHEN Lu (陳露)1, ZHU Juan (朱娟)1, WANG Jian (王箭), WU Haihong (吳海虹)1and YANG Jianguo (楊建國)1,2,**
1Shanghai Key Laboratory of Green Chemistry and Chemical Process, Department of Chemistry, East China Normal University, Shanghai 200062, China2Energy Institue, Department of Materials Science and Engineering, Pennsylvania State University, University Park, PA 16802, USA
The novel efficient procedure has been developed for the conjugate addition of amines to electron deficient alkenes. A series of hydrotalcite-like materials were synthesized as catalyst for the conjugate addition of amines and alkenes. After optimizing the reaction conditions, ZnAl-LDHs (3︰1) was chosen as the best catalyst for the reaction. The results showed that the catalyst worked very well for the conjugate addition of amines to electron deficient alkenes with the excellent yields in several minutes. Operational simplicity, no solvent, low cost of the catalyst, high yields, reusability, excellent chemoselectivity, wide applicability are the key features of this method.
hydrotalcite-like material, conjugate addition, chemoselectivity, solvent-free synthesis
1 INTRODUCTION
The formation of carbon-nitrogen bonds by simple addition of amines to double bonds is a focus of increasing interest and widely used in organic synthesis owing to the importance of the resultant β-amino compounds [1]. These β-amino carbonyl compounds are versatile synthetic intermediates for synthesis of a variety of biologically important natural products, antibiotics and for manufacture of fine chemicals and pharmaceuticals [2, 3]. The conventional method for the preparation of these compounds isthe Mannich reaction. However, it has several shortcomings including long reaction times, low yields and harsh reaction conditions [4]. An alternative method for preparing these compounds isthe Michael addition. It is a very straightforward approach for the synthesis of substituted amines and their derivatives with 100% atom efficiency [5]. In general, this type of conjugated addition reaction requires basic conditions or acidic catalysts. A number of alternative procedures have been reported recently using a variety of reagents such as Pd compounds, InCl3, CeCl3, Yb(OTf)3, Bi(NO3)3, Bi(OTf)3, Cu(OAc)2, LiClO4, clay, silica gel, SnI2, FeCl3, CrCl3, SnCl4,[6-25].
Although these methods are quite useful, many suffer from limitations such as the requirement for large excess of reagents, long reaction time, harsh reaction conditions, and involvement of toxic solvents such as acetonitrile or 1,2-dichloroethane. Hence, the development of less expensive, simpler, ‘greener’ catalysts for the reactions is still highly desired. Aiming to these advantages, we developed a solvent-free protocol for the conjugate addition of amines to electron deficient alkenes using hydrotalcite-like materials as heterogeneous base catalyst. The results showed that the catalysts were efficient for the reaction with excellent yields.
2 EXPERIMENTAL
All organic reagents were commercial products with the highest purity available (>98%) and used for the reaction without further purification.
2.1 Synthesis of the catalyst
All the hydrotalcites were prepared by the co-precipitation method. An aqueous solution (80 ml) containing 0.06 mol Mg(NO3)2·6H2O and 0.02 mol Al(NO3)3·9H2O (total metal nitrates, 0.08 mol with the Mg/Al ratio of 3) was added slowly to a second solution (80 ml) containing NaOH (0.16 mol) and Na2CO3(0.01 mol) under vigorous mechanical stirring, maintaining the pH between 9 and 10. The addition took nearly 0.5 h at 70°C. The mixture was kept at this temperature for 3 h under stirring, after that the product was left standing for 15 h at 353 K. The resulting white precipitate was filtered, washed to eliminate the alkali metal ions and the nitrate ions until the pH of the washing water was 7. Then the catalyst MgAl-LDHs (LDH: layered double hydroxides) was obtained after drying at 353 K for 24 h. The specific surface area of MgAl-LDHs was 51.53 m2·g-1. It was an anionic layered compound. The ZnAl-LDHs and NiAl-LDHs were obtained in the same way using Zn(NO3)2or Ni(NO3)2in stead of Mg(NO3)2, respectively. 5%Fe(III)MgAl- LDHs and 5%CuMgAl-LDHs were obtained by adding 5% Fe or 5% Cu of the total metal nitrates (0.08 mol) to the procedure above.
2.2 The conjugate addition of amines to electron deficient alkenes
Typical procedure for the conjugate addition of amines: The mixture of amines (20 mmol), alkenes (24 mmol) and the catalyst (50 mg) was stirred at room temperature for the certain time. The process of the reaction was monitored by GC analysis (Shimadzu GC-14B). On completion, the catalyst was recycled by filtration.
3 RESULTS AND DISCUSSION
3.1 Characterization of the catalyst
The X-ray diffraction (XRD) patterns (Fig. 1) with sharp and symmetric reflections for the (003), (006), (110), and (113) planes and broad symmetric peaks for the (102), (105), and (108) planes are characteristics of well-crystallized hydrotalcite-like materials.
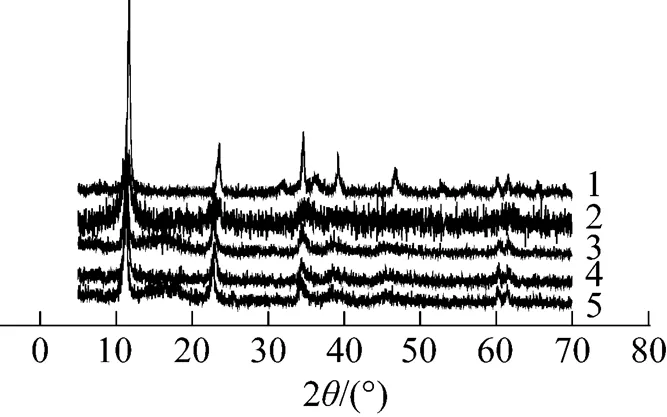
Figure 1 XRD patterns of LDHs
1—Zn︰Al (3︰1); 2—Ni︰Al (3︰1); 3—Mg︰Al (3︰1); 4—FeMgAl [Fe: 5%, Mg︰Al (3︰1)]; 5—CuMgAl [Cu: 5%; Mg︰Al (3︰1)]
3.2 Catalytic activities of different metal oxides
The reaction between cyclohexylamine and methyl acrylate was used as the model to investigate different catalysts (Table 1). As expected, the reaction took place and afforded the corresponding product in high yield. From the results, it was found that the differences between these catalysts were very obvious. The ZnAl-LDHs was the most efficient catalyst for the reaction with the highest conversion, so ZnAl-LDHs was chosen as the catalyst for the reactions below.
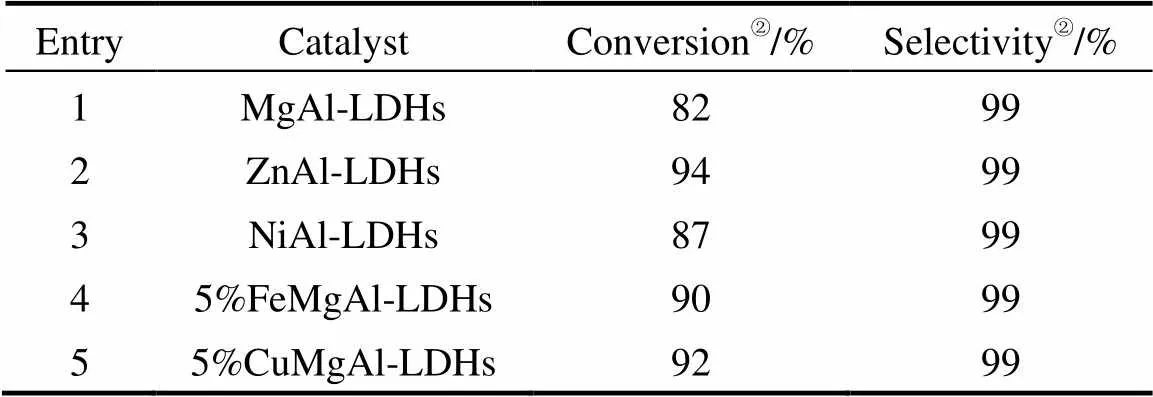
Table 1 Catalytic activities of different metal oxides①
①Reaction conditions: cyclohexylamine 20 mmol, methyl acrylate 24 mmol, catalyst 50 mg, R.T. (25°C) , reaction time 5 min.
②Conversion and selectivity was determined by GC analysis based on cyclohexylamine.
3.3 Catalytic procedure for the conjugate addition
The conjugate additions of various amines with alkenes using ZnAl-LDHs under solvent-free condition were investigated (Table 2). The results showed that the reactions underwent smoothly at room temperature just in several minutes. The primary amines with low steric hindrance easily gave the double substituted products, which decreased the selectivity of the single substituted product (Entries 2, 3, 8). When the cyclohexylamine with large steric hindrance was introduced to the reaction, the selectivities were very high for the single substitute products (Entries 4, 7, 11). The primary amines with low steric hindrance could also give the single substituted products when the alkenes with large space hindrance were involved in the reaction (Entries 8, 9, 10, 13, 14). The secondary amines gave the corresponding products with high conversions and selectivities (Entries 1, 5, 6). But the conversion decreased when the alkenes with large space hindrance were involved in the reaction (Entry 12). As to the alkenes, the reactivity was affected by the electron-withdrawing group (EWG) steric hindrance also had the certain effect on the reaction.

Table 2 The conjugate addition of various amines with alkenes①
② The reaction conditions: amine 20 mmol, alkenes 24 mmol, catalyst 50 mg, reaction time 5 min, R.T. (25°C).
②The conversion and selectivity were determined by GC analysis based on amine.
3.4 Reuse of the catalyst
For the present heterogeneous catalytic process, recovery of the catalyst is very convenient. After reaction, the catalyst was recovered by filtration. The recovered activities were investigated through the reaction of cyclohexylamine with methyl acrylate (Fig. 2). The experimental conditions were the same as in Table 2. The yield changed little after the catalyst had been recycled for sixth times. The novel catalyst showed high catalytic activity perfectly maintained after six recyclings.
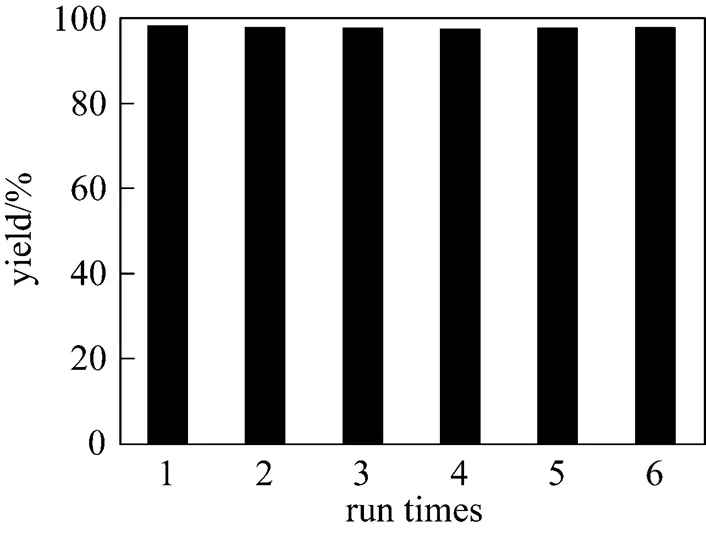
Figure 2 Reuse of the catalyst ZnAl-LDHs
3.5 Chemoselectivity of the catalyst
It is noteworthy that aromatic amines did not produce the corresponding products under the same reaction conditions (Fig. 3). This result indicated that the present protocol could be applicable to the chemoselective addition of aliphatic amines in the presence of aromatic amines.
Figure 3 Chemoselectivity of the catalyst
1 Zhang, D., Wang, G., Zhu, R., “Insight into the mechanism of the Michael addition of malononitrile to a,β-unsaturated imides catalyzed by bifunctional thiourea catalysts”,., 19, 568-576 (2008).
2 Singh, R., Goswami, T., “Acid catalyzed 1, 2 Michael addition reaction: A viable synthetic route in designing fullerene core starlike macromolecule”,...., 21, 225-236 (2008).
3 Wang, C.J., Zhang, Z.H., Dong, X.Q., Wu, X.J., “Chiral amine- thioureas bearing multiple hydrogen bonding donors: Highly efficient organocatalysts for asymmetric Michael addition of acetylacetone to nitroolefins”,.., (12), 1431-1433 (2008) .
4 Xu, L.W., Yang, M.S., Qiu, H.Y., Lai, G.Q., Jiang, J.X., “Efficient iron-catalyzed Sakurai-Michael addition of allyltrimethylsilane to chalcones”,.., 38, 1011-1019 (2008).
5 Miao, T., Wang, L., “Polystyrene-immobilized pyrrolidine as a highly stereoselective and recyclable organocatalyst for asymmetric Michael addition of cyclohexanone to nitroolefins”,., 49, 2173-2176 (2008).
6 Wang, Y., Li, P., Liang, X., Zhang, T.Y., Ye, J., “An efficient enantioselective method for asymmetric Michael addition of nitroalkanes to α,β-unsaturated aldehydes”,.., (10), 1232-1234 (2008) .
7 Bhanushali, M.J., Nandurkar, N.S., Jagtap, S.R., Bhanage, B.M., “Y(NO3)3·6H2O catalyzed aza-Michael addition of aromatic/hetero- aromatic amines under solvent-free conditions”,.., 9 (6), 1189-1195 (2008).
8 Shirakawa, S., Shimizu, S., “Hydrogen-bond-promoted C-C bond-forming reaction: Catalyst-free Michael addition reactions in ethanol”,.., (20), 3160-3164 (2007).
9 Zhu, S., Yu, S., Ma, D., “Highly efficient catalytic system for enantioselective Michael addition of aldehydes to nitroalkenes in water”,,, 47, 545-548 (2008).
10 Chen, F.X., Shao, C., Wang, Q., Gong, P., Zhang, D.Y., Zhang, B.Z., Wang, R., “An enantioselective Michael addition of malonate to nitroalkenes catalyzed by low loading demethylquinine salts in water”,, 49, 1282 (2008).
11 Ni, B., Zhang, Q., Headley, A.D., “Pyrrolidine-based chiral pyridinium ionic liquids (ILs) as recyclable and highly efficient organocatalysts for the asymmetric Michael addition reactions”,, 49, 1249-1252 (2008).
12 Azim, Z.H., Saidi, M.R., “Synthesis of aza-Henry products and enamines in water by Michael addition of amines or thiols to activated unsaturated compounds ”,, 49, 1244-1248 (2008).
13 Li, X., Cun, L., Lian, C., Zhong, L., Chen, Y., Liao, J., Zhu, J., Deng, J., “Highly enantioselective Michael addition of malononitrile to α,β-unsaturated ketones”,, 6, 349-353 (2008).
14 Attanasi, O.A., Favi, G., Filippone, P., Golobic, A., Perrulli, F.R., Stanovnik, B., Svete, J., “Regio- and stereoselective one-pot synthesisof unknown oxazoline-fused pyridazines by’ Michael addition-pyridazine cyclization-oxazoline cyclization’ cascade reactions of 4-chloro- 1,2-diaza-1,3-butadienes with 3-dimethylaminopropenoates”, (19),, 2971-2974 (2007).
15 Micheletti, G., Pollicino, S., Ricci, A., Berionni, G., Cahiez, G., “Michael addition of manganese enolates to nitroolefins”,, (18), 2829-2832 (2007).
16 Esteban, J., Costa, A.M., Gomez, A., Vilarrasa., J., “Michael addition-elimination reactions of chiral enolates with ethyl 3-halopropenoates”,, 10, 65-68 (2008).
17 Bi, X., Zhang, J., Liu, Q., Tan, J., Li, B., “Intramolecular aza-anti-michael addition of an amide anion to enones: A regiospecific approach to tetramic acid derivatives”,, 349, 2301-2306 (2007).
18 Chen, F.X., Shao, C., Wang, Q., Gong, P., Zhang, D.Y., Zhang, B.Z., Wang, R., “An enantioselective Michael addition of malonate to nitroalkenes catalyzed by low loading demethylquinine salts in water”,, 48, 8456-8459 (2007).
19 Luo, S., Zhang, L., Mi, X., Qiao, Y., Cheng, J., “Functionalized chiral ionic liquid catalyzed enantioselective desymmetrizations of prochiral ketonesasymmetric Michael addition reaction”,, 72, 9350-9352 (2007).
20 Fustero, S., Chiva, G., Piera, J., Volonterio, A., Zanda, M., Gonzalez, J., Ramallal, M., “The role of fluorine in the stereoselective tandem aza-Michael addition to acrylamide acceptors: An experimental and theoretical mechanistic study”,.., 13, 8530-8542 (2007).
21 Deb, I., John, S., Namboothiri, I.N.N., “Synthesis of benzo-fused medium ring cyclic ethersa Michael addition-ring closing metathesis strategy involving nitroaliphatic compounds”,, 63, 11991-11997 (2007).
22 Sharma, Y.O., Degani, M.S., “Green and mild protocol for hetero-Michael addition of sulfur and nitrogen nucleophiles in ionic liquid”,...., 277, 215-227 (2007) .
23 Xu, D.Q., Luo, S.P., Wang, Y.F., Xia, A.B., Yue, H.D., Wang, L.P., Xu, Z.Y., “Organocatalysts wrapped around by poly(ethylene glycol)s (PEGs): A unique host-guest system for asymmetric Michael addition reactions”,.., (42), 4393-4395 (2007).
2009-03-05,
2009-07-28.
the National Key Project of Scientific and Technical Supporting Programs Funded by Ministry of Science & Technology of China (2006BAE03B06), Shanghai Leading Academic Discipline Project (B409) and Shanghai International Cooperation of Science and Technology Project (06SR07101).
** To whom correspondence should be addressed. E-mail: jgyang@chem.ecnu.edu.cn; jzy2@psu.edu
 Chinese Journal of Chemical Engineering2009年5期
Chinese Journal of Chemical Engineering2009年5期
- Chinese Journal of Chemical Engineering的其它文章
- Effect of Relative Humidity on Catalytic Combustion of Toluene over Copper Based Catalysts with Different Supports*
- Prediction of Critical Endpoints Based on the PR Equation of State*
- Kinetic Studies on Wheat Straw Hydrolysis to Levulinic Acid*
- Calculation of Transport Properties of CF4+Noble Gas Mixtures
- Synthesis of 4,4′-MDC in the Presence of Sulfonic Acid-functionalized Ionic Liquids*
- A New Study on Combustion Behavior of Pine Sawdust Characterized by the Weibull Distribution*
