Synthesis of Didodecyl Carbonate via Transesterification Catalyzed by KF/MgO*
FAN Yanping (范燕平), WANG Qingyin (王慶印), YANG Xiangui(楊先貴), YAO Jie (姚潔) and WANG Gongying (王公應(yīng)),**
?
Synthesis of Didodecyl CarbonateTransesterification Catalyzed by KF/MgO*
FAN Yanping (范燕平)1,2,3, WANG Qingyin (王慶印)1, YANG Xiangui(楊先貴)1, YAO Jie (姚潔)1,2and WANG Gongying (王公應(yīng))1,2,**
1Chengdu Institute of Organic Chemistry, Chinese Academy of Sciences, Chengdu 610041, China2Changzhou Institute of Chemistry, Changzhou 213164, China3Graduate School of Chinese Academy of Sciences, Beijing 100039, China
The catalytic properties of KF/MgO for the synthesis of didodecyl carbonate (DDC) by transesterification from dimethyl carbonate (DMC) and dodecanol were studied. The effects of loading amount of KF and calcining temperature were systemically investigated. The phase structure was characterized by X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FTIR). Interaction between KF and the carrier MgO occurred in the process of calcination, and a new phase K2MgF4formed when calcining temperature was 673 K or above. FTIR results showed that K2CO3was observed when catalysts calcined in air. When calcining temperature was 873 K and the loading mass amount of KF was 30%, the KF/MgO catalyst exhibited the optimal catalytic properties and the yield of DDC was maximized to 80%. The excellent catalytic properties of KF/MgO was ascribed to the formation of K2MgF4+K2CO3during the calcination in air.
didodecyl carbonate, dimethyl carbonate, transesterification, supported KF catalyst
1 INTRODUCTION
Long chain alkyl carbonates have been found numerous applications in lubricant, cosmetic, pharmaceuticals, and fuel additives [1-3]. Especially as the lubricant or additive in the lubricant, these carbonates show superiority to only mineral oil and traditional synthetic esters due to their promising properties such as lower pour point, less thermal oxidative degradation, higher wear and corrosion resistant properties, and higher self-cleaning activity [4]. The commercial dialkyl carbonates have been produced by using highlytoxic phosgene as raw material [5]. The obvious disadvantagesof the phosgene process are pollution to environment and corrosion of equipments. Non-phosgene methods have been proposed, such as oxidative carbonylation of alcohol with carbon monoxide [6], reaction of urea with alcohol [7, 8], transesterification between short chain dialkyl carbonates and higher alcohol [9, 10], transesterification of dimethyl carbonate (DMC) with long chain alcohol, and so on. It is most attractive to develop the transesterification of dimethyl carbonate (DMC) with long chain alcohol to prepare long chain dialkyl carbonates due to the usage of environmentallyfriendly DMC as raw material, no pollutionand mild reaction conditions. However, up to now, only a few reports have been published on the long chain dialkyl carbonate synthesis with low yield and the catalysts used are mostly homogeneous catalysts, such as Bu2SnO [10], NaOCH3[11], K2CO3[12, 13], and so on. It is well known that the separation and recovery of homogeneous catalysts are very difficult in the industrial process, and it is desirable to develop active solid catalysts to improve the yield of long chain dialkyl carbonate.
In this work, an environmentally friendly solid base heterogeneous catalyst has been introduced to prepare a long-chain dialkyl carbonate (didodecyl carbonate, DDC) with satisfactory yields and selectivity. The synthesis of DDC is usually in two steps. In the first step, dodecanol is converted into methyl dodecyl carbonate (MDC) Reaction (1), and in the second step, MDC is converted into DDC by the reaction with dodecanol or by disproportion Reactions (2) and (3):
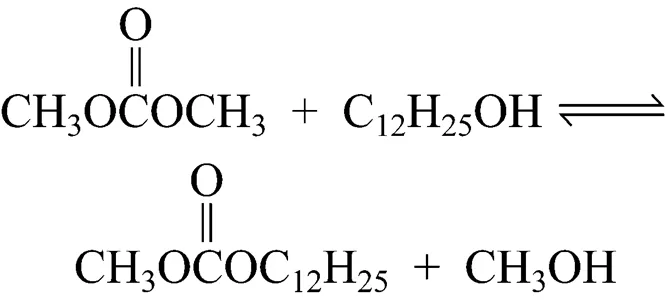
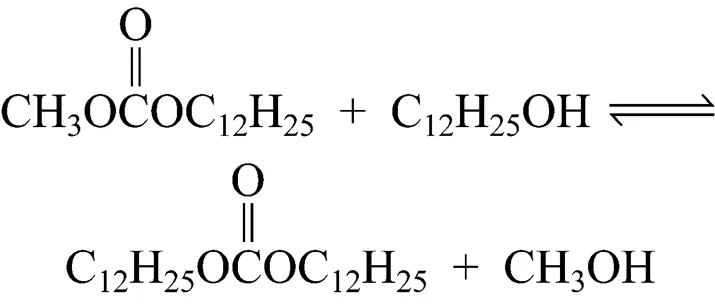
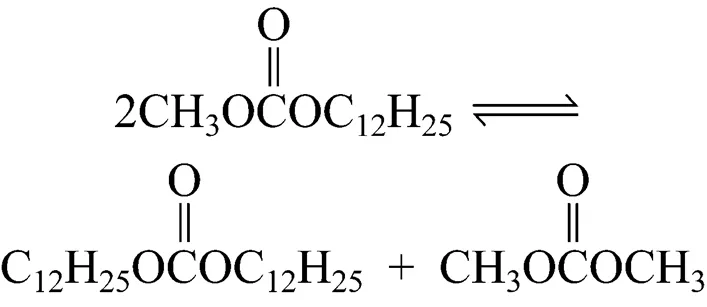
2 EXPERIMENTAL
2.1 Chemical reagents
KF·2H2O, DMC and dodecanol (DoOH) were of analytical grade, all reagents were purchased from local manufactures. MgO (Chengdu Kelong Chemical Reagent Plant, AR, powder, BET surface 13.8 m2·g-1, measured by the nitrogen adsorption on a constant volume adsorption apparatus (ASAP2010C) at the liquid nitrogen temperature).
2.2 Catalyst preparation
The catalysts were prepared by the conventional impregnation method, which is described as follows. Prior to impregnation, commercial carrier MgO was calcined at 673 K for 6 h for dehydration. The supported KF catalyst was prepared by impregnation with an aqueous solution of KF·2H2O. 10 g MgO support was impregnated with 30 ml solution, KF mass loadings were varied in the range of 10%-40%. After evaporation of the solvent in a rotary evaporator at 353 K, the impregnation sample was dried in an oven at 393 K overnight and then calcined in a muffle furnace at 873 K for 5 h.
2.3 Characterization
2.4 Catalytic test
The reaction was carried out in a 250 ml three-neck round-bottomed ?ask, equipped with a magnetic stirring bar, a thermometer, a nitrogen inlet, a dropping funnel, and a fractionating column connected to a liquid dividing head. Dodecanol (111.8 g, 0.6 mol) was added followed by KF/MgO(1.25 g) under a stream of nitrogen then heated to 413 K, DMC (12.7 ml, 0.15 mol) was added dropwise over a period of 30 min to the reaction mixture. The pot temperature was between 403 K and 423 K after complete addition of DMC. After the reaction, the mixture was cooled to room temperature and the catalyst was ?ltered and the ?ltrate was analyzed by gas chromatograph.
3 RESULTS AND DISCUSSION
3.1 Effect of calcination temperature of catalyst on the reaction
The catalytic properties of KF, MgO (unimpregnated) and KF/MgO calcined at different temperature were listed in Table 1. It can be seen that the catalytic properties strongly depended on the calcining temperature. Clearly, both conversion of DMC and selectivity of DDC first increased and then decreased with increasing the calcining temperature. The catalyst calcined at 873 K exhibited the highest catalytic activity for the transesterification reaction, and maximum DMC conversion (84.2%) and the selectivity of DDC (95.0%) were obtained.
Table 1 The catalytic properties of KF/MgO catalysts atdifferent calcined temperatures [Reaction conditions: KF mass loading amount 30%, catalyst 1.0%, n(dodecyl alcohol) ︰n(DMC)4︰1, reaction time 4 h]
① KF mass dosage was 0.3%.
② KF/MgO was calcined in N2(99.99%) atmosphere.
In order to find the reason of excellent catalytic properties, the XRD patterns and FTIR spectra of KF/MgO calcined at different temperatures were analyzed. Fig. 1 showed the XRD patterns of KF/MgO calcined at different temperatures. The peaks of KF/MgO calcined at 573 K corresponded to MgO and Mg(OH)2respectively. Mg(OH)2should be created by impregnating MgO in the aqueous solution of KF. In addition, the KF/MgO catalyst calcined should contain KF. No peaks corresponding to KF may be attributed to good dispersion of KF and no large clusters in the monolayer coverage. The phenomenon was similar to Ref. [14]. When calcining temperature was 673 K or more than, the KF/MgO catalyst contained K2MgF4and MgO. The formation of K2MgF4may be ascribed to the reaction between KF and MgO. The similar interaction between KF and Al2O3[14] or KF and SiO2[15] has been reported. The lack of MgOH should be ascribed to the conversion of Mg(OH)2to MgO at higher calcining temperature. With higher calcining temperature, the intensity of peaks corresponding to K2MgF4first increased and then decreased. The intensity of peaks corresponding to K2MgF4was highest when calcining temperature was 873 K.
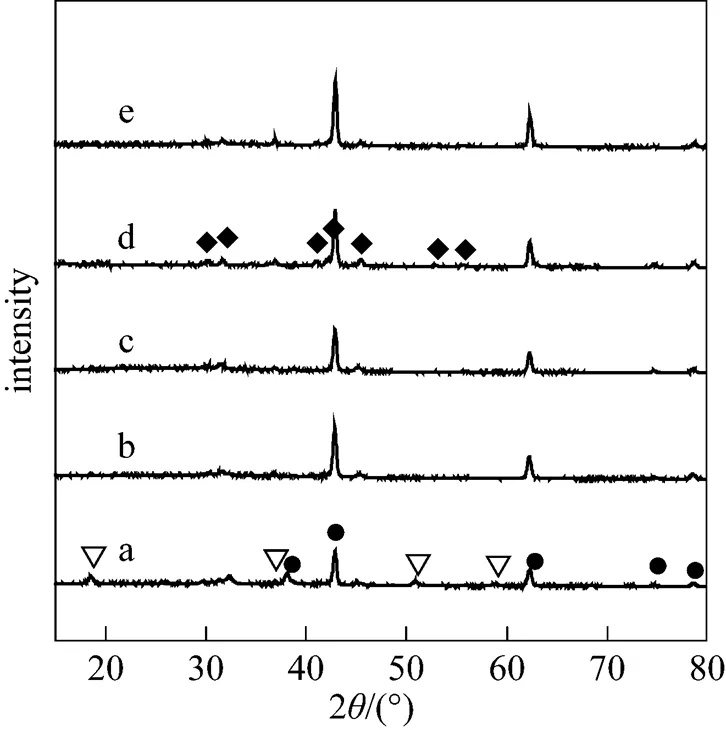
Figure 1 XRD patterns of KF/MgO calcined at different temperatures with KF mass loading amounts 30%
a—573 K; b—673 K; c—773 K; d—873 K; e—973 K
▽?Mg(OH)2; ◆?K2MgF4; ●?MgO


Figure 2 FTIR spectra of the KF/MgO calcined at different temperatures with KF mass loading amounts 30%
a—MgO; b—573 K; c—673 K; d—773 K; e—873 K; f—973 K
To make sure that K2MgF4+K2CO3is responsible for the higher DMC conversion and DDC selectivity, the KF/MgO catalyst was calcined under N2(99.99%) atmosphere and the activity was also listed in Table 1. Clearly the conversion of DMC of the catalyst calcined under N2was similar to that of the catalyst calcined in air. The selectivity of DDC of the catalyst calcined under N2is only 89.2%, obviously less than that of the catalyst calcined under air (95.0%). Evidently the formation of K2CO3when KF/MgO was calcined in air was in favor of the selectivity of DDC.
3.2 Effect of KF loading amounts on the reaction
Table 2 showed the catalytic properties of KF/MgO calcined at 873 K with different KF loadings for the transesteri?cation of DMC with dodecanol. Catalytic properties of KF/MgO were obviously better than that of MgO or KF only. The conversion of DMC and selectivity of DDC were improved greatly when KF loading on the carrier MgO. The conversion of DMC of KF/MgO was more than 84%. Both selectivity and yield of DDC first increased and then decreased with increasing KF loading amount. When KF mass loading amount was 30%, both selectivity and yield of DDC were maximum, and were 95% and 80%, respectively.
Figure 3 showed the XRD patterns of KF/MgO calcined at 873 K with different KF loadings. It can be seen that XRD patterns of KF/MgO contained MgO and K2MgF4. The intensity of the peaks corresponding to K2MgF4enhanced with increasing the loading amount of KF, which implied the increasing of KF loading amount would contribute to the increasing of K2MgF4. Fig. 4 showed FTIR spectra of the catalysts calcined at 873 K with different KF loadings. Clearly the bands of K2CO3can be found and the intensity of peaks increased with increasing the amount of KF loading. This implied that K2CO3was formed in KF/MgO and the amount of K2CO3increased with increasing the amount of KF loading. The high yield of DDC of KF/MgO further confirmed that the formation of K2MgF4+K2CO3was very important reason of excellent catalytic properties of KF/MgO catalysts. The yield of DDC first increased and then decreased with increasing the amount of K2MgF4+K2CO3, which implied other factors also affect the selectivity of DDC besides the amount of K2MgF4+K2CO3. Further investigation was in process.
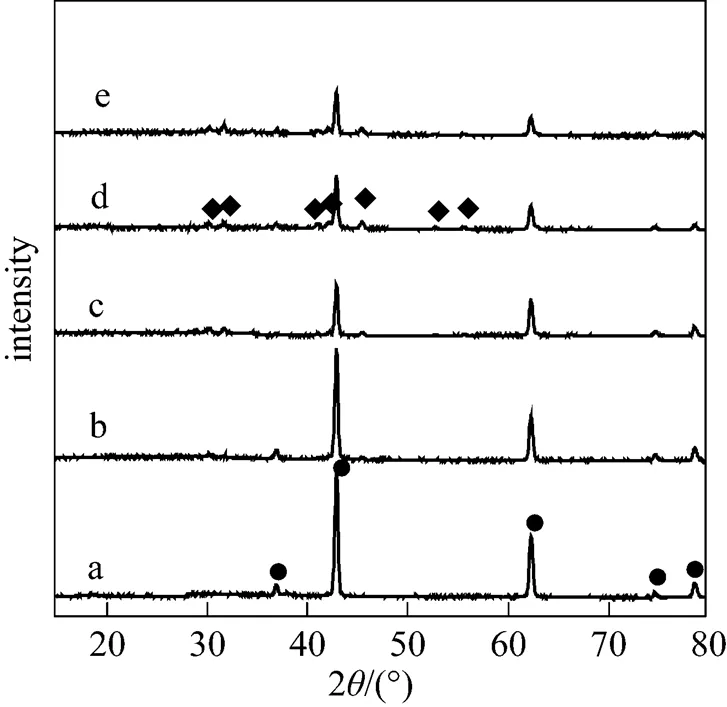
Figure 3 XRD patterns of KF/MgO with different KF loadings calcined at 873 K
a—MgO; b—10% KF; c—20% KF; d—30% KF; e—40% KF
◆?K2MgF4; ●?MgO
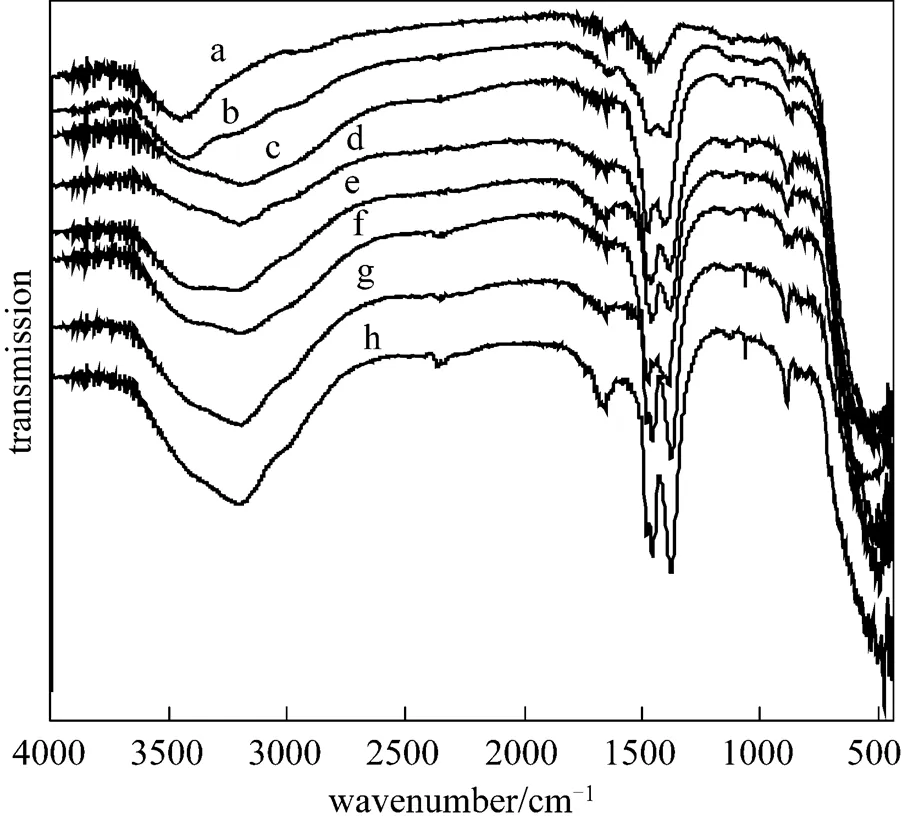
Figure 4 FT-IR spectra of KF/MgO with different KF loadings calcined at 873 K
a—MgO; b—10% KF; c—15% KF; d—20% KF; e—25% KF; f—30% KF; g—35% KF; h—40% KF
Qian and Wu. [12, 13] reported homogeneous catalyst K2CO3exhibited best catalytic activity for transesterification from dimethyl carbonate (DMC) and dodecanolamong the conventional Lewis alkali or acid. The catalytic properties of K2CO3were also listed in Table 2. The superiority of KF/MgO was more outstanding. The conversion of DMC using 30% (by mass) KF/MgO was obviously higher than that using K2CO3, while the selectivity of DDC using 30% (by mass) KF/MgO was similar to that using K2CO3. The higher the conversion of DMC using 30% (by mass) KF/MgO was mainly ascribed to better catalytic properties of K2MgF4. Furthermore, KF/MgO can be separated by simply filtering from the reaction system. However, part of K2CO3dissolved in the reaction process, and the remainder was difficultly separated. The re-usability of KF/MgO was so far better than that of K2CO3.
In conclusion, an efficient and friendly heterogeneous catalyst KF/MgO has been employed for transesterification of DMC with dodecyl alcohol. The 30% (by mass) KF/MgO calcined at 873 K exhibited much better catalytic properties than conventional homogeneous catalyst. Especially, the conversion of DMC was obviously improved without the loss of selectivity of DDC. Moreover, the decrease of DMC conversion was negligible when recovered catalyst was used. KF/MgO was promising to be used in the industrial product due to excellent catalytic properties and re-usability. The study on the mechanism of KF/MgO catalysis is in progress.
1 Shaikh, A.G., Sivaram, S., “Organic carbonates”,.., 96, 951-976 (1996).
2 Bruening, S., Ansmann, A., Gondek, H., “Emulsifier mixture”, U.S. Pat., 0234563 (2004).
3 Achim, A., Ulrich, I., Bruening, S., Bettina, J., “Wax-based compositions”, W.O. Pat., 03005981 (2003).
4 Gryglewicz, S., Oko, F.A., Gryglewicz, G., “Synthesis of modern synthetic oils based on dialkyl carbonates”,...., 42, 5007-5010 (2003).
5 Choppin, A.R., Rogers, J.W., “The preparation of di--butyl carbonate and-butyl chlorocarbonate”,...., 70, 2967 (1948).
6 Cavinato, G., Toniolo, L., “New aspects of the synthesis of dimethyl carbonatecarbonylation of methyl alcohol promoted by methoxycarbonyl complexes of palladium (II) ”,..., 444, C65-C66 (1993).
7 Doya, M., Kimizuka, K., Kanbara, Y., “Process for the production of dialkyl carbonate”, U.S. Pat., 5489702 (1996).
8 Wang, C., Wang, Y. , Yao, J., “Synthesis of long chain aliphatic carbonic esters by catalytic alcoholysis of urea”,, 20, 879-882 (2003). (in Chinese)
9 Shaikh, A.G., Sivaram, S., “Dialkyl and diaryl carbonates by carbonate interchange reaction with dimethyl carbonate”,...., 31, 1167-1170 (1992).
10 Kenar, J A., Knothe, G.H., Copes, A.L., “Synthesis and characterization of dialkyl carbonates prepared from mid-, long-chain, and guerbet alcohols”,...., 81, 285-291 (2004).
11 Koch, P., Rom, U., “Synthesis of higher alcohol carbonates and their use as synthetic lubricants”, Eur. Pat., 0089709 (1983).
12 Qian, J.H., Wang, Q.Y., Wang, G.Y., Suo, J.S., “Synthesis of didodecyl carbonate by catalytic transesterification”,, 23, 403-406 (2006). (in Chinese)
13 Wu, J.Q., Tian, H.S., Zhu, Y.F., “Synthesis of didodecyl carbonate by catalytic transesterification”,, 34, 12-15 (2007). (in Chinese)
14 Ando, T., Brown, S.J., Clark, J.H., Cork, D.G., Hanafusa, T., Ichihara, J., Miller, J.M., Robertson, M.S., “Alumina-supported fluoride reagents for organic synthesis: Optimisation of reagent preparation and elucidation of the active species”,...,., 8, 1133-1139 (1986).
15 Zhu, J.H., Chun, Y., Qin, Y., Xu, Q.H., “An investigation of KF modification to generate strong basic sites on NaY zeolite”,..., 24, 19-28 (1998).
2009-04-01,
2009-07-15.
the PetroChina Company Limited
** To whom correspondence should be addressed. E-mail: gywang@cioc.ac.cn
 Chinese Journal of Chemical Engineering2009年5期
Chinese Journal of Chemical Engineering2009年5期
- Chinese Journal of Chemical Engineering的其它文章
- Effect of Relative Humidity on Catalytic Combustion of Toluene over Copper Based Catalysts with Different Supports*
- Prediction of Critical Endpoints Based on the PR Equation of State*
- Kinetic Studies on Wheat Straw Hydrolysis to Levulinic Acid*
- Calculation of Transport Properties of CF4+Noble Gas Mixtures
- Synthesis of 4,4′-MDC in the Presence of Sulfonic Acid-functionalized Ionic Liquids*
- A New Study on Combustion Behavior of Pine Sawdust Characterized by the Weibull Distribution*
