香草醛縮氨基硫脲合Ni(II)配合物的合成及晶體結構
吳雪立,劉尚遠,史學芳
(天津師范大學化學學院,天津 300387)
香草醛縮氨基硫脲合Ni(II)配合物的合成及晶體結構
吳雪立,劉尚遠,史學芳
(天津師范大學化學學院,天津 300387)
利用X-射線單晶衍射技術測定了香草醛縮氨基硫脲合鎳(II)配合物甲醇溶劑化物Ni(C9H10N3O2S)2·4CH3OH)的單晶結構。測定結果表明,該晶體屬單斜晶系,C2/c空間群,晶胞參數分別為a=2.571 2(6),b=0.730 41(18),c=1.656 3(4)nm,β=104.212(4)°,V=3.015 4(13)nm3,Z=4.在晶體結構中,中心離子 Ni(II)與2個配體中的N、S原子形成四配位的平面四邊形結構,配體在與Ni(II)配位時,其結構發生硫酮式與硫醇式互變異構,最終以硫醇陰離子形式與Ni(II)配位,因而得到中性配位單元.相鄰配位單元通過N3-H3A…S1氫鍵形成一維鏈狀結構;甲醇分子既作為質子的受體又作為質子的給體,每個配位單元通過多重O-H…O、N-H…O氫鍵形成溶劑化物,生成沿ab平面的氫鍵二維結構;計算表明,包結在其中的甲醇客體構成一維孔道結構,占據晶格體積的35.4%.
鎳配合物;互變異構;孔道結構;氫鍵
The thiosemicarbazones have been detected to form comp lexesw ith variousmetal ions in the form of neutralor deprotonated ligands[1-2].M any metal comp lexes exhibit interesting biological behavior[3-5],in w hich both coordination bond and hydrogen bond play an important role.Containing additional-NH2,-OH,o r-SH(o r>C=S)groups at the substituent,the ligandsareof special interest[6].Vanillin thiosemicarbazone ligand coo rdinates in a bidentate fashion,resulting in a significant increase of stability of the comp lexes[7-9].W hile having plenty of active hydrogen bonding sites,the ligands act as both p roton accep tors and dono rs.This p roperty makes it be used as a hosttemp late to encapsulate solvent aggregates w ithin its crystal lattice to study solvent-induced p roperty.In this paper,a new comp lex is synthesized and structurally determ ined by X-ray diffraction analysis,[Ni(C9H10N3O2S)2]·4CH3OH,I,the porous crystal structureof Ni(II)comp lex w ith vanillic thiosemicarbazone ligand and methanol solvates assemblingviahydrogen bonds.The void space size of formed channels corresponds to 35.4%of the unit-cell volume,which depends on the type of substituents on phenyl group of ligand and the volume of the solvent.
1 EXPERIM ENTAL
1.1 Materialsand generalmethods
All chemical reagentswere commercially available and purified by standard methods p rior to use.M elting points were measured on a Yanagimoto M P-500 apparatus(uncorrected).Elemental analyses were perfo rmed on a Perkin-Elmer 2400 analyzer.
1.2 Preparations
The vanillin thiosemicarbazone ligand,L,w as synthesized acco rding to a literature method[10]by the reaction of vanillin and thiosemicarbazide in the yield of 75%,m.p.:196~198 ℃.Elemental analysis:Calcd.for C14H13N3O3(%):C,47.99;H,4.92;N,18.65;Found:C,47.81;H,4.95;N,18.23.
The comp lex Iw as p repared as follow s:A solution of Ni(Ac)2·4H2O(45.4 mg,0.2 mmol)in CH3OH(10 mL)w as added L(54.25 mg,0.2 mmol)in CH3OH(10 m L)w ith stirring fo r 0.5 hour.The green solution w as filtered.A fter one week,the green histogram single crystalswere obtained upon slow evapo ration of the solvent w ith a yield of 60%.Elemental analysis:Calcd.for C22H36N6NiO8S2(%):C,41.59;H,5.71;N,13.23;Found:C,41.45;H,5.52;N,13.49.
1.3 X-Ray crystallographic study
Single crystal X-Ray diffraction measurements were carried out on a Bruker Smart 1000 CCD diffractometer equipped with a graphite monochromatized MoKαradiation(λ=0.071 073 nm)w ithφandωscan mode at 294(2)K.Semi-empirical absorp tion corrections were app lied using SABABS p rogram.A ll structures w ere solved by direct method and successive difference Fourier syntheses(SHELXS-97),and refined by full-matrix leastsquares onF2w ith anisotropic thermal parameters for all non-hydrogen atom s(SHELXL-97)[11].Crystallographic data and experimental details for structural analysesare summarized in Table 1.The selected bond lengths and angles are in Table 2.

Table 1 Crystal data and structure refinement summary for complex I
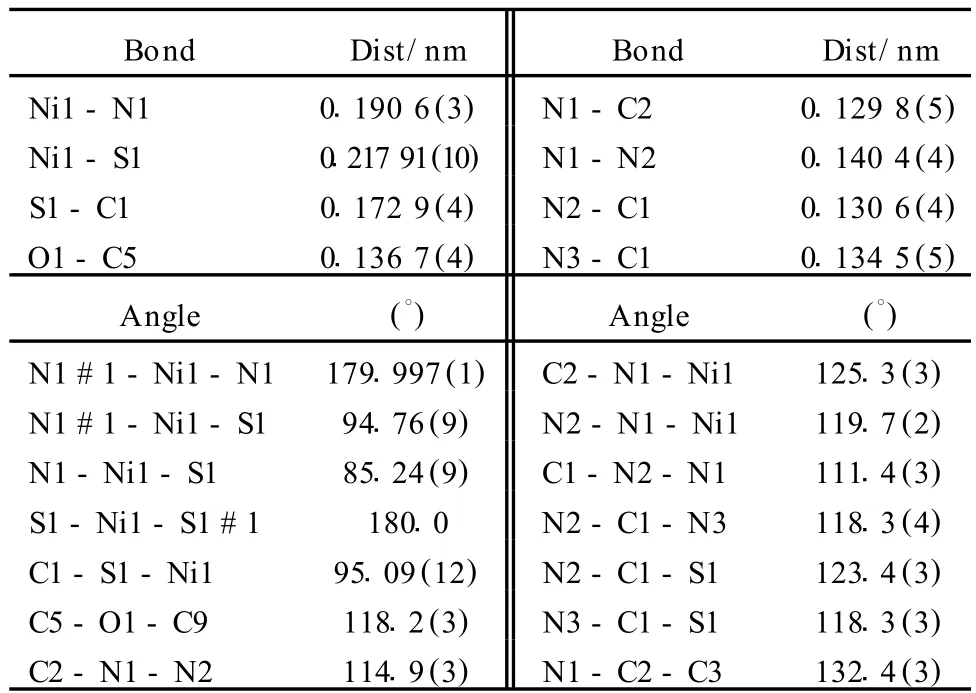
Table 2 Selected Bond Lengths(nm)and Bond Angles(°)for complex I
2 RESULTSAND D ISCUSSION
2.1 Bonding modes of L igand
Comp lex Iwasp repared by using vanillin thiosem icarbazone ligand with Ni(Ac)2·4H2O in methanol.In the solution,acting as strong alkaline the acetate anions had the ability of despoiling p roton from thione-thiol tautomers.As a result,the ligand bonded to nickel ion centre in the anionic form.
2.2 Descr iption of the complex I
The title compound[Ni(C9H10N3O2S)2]·4CH3OH,I,which isa neutral comp lex,crystallizes in a monoclinic system with space groupC2/c.The Ni(II)ion coordinates with two N atoms and two S atom s from the thiosemicarbazide groups of two vanillin thiosemicarbazone ligands,L,performing a normative quadrangle coordination environment.The coo rdination angles lie in the range of 85.24(9)~94.76(9)°.The two L coordinate to the Ni(II)ion in bidentatemode through N atoms and S atoms,and the Ni—N and Ni—S bond distances are 0.190 6(3)nm and 0.217 91(10)nm respectively.The structure of forming two N—N—C—Ni—S fivemembered chelated rings is show nin Fig.1.

Fig.1 Ball and stick drawing of[Ni(C9 H10 N3O2 S)2]·4CH3 OH
Having—NH2, —OH,and—SH groups at the substituent,the vanillin thiosemicarbazone ligand acts as po tential hydrogen bonding accep to r and dono r,not only betw een comp lexes but also between complex and solvent molecules.The X-ray diffraction result show s that each monoclinic unitcell contains fourmoleculesof the solvatemethanol to give a formula of[Ni(C9H10N3O2S)2]·4CH3OH.The hydrogen bonds parameters are listed in Table 3.

Table 3 Hydrogen bonds lengths(nm)and angles(°)for complex I
Firstly,the ligands from comp lexes p roduce double N3-H3A…S1 hydrogen bonds.The ge-ometry is:H3A…S1=0.264 8(12)nm,N 3…S1=0.351 6(4)nm and N 3-H3A …S1=166(3)°,respectively,w ith symmetry code:-x+2,y,-z+5/2.The comp lexes arrange side by side in o rder to allow this hydrogen bond to conform.Furthermore,two neighbo ring moleculesof L forme a centrosymmetric R22(8)dimer in AD DA(A:hydrogen bond accep tor,D:hydrogen bond donor)fashion through intermolecular N—H…S hydrogen bonds show n in Fig.2.There is a N3-H3B…O2 hydrogen bond between the oxygen atom of the phenol and the hydrogen atoms of amidogen from neighboring ligands.The geometry of the N 3-H3B…O2 is:H3B…O2=0.209 2(15)nm,N3…O2=0.296 5(5)nm and N3-H3B …O2=166(3)°,respectively,with symmetry code:-x+3/2,-y+1/2,-z+2.

Fig.2 Hydrogen-bonded 1D chains of the complex,form ing by R22(8)dimer through intermolecular N-H…S hydrogen bonds
Secondly,four methanol guest molecules act as hydrogen bridges betw een the comp lex molecules and two symmetry-related methanol molecules form a two dimensional arrangement.The hydrogen bonds between the molecules lead to a 3-D microporous host latteic with 1-D channels,in which the guest methanol molecules are located.The void space of this host was computed using the PLA TON program,which corresponds to 35.4%of the unit-cell volume,as a matter of fact,the methanol solvents are accommodated in the voids(Fig.3).

Fig.3 Packing diagram of the crystal from ab plane,all the methanol guest have been removed for viewing them icroporous lattice clearly.
In summary,a new porous crystal structure of Ni(II)comp lex was p repared and structurally characterized.The ligands take p lace thione-thiol tautomerization w hile coordinating to Ni(II)center as fashion of anionic fo rm.In the crystal,the neighbo ring ligands produce hydrogen-bonded interaction forming R22(8) rings conformation.Comp lex Iis arranged through the N-H…S,NH…O and O-H…O hydrogen bonds into a 2D framework.The ligands act as either p roton acceptor or donor encapsulated methanol solvent aggregates w ithin its crystal lattice,resulting in a po rous crystal structure of Ni(II)comp lex,the computation of the size of formed channels corresponds to 35.4%of the unit-cell volume,w hich depends on the type of substituents on phenyl group of ligand and the volume of the solvent.
2.3 Thermal analysis
The thermal analysis curve(TG)of the title comp lex(Fig.4)show that there exist three obvious weight-loss steps.The first weight loss begins at 80℃that is attributed to the loss of four uncoordinated methanol molecules (measured value 20.9%,calculated value 20.2%),accompanying two endotherm ic peaks.W ith increasing temperature,the decomposition continues.DTG curve at 520℃hasa strong absorp tion peak,and TG curve in the region 265~555 ℃exists weight loss,w ith rate of 59.08%,thatmay corresponds to the Ni—N and the S—C bond breaking and ligand decomposition(theoretical value 60.78%),remnants of this p rocess is NiS2.Upon subsequent heating,the weight loss of other coo rdinated parts is observed.The final residue may be NiS(measured value 14.4%,calculated value 14.3%).

Fig.4 TG/DTG diagram of the title com plex.
3 CONCLUSION
In this paper,a new comp lex is synthesized from Ni(Ac)2and the ligand of Schiff base.A single crystal isobtained and determined by X-ray diffraction(Fig.1).The result shows that the Ni(II)is four-coordinated with two Natom sand two Satom s from two vanillin thiosemicarbazone ligands giving a quadrangle coo rdination structure.With thione-thiol tautomerization taking place,the ligands coo rdinate to Ni(II)center in the anionic fo rm.Having p lenty of active hydrogenbonding sites,the ligands act as either p roton accep tor or donor encapsulated methanol solvent aggregates w ithin its crystal lattice by hydrogen bonds.Resulting in a porous crystal structure of Ni(II)comp lex,the size of formed channels corresponds to 35.4%of the unit-cell volume,which depends on the type of substituents on phenyl group of ligand and the volume of the solvent.
[1] Lobana T S,Sharma R,Bawa G,et al.Bonding and structure trends of thiosemicarbazone derivatives of metals-An overview[J].Coo rd Chem Rev,2009,253(7-8):977-1055.
[2] Casas J S,Casta?o M V,Castellano E E,et al.Metal-induced cyclization of thiosemicarbazones derived fromβ-keto amides andβ-keto esters:Open-chain and cyclized ligands in zinc(II)comp lexes[J].Inorg Chem,2002,41(6):1550-1557.
[3] Lobana T S,Khanna,S,Butcher R J,et al.Synthesis,crystal structures and multinuclear NMR spectroscopy of copper(I)complexes with benzophenone thiosemicarbazone[J].Polyhedron,2006,25(14):2755-2763.
[4] Kasuga N C,Sekino K,Ishikawa M,et al.Synthesis,structural characterization and antimicrobial activities of 12 zinc(II)complexes with four thiosemicarbazone and two semicarbazone ligands[J].J Inorg Biochem,2003,96(2-3):298-310.
[5] Ferrari M B,Bisceglie F,Fava G G,et al.Synthesis,characterization and biological activity of two new polymeric copper(II)complexes withα-ketoglutaric acid thiosemicarbazone[J].J Inorg Biochem,2002,89(1-2):36-44.
[6] Santos IG,Abram U,Alberto R,et al.Tricarbonylrhenium(I) complexes with thiosemicarbazone derivatives of 2-acetylpyridine and 2-pyridine formamide showing two unusual coordination modesof tridentate thiosemicarbazone ligands[J].Ino rg Chem,2004,43(6):1834-1836.
[7] Gatto C C,Lang E S,Kupfer A,et al.Mono-,di-and trinuclear dioxo complexes of uranium containing hydrazonato and azomethine ligands[J].Z Anorg Allg Chem,2004,630(8-9):1286-1295.
[8] Ortner K,Abram U.Reactions of dichloro[2-(dimethylaminomethyl)phenyl-C1,N]gold(III),[Au(damp-C1N)Cl2],with aromatic thiosemicar bazones.Structures and spectroscopical data of the first gold(III)thiosemicarbazone complexes[J].Inorg Chem Commun,1998,1(7):251-253.
[9] Akinchan N T,Abram U.Bis(4-hydroxy-3-methoxybenzaldehyde thiosemicarbazonato-N1,S)nickel(II)tetraethanol solvate[J].Acta Crystallogr Sect C:Cryst Struct Commun,2000,56(5):549-550.
[10] Abram U,Ortner K,Gust R,et al.Gold complexes with thiosemicarbazones:reactions of bi-and tridentate thiosemicarbazones with dichloro[2-(dimethylaminomethyl)phenyl-C1,N]gold(III),[Au(damp-C1,N)Cl2][J].J Chem Soc,Dalton Trans.2000,5:735-744.
[11] Sheldrick G M.SHELXTL V 5 Reference Manual,Siemens Analytical X-ray Sysytems Inc[M].USA Madison WisPdnsin,1997.
[12] Zhao J R,Sun P P.Crystal structure and characterization of thiosemicarbazone nickel(II)sulfate water containing complex[J].J Qingdao Univer Sci&Techn,2004,25(3):17-21.
A porous crystal of Ni(II)com plex with van illic thiosem icarbazone ligandsand methanol solvate assembling via hydrogen bonds
WU Xueli,L IU Shangyuan,SH I Xuefang
(College of Chemistry,Tianjin Normal University,Tianjin 300387,China)
The title compound,[Ni(C9H10N3O2S)2]·4CH3OH is synthesized and characterized by X-ray diffraction analysis.The crystal ismonoclinic space groupC2/cwitha=2.571 2(6),b=0.730 41(18),c=1.656 3(4)nm,β=104.212(4)°,V=3.015 4(13)nm3,Z=4.The results show that the Ni(II)is four-coo rdinated w ith two N atom s and two S atom s from two vanillin thiosemicarbazone ligands giving a quadrangle coordination structure.With thione-thiol tautomerization taking p lace,the ligands coo rdinate to Ni(II)center in the anionic form.Having p lenty of active hydrogenbonding sites,the ligands act as either proton acceptor or donor encapsulated methanol solvent aggregates within crystal lattice by hydrogen bonds.Resulting in a po rous crystal structure of Ni(II)comp lex,the size of fo rmed channels corresponds to 35.4%of the unit-cell volume,w hich dependson the type of substituentson phenyl group of ligand and the volume of the solvent.
nickel comp lex;tautomerization;porous;hydrogen bond
date:2009-11-21
WU Xueli(1984—),Femaie,MA candidate,majoring in organic synthesis.
SH IXuefang(1961—),Female,p rofessor,majoring in heterocyclic chem istry and organic materials chemistry.
O641
A
1671-1114(2010)03-0064-05
E-mail:xuefangshi@126.com
(責任編校 李宏偉)
- 天津師范大學學報(自然科學版)的其它文章
- A r/N2氣體比例對ZrN/W TiN納米多層膜的影響

