Caffeine Crystallization Induction Time Measurements Using Laser Scattering Technique and Correlation to Surface Tension in Water and Ethanol
HAN Jiabin (韓佳賓)* and WANG Jingkang (王靜康)
1 School of Chemical Engineering & Technology, Tianjin University, Tianjin 300072, China
2 Earth and Environmental Science Division, Los Alamos National Laboratory, Los Alamos, New Mexico, USA
1 INTRODUCTION
Crystallization is an important manipulation in industrial chemistry, where it is used extensively in purification and separation processes [1]. The kinetics of crystal nucleation and growth is an important parameter for batch crystallization processes. Crystallization kinetics is typically parameterized by the onset time required for measurable crystal nucleation and growth to occur, which is also known as the induction time. The induction time is dependent on a number of parameters including temperature, concentration, solvent, and stirring rate/fluid dynamics [2].
Caffeine is widely used as alternative medicine of addictive drugs for medical purposes [3]. Caffeine crystallization thermodynamics (solubility [4], super-saturation and meta-stable zone [5]) and kinetics(induction time [6, 7],etc.) are crucial factors in design and optimization of batch preparation of this compound. This paper focuses on measuring caffeine induction times in water and ethanol solvents at 30 °C and 40 °C using laser light scattering [8]. Surface tension was calculated from this data based on Mullin’s model [9]. This is an important parameter to solvent selection for crystallization operations. Low surface tension facilities nucleation.
2 EXPERIMENTAL
The experiment setup for induction time measurements of caffeine crystallization is depicted in Fig. 1.This setup is composed of crystallization, laser recording, stirring and heating systems.
Caffeine crystallization induction times were measured at 30 °C and 40 °C in stagnant water and ethanol solvents. The supersaturation defined by the ratio of the experimental solution concentration under the given conditions to its saturated concentration of caffeine in both solvents was controlled between 1 and 4.
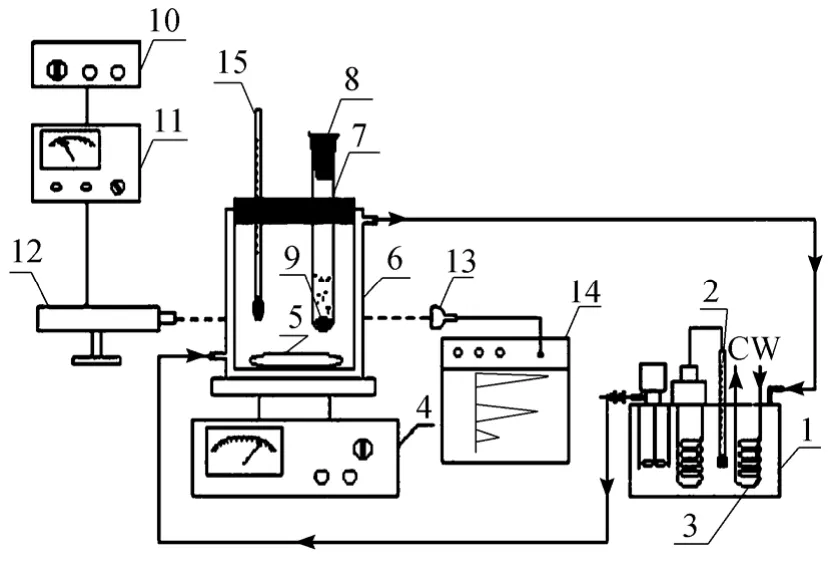
Figure 1 Setup for caffeine crystallization induction time measurement1—water bath; 2—thermometer; 3—cooling coil; 4—magnetic motor control; 5—magnetic stirring bar; 6—glass jacket kettle; 7—glass tube; 8—rubber stopper; 9—magnetic stirring bar; 10—electricity power control; 11—laser power control;12—laser generator; 13—laser receiver; 14—signal recorder;15—thermometer
The temperature was held at the desired value with fluctuations of ±1 °C. The saturated caffeine solution was initially prepared as a hot solution. Supersaturation 1-4 in the experimental solution was achieved by cooling the solution to either at 30 °C or 40 °C by immediately bathing the hot solution in the jacket kettle. And this starting point is recorded as time=0. The supersaturated solution was initially transparent. Solution transparency changes that occurred as the solid phase precipitated were measured by the decrease in laser intensity at the detector. The onset of crystal nucleation and growth was determined from the inflection point in the time dependent laser intensity. Kinetically speaking, there should be no incubation period necessary for new solid phase (precipitates) appearing from homogeneous liquid phase(solution) since these phases are in dynamic equilibrium.Due to the limited sensitivity of optical scattering techniques, such as that used here, the solid phase can only be observed when enough precipitation has accumulated to result in a measurable effect. Thus, the onset time to achieve the minimum detection limit(e.g., the inflection of the time dependent laser intensity) is related to the rate of accumulation of precipitate in the solution, and is directly to the surface tension of the crystallizations.
3 RESULTS AND DISCUSSION
3.1 Induction time measurements
Induction times for caffeine crystallization in water and ethanol are depicted in Fig. 2 at 30 °C.The induction time in ethanol is less than that measured in water. This result indicates that caffeine nucleation is easier in ethanol than in water.
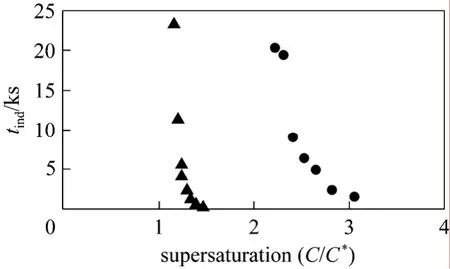
Figure 2 Nucleation induction time vs. supersaturation in water and ethanol at 30 °C● water; ▲ ethanol
The induction time in water for caffeine varies between 0.5 h and 5.7 h at supersaturations of 3.1 and 2.2, respectively, while in ethanol it varies between 3 min and 6.5 h at supersaturations of 1.5 and 1.1, respectively. Giftet al. [10] measured the caffeine induction time to be 8 min in water at 25 °C with a calculated supersaturation of ~6. Extrapolation of the measured data in this paper to 30 °C and a caffeine supersaturation of 6 gives reasonable agreement with this value, yielding an induction time of 4 min. Considering that the induction time can be longer at lower temperature,i.e. 25 °C, the data in this paper are in agreement with previous literature.
3.2 Determination of surface tension
Surface tension between solid and liquid phases can be obtained by crystallization induction time measurements. The homogeneous nucleation rate can be calculated from the classic nucleation theories [7, 9, 11]:
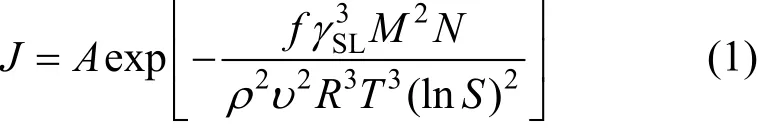
whereAis a constant,
fis the shape factor,γSLis the tension of solid-liquid phase,Mis molecular weight,
Nis the Avogadro constant, 6.02×1023mol-1,ρis the crystal density,
υis the ion number every mol matter,
Ris gas constant with the value of 8.314 J?mol-1?K-1,
Tis the absolute temperature,Sis the ratio of supersaturation.
The nucleation rate is inversely proportional with induction time as expressed below [7, 9]:

whereKis a constant andtindis induction time.
Combining Eqs. (1) and (2), one can obtain [7, 9]:

whereBis a constant equal toK/A.
From the theoretical framework expressed above,ln(tind) of caffeine is linearly related to (lnS)-2as shown in Fig. 3 at 30 °C.
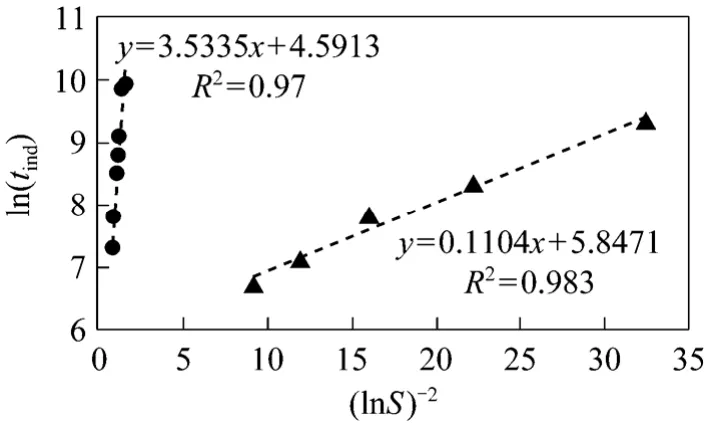
Figure 3 ln(tind) plotted vs. (lnS)-2 for caffeine in water and ethanol at 30 °C(The data in Fig. 3 is calculated based on the data in Fig. 2)● water; ▲ ethanol
As depicted in Fig. 3, the data for induction times in water and ethanol can be linearly fit using the formula derived from Eq. ( 3) as illustrated below:
In water solvent:
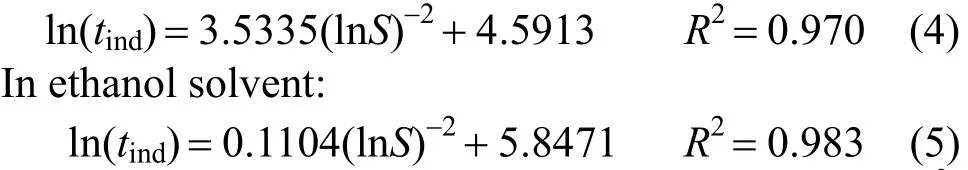
The density of caffeine,ρ=1308.665 kg·m-3,was measured using the volume difference method.The other required parameters are known:ν=1,R=8.314 kJ·kmol-1?K-1,T=303.15 K,f=0.0235,M=212.21 kg·kmol-1, andN=6.023×1026kmol-1. Surface tensionγSLW(water-caffeine interface) andγSLE(ethanol-caffeine interface) can then be calculated:
γSLW=5.337×10-5kJ·m-2andγSLE=1.681×10-5kJ·m-2.
3.3 Temperature and supersaturation effects on induction time
The effects of supersaturation and temperature on the induction times for caffeine crystallization in water and ethanol are demonstrated in Fig. 2. The induction time decreased as the supersaturation ratio in water and ethanol solvents increased. This effect observed in the ethanol system is shown in Fig. 4 at both 30 °C and 40 °C. A shorter nucleation induction time was observed at higher temperature. The surface tension for caffeine-ethanol at 40 °C was calculated to be 1.038×10-5kJ·m-2, which is smaller than the value at 30 °C (determined above). This result indicates that the overall energy barrier for nucleation is lower at higher temperature.
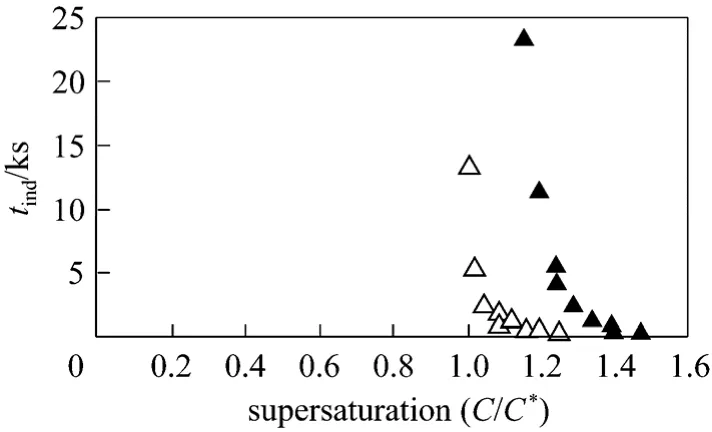
Figure 4 Comparison of nucleation induction time vs.supersaturation in ethanol solutions at 40 °C▲ 30 °C; △ 40 °C
4 CONCLUSIONS
Caffeine crystallization induction times were measured between supersaturation ratios of 1 to 4 in the temperature range of 30-40 °C in both water and ethanol. Surface tensions were calculated based on induction time measurements. The induction time decreased with increasing supersaturation ratio and temperature, with overall shorter induction times observed in ethanol solution. The surface tension decreased with increasing temperature and a smaller surface tension is observed in ethanol than in water.This indicates that ethanol can be an alternative solvent to water if crystal nucleation is important step during crystallization.
ACKNOWLEDGEMENTS
The authors would like to thank the anonymous reviewers and the editor for their insight comments and suggestions.The authors thank Dr.Jonathan L.Cape at Los Alamos National Laboratory for his kind editorial help.Some of the valuable sentences from reviewer are incorporated in the paper and should be acknowledged.
1 Garside, J., “Industrial crystallization from solution”,Chem.Eng.Sci., 40, 3-20 (1985).
2 Hua, H., Halea, T., Yanga, X., Wilsonb, L.J., “A spectrophotometer based method for crystallization induction time period measurement”,Journal of Crystal Growth, 232, 86-92 (2001).
3 Han, J., Wang, J.K., “The measurement and correlation of the solubility of caffeine in water and ethanol”,J.Chem.Ind.Eng., 55 (1),125-128 (2004).
4 Han, J., Chen, J., Liu, X., Wang, J.K., “The model of the solubility of caffeine based on the artificial neural networks”,Chem.Ind.Times, 17 (4), 26-28 (2003).
5 Han, J., Wang, J.K., “The metastable region of caffeine in water and ethanol”,Journal of Tianjin University:Science and Technology, 36(6), 765-768 (2003).
6 Byrappa, K., Ohachi, T., Crystal Growth Technology, William Andrew Inc., Norwich, New York (2003).
7 Richardson, J.F., Harke, J.H., Backhurst, J.R., Coulson and Richardson’s Chemical Engineering, Volume 2 (5th edition): Particle Technology and Separation Processes, Butterworth-Heinemann,Oxford (2002).
8 Kashchiev, D., Verdoes, D., Van Rosmalen, G.M., “Induction time and metastability limit in new phase formation”,Journal of Crystal Growth, 110 (3), 373-380 (1991).
9 Mullin, J.W., Crystallization, 4th edition, Butterworth-Heinemann,Oxford (2001).
10 Gift, A.D., Luner, P.E., Luedeman, L., Taylor, L.S., “Influence of polymeric excipients on crystal hydrate formation kinetics in aqueous slurries”,Journal of Pharmaceutical Sciences, 97 (12), 5198-5211(2008).
11 Yang, C.F., Xu, D.Q., Shen, Z.Q., “Theoritical analysis and experimental study of the induction period of calcium carbonate scaling”,J.Chem.Ind.Eng., 45 (2), 199-205 (1994).
 Chinese Journal of Chemical Engineering2010年5期
Chinese Journal of Chemical Engineering2010年5期
- Chinese Journal of Chemical Engineering的其它文章
- Synthesis of Petroleum Sulfonate Surfactant by Different Sulfonating Agent with Application of HIGEE Technology*
- Coupled Reaction/Distillation Process for Hydrolysis of Methyl Acetate*
- On-line Measurement for Ohmic Resistance in Direct Methanol Fuel Cell by Current Interruption Method*
