Dysadherin基因沉默對胰腺癌細胞侵襲移行能力的影響
杜冀暉 張厚德 高燕 謝宏民
·論著·
Dysadherin基因沉默對胰腺癌細胞侵襲移行能力的影響
杜冀暉 張厚德 高燕 謝宏民
目的探討靶向封閉dysadherin基因對胰腺癌細胞PANC1、BxPC3體外侵襲移行能力的影響。方法應用脂質體轉染方法將小分子RNA(siRNA)轉染細胞。實驗分為dysadherin-siRNA轉染(dysa)組、陰性對照siRNA轉染(HK)組、脂質體對照(對照)組、。采用RT-PCR、免疫組化方法檢測轉染細胞的dysadherin mRNA及蛋白表達;采用Transwell侵襲小室檢測轉染細胞體外侵襲移行能力。結果轉染dysadherin-siRNA(5 nmol/L)的PANC1和BxPC3細胞的dysadherin mRNA表達較HK組細胞分別下降95.4%、52.1%(P<0.05);dysadherin蛋白表達亦分別降低91.2%、83.6%(P<0.01)。PANC1細胞的對照組、HK組和dysa組穿膜細胞數分別為163.2±15.5、154.4±17.3和53.6±7.9;BxPC3細胞的對照組、HK組和dysa組穿膜細胞數分別為30.7±3.2、27.5±2.8和4.7±2.4。dysa組顯著低于HK組和對照組(P值均<0.01)。結論應用RNA干擾技術沉默人胰腺癌細胞株PANC1、BxPC3的dysadherin基因可使細胞的侵襲移行能力下降。
胰腺腫瘤; RNA干擾; 癌,移行細胞; Dysadherin
近年來的研究表明,癌相關細胞膜糖蛋白dysadherin在腫瘤細胞的過表達能降低細胞間黏附、促進細胞運動和轉移[1-2]。本研究應用RNA干擾技術沉默人胰腺癌細胞株PANC1、BxPC3的dysadherin基因,探討其對胰腺癌細胞侵襲移行能力的影響,為臨床應用提供實驗依據。
材料與方法
一、實驗分組
胰腺癌細胞株PANC1、BxPC3 購自中國科學院上海生科院細胞資源中心,常規培養傳代后收集對數生長期PANC1和BxPC細胞,分別接種于6孔板,每孔(0.8~3.0)×105個細胞,分為脂質體對照組、陰性對照siRNA轉染(HK)組和dysadherin-siRNA轉染(dysa)組。當培養細胞達到50%~80%融合時,HK組和dysa組分別應用脂質體轉染HK siRNA和dysadherin 141 siRNA,dysadherin 141 siRNA(序列為GCGCCTGCACCCCGGAGCG)及通用陰性對照HK siRNA(序列為GACTTCATAAGGCGCATGC)由武漢晶賽生物工程技術有限公司提供。對照組僅加脂質體。每組設3個復孔。轉染后繼續培養24 h,收集各組細胞。
二、RT-PCR 檢測細胞dysadherin mRNA表達
采用Trizol試劑抽提各組細胞總RNA,常規進行RT-PCR。dysadherin正義引物為5′-CCTGTGTCTTCTCACCATCG-3′,反義引物為5′-AGGAGGTTGTCAGCTCCTGT-3′,擴增產物為551 bp;內參GAPDH正義引物為5′-AACGTGTCAGTGGTGGACCT-3′,反義引物為5′-AGGGGAGATTCAGTGTGGTG-3′, 擴增產物為400 bp。PCR擴增條件:94℃ 3 min,94℃ 45 s、54℃ 45 s、72℃ 1 min,30個循環,最后72℃ 10 min。產物經瓊脂糖凝膠電泳分離,采用凝膠成像系統(法國Vilber Lourma Bio-vision1000)測定條帶灰度。以目的條帶與GAPDH灰度值之比作為dysadherin mRNA相對表達量,表達抑制率=(1- 轉染組表達量/ 對照組表達量)×100%。
三、免疫組化檢測細胞dysadherin蛋白表達
制備細胞爬片,同上述方法轉染細胞,繼續培養24 h后,進行免疫組化檢測。由病理科醫師在高倍鏡下隨機觀察5個視野,計數細胞并對每個細胞進行評分。細胞不著色為0分,淡黃色為1分,棕黃色為2分,深棕黃色為3分。將評分之和除以細胞數作為dysadherin蛋白表達強度,表達抑制率= (1-轉染組表達強度/對照組表達強度) ×100%。
四、Transwell轉移小室測定細胞侵襲移行能力
將Transwell小室放入24孔培養板中,上室加300 μl預溫的無血清培養基,室溫下靜置1 h使基質膠再水化,吸去剩余培養液,分別接種300 μl用無血清培養基制備的各組細胞懸液[(0.5~1.0)×106/ml],下室加500 μl含有20%胎牛血清的培養基,常規培養24 h后用棉頭拭子擦去基質和聚碳酸酯膜上方的細胞,加入500 μl結晶紫染液浸泡小室20 min,用蒸餾水洗去多余染液,晾干。在顯微鏡下隨機觀察10個視野,計數每個視野內穿過膜的細胞數,表示腫瘤細胞的侵襲移行能力。
五、統計學處理
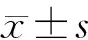
結 果
一、腫瘤細胞dysadherin mRNA及蛋白的表達
PANC1的dysa組和HK組dysadherin mRNA表達量分別為8.5和183,BxPC分別為102和213,dysa組兩株細胞dysadherin mRNA表達抑制率分別為95.4%、52.1%(P<0.05)。兩細胞對照組的dysadherin mRNA表達量分別為187和213,與HK組無顯著性差異(圖1)。
PANC1和BxPC3的dysa組的dysadherin蛋白表達均較HK組明顯降低,蛋白表達抑制率分別為91.2%、83.6%(P<0.01);HK組與對照組比較,其抑制率僅為1.8%、0.7% (圖2)。
二、轉染細胞體外侵襲移行能力的變化
dysa組穿過膜的PANC1、BxPC3細胞明顯減少(圖3)。PANC1的對照組、HK組和dysa組穿膜細胞數分別為163.2±15.5、154.4±17.3和53.6±7.9;BxPC3分別為30.7±3.2、27.5±2.8和4.7±2.4。dysa組顯著低于HK組和對照組(P值均<0.01)。
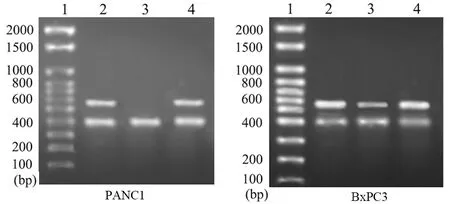
l.DNA marker;2.對照組;3.dysa組;4.HK組
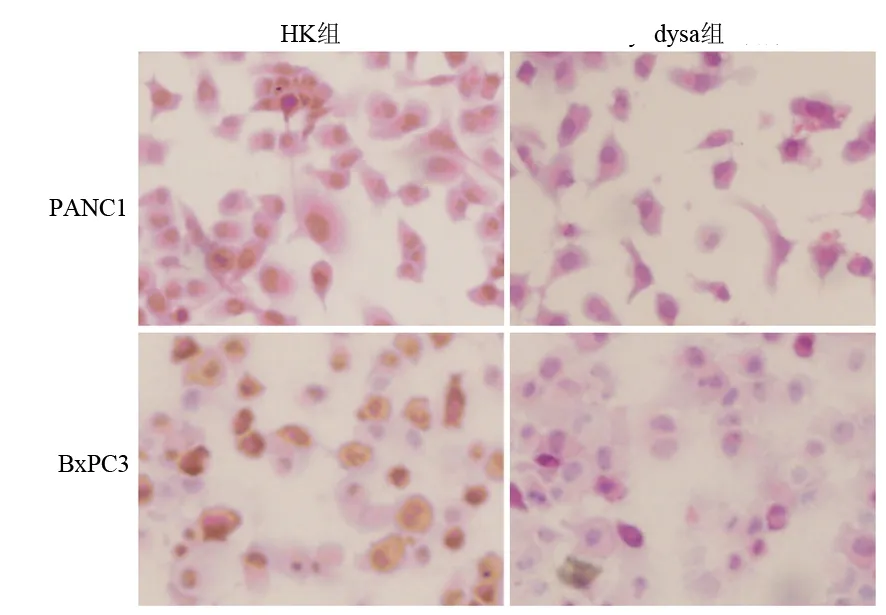
圖2各組細胞dysadherin蛋白表達的變化(免疫組化 ×400)
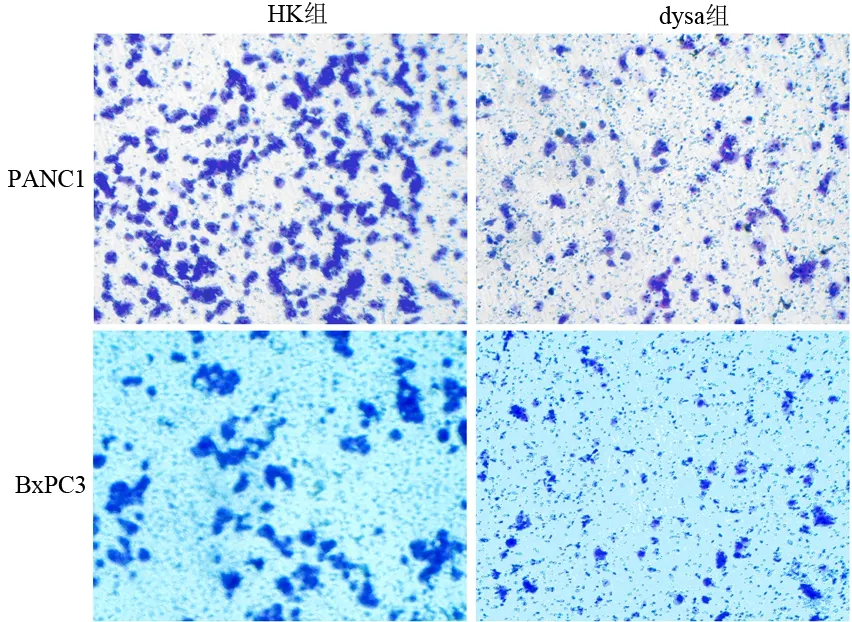
圖3 兩組細胞的穿膜細胞量( ×100)
討 論
Dysadherin是位于細胞膜的一種糖蛋白,是一種腫瘤相關性抗原[3]。近年研究發現,dysadherin可能在癌細胞的浸潤及轉移方面發揮重要作用。Dysadherin主要表達于包括胰腺癌在內的各種不同類型的腫瘤細胞,僅在少數幾種正常人體細胞表達[4-6]。Shimamara等[4]報道,dysadherin的表達與胰腺癌的遠處轉移及不良預后明顯相關,且表達dysadherin的胰腺癌細胞在癌巢中所占比例與胰腺癌的組織病理分級明顯相關。Ino等[1]報道,dysadherin在人胰腺癌細胞株Capan-1和HPAF-Ⅱ低表達,在PANC1和Miapaca-2中等量表達,在Mpanc-96和BxPC3高表達。將轉染dysadherin基因的Capan-1細胞種植于裸鼠胰腺,35 d后裸鼠肝轉移明顯較對照組增多。
PANC1、BxPC3細胞dysadherin基因沉默后,通過Transwell侵襲小室的細胞數明顯減少,提示下調dysadherin的表達能明顯抑制胰腺癌細胞的侵襲能力,表明dysadherin 表達可能與胰腺癌細胞的侵襲能力有一定的相關性,與Ino等[1]的研究相似。
腫瘤發生侵襲轉移是多因素作用的結果,包括促進細胞運動及侵襲細胞外基質。研究表明,dysadherin可通過抑制E-cadherin介導的細胞間黏附等多種機制降低細胞間黏附、促進細胞運動和轉移及腫瘤進展[1,4,7]。此外,dysadherin陽性細胞分泌趨化因子配體CCL2也可能是dysadherin促進腫瘤發生、發展和轉移的機制之一[8]。因此,有關以dysadherin為靶點的基因治療在胰腺癌中的應用及其機制尚需進一步地深入研究。
[1] Ino Y,Gotoh M,Sakamoto M,et al.Dysadherin,a cancer-associated cell membrane glycoprotein,down-regulates E-cadherin and promotes metastasis.Proc Natl Acad Sci USA,2002,99:365-370.
[2] Shimamura T,Yasuda J,Ino Y,et al.Dysadherin expression facilitates cell motility and metastatic potential of human pancreatic cancer cells.Cancer Res,2004,64:6989-6995.
[3] Tsuiji H,Takasaki S,Sakamoto M,et al.Aberrant O-glycosylation inhibits stable expression of dysadherin,a carcinoma-associated antigen,and facilitates cell-cell adhesion.Glycobiology,2003,13:521-527.
[4] Shimamura T,Sakamoto M,Ino Y,et al.Dysadherin overexpression in pancreatic ductal adenocarcinoma reflects tumor aggressiveness: relationship to e-cadherin expression.J Clin Oncol,2003,21:659-667.
[5] Shimada Y,Yamasaki S,Hashimoto Y,et al.Clinical significance of dysadherin expression in gastric cancer patients.Clin Cancer Res,2004,10:2818-2823.
[6] Kyzas PA,Stefanou D,Batistatou A,et al.Dysadherin expression in head and neck squamous cell carcinoma:association with lymphangiogenesis and prognostic significance.Am J Surg Pathol,2006,30:185-193.
[7] Sato H,Ino Y,Miura A,et al.Dysadherin: expression and clinical significance in thyroid carcinoma.J Clin Endocrinol Metab,2003,88:4407-4412.
[8] Nam JS,Kang MJ,Suchar AM,et al.Chemokine (C-C motif) ligand 2 mediates the prometastatic effect of dysadherin in human breast cancer cells.Cancer Res,2006,66:7176-7184.
2009-05-08)
(本文編輯:屠振興)
EffectofdysadheringenesilencingmediatedbyRNAinterferenceonmetastasisandinvasionofpancreaticcancercells
DUJi-hui,ZHANGHou-de,GAOYan,XIEHong-min.
CentralLaboratory,NanshanHospital,GuangdongMedicalCollege,Shenzhen518052,China
ZHANGHou-de,Email:szkjk@126.com
ObjectiveTo investigate the effect of dysadherin gene silencing on metastasis and invasion in pancreatic cancer cell line PANC1, BxPC3 in vitro.MethodsPANC1 and BxPC3 cells were divided into dysa group, negative siRNA control group (HK), liposomes control group(control). dysa group and HK group were tranfected by dysadherin siRNA and Negative siRNA, respectively.The expression of dysadherin mRNA and protein were detected by RT-PCR and immunohistochemical method. Transwell test was used to evaluate the invasion ability of pancreatic cancer cells.ResultsAfter transfected by dysadherin siRNA, the dysadherin mRNA levels in PANC1 and Bxpc3 cells were decreased by 95.4% and 52.1%. The expression of dysadherin protein was also down-regulated by 91.2% and 83.6%, respectively, when compared with HK groups (P<0.05). The numbers of invasive cells migrated in Transwell in PANC1 cells control group, HK group and dysa group were 163.2±15.5, 154.4±17.3 and 53.6±7.9; the numbers of invasive cells in BxPC cells control group, HK group and dysa group were 30.7±3.2, 27.5±2.8 and 4.7±2.4, respectively. The numbers in dysa group were significantly lower than those of HK group and control group (P<0.01).ConclusionsSilencing the dysadherin gene of PANC1, BxPC3 by RNA interference could significantly inhibit the invasive and migratory ability of canceroas cells.
Pancreatic neoplasms; RNA interference; Carcinoma,transitional cell; Dysadherin
10.3760/cma.j.issn.1674-1935.2010.01.009
深圳市科技計劃資助項目(JH200505260369A)
518052 深圳,廣東醫學院附屬南山醫院中心實驗室(杜冀暉),消化內科(張厚德、高燕、謝宏民)
張厚德,Email: szkjk @126.com

