酞菁氧釩分子及納米簇在高定向石墨表面的自組裝
王永鋒 張鑫然 葉迎春 梁德建 王 遠 吳 凱
(北京大學化學與分子工程學院,分子動態與穩態結構國家重點實驗室,北京分子科學國家實驗室,北京 100871)
酞菁氧釩分子及納米簇在高定向石墨表面的自組裝
王永鋒 張鑫然 葉迎春 梁德建 王 遠*吳 凱*
(北京大學化學與分子工程學院,分子動態與穩態結構國家重點實驗室,北京分子科學國家實驗室,北京 100871)
報導了酞菁氧釩(VOPc)分子及其納米簇在高定向石墨(HOPG)表面的自組裝.在室溫下,將HOPG浸入含有VOPc納米簇(2-20 nm)和VOPc分子(約為10-3g·L-1)的1,2-二氯乙烷膠體溶液中,VOPc分子在HOPG表面自組裝形成單分子層(SAM),VOPc納米簇在上述SAM表面進行尺寸選擇性自組裝.組裝于VOPc單分子層表面的納米簇的粒徑為(4.60±0.47)nm.掃描隧道顯微鏡研究表明,隨著酞菁氧釩膠體溶液濃度由2.5×10-2g·L-1增至2.5×10-1g·L-1,組裝于SAM表面的VOPc納米粒子的數量逐漸增多,最終形成稠密的單層粒子組裝體.本文提供的自組裝結構及方法在發展光電功能體系等方面具有潛在應用價值.
自組裝;酞菁氧釩;納米粒子;高定向石墨;掃描隧道顯微鏡;VOPc
Assembly of nanoparticles is of significance in the fabrication of novel nanostructured materials and devices.Great efforts have been made to fabricate ordered nanostructures using inorganic semiconductor nanoparticles for developing functional systems such as photonic materials,high-density magnetic data storage devices,microchip reactors and sensors[1-8].
In contrast with many inorganic semiconductors,organic semiconductors,such as phthalocyanines(Pcs),have narrow band gaps that limit their spectral sensitivities to corresponding regions and facilitate their use as photoconductors in visible and near-infrared diode laser-based devices[9-11].Pcs are a very important class of organic functional dye with many applications due to their photochemical,photophysical and electrochemical properties,biological functions,high stability and innocuity. They mainly have two characteristic light absorption bands: Soret band(300-400 nm)and Q-band(600-800 nm).The molecular structure of VOPc is shown in Fig.1.Pcs colloidal particles or thin films have been widely applied in xerographic technologies as the charge generation materials[12-14],and are also promising for applications in light emitting devices[15-16],photodetectors[17],image sensors[18],solar cells[19-20],gas sensors[21-22], optical recorders[23-24],and nonlinear optical materials[25-26].
Traditionally,colloidal particles of photoconductive Pcs are produced,in both industry and laboratory,by complicated milling techniques that give a wide particle-size distribution (20-500 nm).To narrow the size distribution,Jenekhe and Yi[17]used phase-separation induced aggregation of metallophthalocyanines in blends with a polymer as a new approach to obtain oxotitanium phthalocyanine(TiOPc)nanospheres of 70-110 nm in diameter with a good photoconductivity.Actually prior to this we already reported the preparation of stable water colloidal solutions containing much smaller VOPc nanoparticles with average diameters of 2.3-26 nm[27-28],and the VOPc nanoparticles separated from the aqueous colloidal solution exhibited promising charge-generation properties in a dual-layer photoreceptor[29].
Very recently,we reported the preparation of stable C2H4Cl2colloidal solutions of highly concentrated solvent-stabilized VOPc and TiOPc nanoparticles without any additives[29-31].The stability of the nanoparticles was attributed to the solvation of the nanoparticle surfaces and the repulsive electrostatic forces between the positively charged nanoparticles[29,31].The VOPc nanoparticles were then embedded in polycarbonate resins to form single-layered photoreceptors on Al substrates that exhibited satisfying xerographic properties in the positively charged mode.According to the light-assisted scanning tunneling spectroscopy(STS)measurements,we proposed thatlight-induced enhancement of the electron tunneling through the VOPc nanoparticle/insulator junctions was responsible for the excellent photoconductivity of the prepared single-layered photoreceptors in the absence of other charge transport materials.
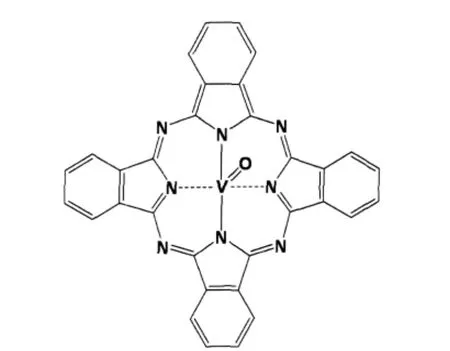
Fig.1 Schematic molecular structure of VOPc
To explore the surface structure and electronic properties of SAMs at the atomic and molecular levels,scanning tunneling microscopy(STM)has been widely used.This technique has greatly enhanced our understanding of the assemblies of phthalocyanines and their derivatives on various substrates.CuPc on polycrystalline silver was first studied by Gimzewski et al.[32]. Lippel and co-workers[33]reported the first STM observation of the internal structure of an isolated Pc molecule.In the systematic studies on various metal-coordinated Pcs such as CuPc,CoPc, NiPc,FePc,and VOPc on Au(111)surface under ultra-high vacuum(UHV)conditions,Hipps et al.[34-36]observed in STM images a depression at the molecular centers of NiPc and CuPc, and a protrusion at the molecular centers of FePc and CoPc, showing the possibility of molecular identification with STM. Bai and co-workers[37-39]reported copper(II)octaalkoxyl-substituted phthalocyanine(CuPcOC8)layers formed on HOPG, CuPc layers on n-alkane-adsorbed and tridedycelamine-adsorbed HOPG.Itaya and collaborators investigated highly ordered arrays of water-soluble 5,10,15,20-tetrakis(N-methylpyridinium-4-yl)-21H,23H-porhine tetrakis(p-toluenesulfonate) (TMPyP)on iodine-modified Pt(100)[40]and sulfur-modified Au (111)[41]electrodes in solution.They also obtained highly ordered molecular arrays of wateinsoluble 5,10,15,20-tetraphenyl-21H, 23H-porphine cobalt(II)and copper(II)(CoTPP and CuTPP)on Au(111)surface[42].Itaya et al.[43]further studied the electrocatalytic activity of oxygen at CoPc-and CuPc-covered Au(111) surfaces.In a further step,Ho et al.[44-45]carefully measured the electronic density of states(DOS)of metal-CuPc-metal junctions and vibronic states of individual CuPc molecules.
Although Pc SAMs have been extensively studied by STM, non-substituted Pc SAMs,such as methal-free pathalocyanine (H2Pc),metal phthalocyanine(MPc)or metal oxide phthalocyanine(MOPc),have never been realized from solution sources on HOPG at room temperature.The main difficulty was that the solubility of these molecules in most solvents was extremely low.Up to date,unmodified Pcs could only be imaged at low temperatures or on pre-modified HOPG[46].
In this paper,we report the successful room-temperature selfassembly of the VOPc molecules and size-selective assembly of the VOPc nanoparticles on HOPG from prepared VOPc/C2H4Cl2colloidalsolutions containing VOPc nanoparticles(2-20 nm)and dissolved VOPc molecules.The assembled VOPc nanoparticles are found to exist on top of the VOPc SAM and have a very narrow size-distribution,(4.60±0.47)nm,with a standard deviation of ca 10%.
1 Experimental
1.1 Preparation of the organic sols of solventstabilized VOPc nanoparticles
Starting material of VOPc powder(B-VOPc)was kindly supplied by Professor Lianming Yang at the Institute of Chemistry, Chinese Academy of Sciences.Elemental analyses showed that the composition of the powder was in accord with that of theoretical values.Water was purified through an Ultrapure Water System(Beijing Epoch Company).C2H4Cl2was purchased from Beijing Chemical Corporation and redistilled before use.
Details for the preparation and characterization of the stable VOPc/C2H4Cl2colloidal solution were described in our previous report[29],in which the C2H4Cl2-stabilized VOPc nanoparticles had a size distribution of 2-20 nm.The VOPc nanoparticle concentration in the colloidal solution could be as high as 100 g·L-1, while the dissolved VOPc molecule content was about 1×10-3g· L-1.The X-ray powder diffraction pattern of these VOPc nanoparticles showed diffraction peaks at Bragg angles(2θ)of 7.4°, 10.2°,12.6°,22.6°,24.2°,25.5°,and 28.7°for the prepared nanoscopic VOPc powder[29],indicating that theywere in phase II crystal form,and the small particle size caused the broadening of the diffraction peaks[47].
1.2 Preparation of the VOPc assemblies and STM characterization
The HOPG substrate(ZYH grade,purchased from Digital Instruments)was freshly prepared by peeling-off its top layers with an adhesive tape[48].It was then vertically immersed for about 16 h in the VOPc sols of different concentrations(from 2.5×10-4to 10 g·L-1)described in preceding section.This would generate the assemblies of the VOPc molecules and nanoparticles on the HOPG substrate.After dried in vacuum,the self-assembly on HOPG was immediately introduced into an ultra-high vacuum (UHV)chamber.STM images of the samples were collected with an Omicron variable temperature STM in UHV with a base pressure of ca 7×10-9Pa.Tungsten tips were used to take the measurements.In imaging,constant current mode was employed.All experiments were taken at room temperature.Some measurements were double-checked with a Nanoscope IIIA SPM(Digital Instruments)using Pt/Ir tips.
2 Results and discussion
2.1 Assemblies of VOPc molecules and nanoparticles
Fig.2(a)shows the STM images of the VOPc nanoparticles dispersed on the HOPG substrate.This sample was prepared by dropping on to the HOPG substrate a small amount of the colloidal solution containing VOPc nanoparticles,which gave rise to either isolated or congregated VOPc nanoparticles.Uncontrolled aggregation of the VOPc nanoparticles on the substrate is difficult for their further characterization and utilization.
Fig.2(b)shows the assembled VOPc nanoparticles dispersed on the surface,prepared by the method described in Section 1.2. A closer look at the surface structure revealed that these VOPc nanoparticles were actually seated on a molecular layer of VOPc.This molecular assembling structure with a unit size of ca 1.50 nm(Fig.2(c)),sampled from the circled area marked in Fig. 2(b),is very similar to that obtained by molecular beam epitaxy (MBE)of VOPc molecules on gold[49-50].The VOPc SAM should be monolayer because the height of the film is around 0.25 nm, which is the typical height of a Pc molecule on surfaces.Note that in our experiment,the HOPG substrate was simply dipped in the VOPc colloidal solution containing dissolved VOPc molecules.This is actually the first example for a VOPc self-assembled monolayer(SAM)from a colloidal solution.
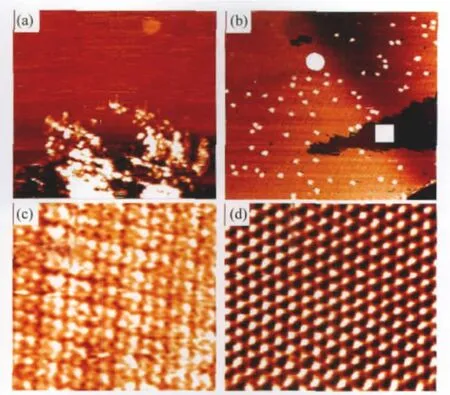
Fig.2 STM images of VOPc species on HOPG(a)randomly dispersed and piled VOPc particles on HOPG(Vsample=1.1 V,I=0.10 nA;200 nm×200 nm);(b)assemblies of VOPc molecules and particles from VOPc colloidal solutions(c=2.5×10-2g·L-1,Vsample=-0.48 V,I=0.31 nA;200 nm×200 nm); (c)the VOPc SAM sampled from the circle area in(b)(Vsample=-0.74 V,I=0.29 nA; 12 nm×13 nm);(d)the HOPG lattice structure sampled from the square area in(b) (Vsample=0.30 V,I=0.80 nA;3.8 nm×3.8 nm)
No VOPc nanoparticles could be observed in bare HOPG substrate areas,i.e.,in the big dark triangular area in Fig.2(b).This bare area did not carry anything visible and its atomic image (Fig.2(d))sampled from the square area clearly displayed a perfect HOPG structure.
The underlying driving force for the formation of VOPc SAMs in our experiments is likely that the interaction between HOPG surface and VOPc molecules is stronger than that between the solvent and VOPc molecules,and the ordered structure of the VOPc SAM further decreases the mobility of the adsorbed molecules.We have proven[29]that in the VOPc colloidal solution,equilibrium was established between VOPc molecules in the solution and at the nanocrystal surface.Albeit the solubility of VOPc in C2H4Cl2was quite low(ca 10-3g·L-1),the concentration of the VOPc molecules in the colloidal solution did not change much during the formation of the VOPc SAM on the HOPG substrate.These would facilitate the formation of a largearea and ordered VOPc SAM.
The VOPc nanoparticles can really“stick”to the VOPc SAMs at the surface.The exact reason for this phenomenon is still elusive and needs further investigation.One possibility is that the VOPc SAMs with their V=O groups pointing up-wards[49-50]provide a structural complement for the assembly of the VOPc nanocrystals with their specific crystal face attached to the VOPc SAM.
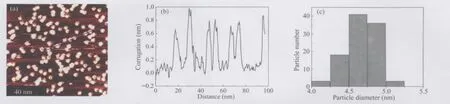
Fig.3 (a)STM image of the assembled VOPc nanoparticles at room temperature;(b)line scan profile along the line in(a); (c)size-distribution of the VOPc nanoparticles according to the statistical analysis of 101 nanoparticles in(a)
2.2 Assembling-induced size-selection of the assembled VOPc nanoparticles
Generally speaking,the smaller the nanoparticles,the more difficult to achieve a narrow size-distribution.The lateral sizes of the assembled particles were quite uniform(Fig.3(a,b))and calibrated with the HOPG substrate lattice constant and the VOPc molecular size.The particle sizes of the VOPc nanoparticles were measured to be(4.60±0.47)nm(Fig.3(c))with a standard deviation of ca 10%according to the statistical analysis of 101 particles.This provides an excellent example for the monodispersed organic semiconductor nanoparticles.
The size distribution of the VOPc nanoparticles in the original colloidal solution was in the range of 2-20 nm,as measured by TEM[29],while the STM measurments only found the near-monodispersed VOPc nanoparticle in the present assembly(Fig.3). The unusual size-selective assembling of the VOPc colloidal nanoparticles on the VOPc-SAM/HOPG substrates has not been well understood at the moment.Since the VOPc nanoparticles possess excellent photoconductivity,the assembling-induced sizeselection may provide a novel approach to the fabrication of nanostructured photoelectric devices with a high resolution.
With the increase of the VOPc nanoparticle concentration in the colloidal solution,more and more VOPc nanoparticles were dispersed on the molecular layer(Fig.4)and in the end,a dense VOPc nanoparticle monolayer formed.Note that the sizes of VOPc nanoparticles on the substrates did not change upon increasing the VOPc concentration of the colloidal solutions,confirming that the VOPc nanoparticles on the substrate were really size-selectively assembled from the colloidal solution.This is quite intriguing because the“dual-layer”structure composed of a uniform VOPc nanoparticle layer and an ordered assembled VOPc molecular layer can be easily prepared in this way.
2.3 Imaging and stability of the VOPc SAM
In Fig.4(a),one can immediately notice that the STM image of the underlying VOPc SAM appears ridge-like.Does this mean that this ridge-like VOPc SAM is different from that shown in Fig.2(c)in structure?To uncover this,we did a series of scanning experiments and the results were compiled in Fig.5. In Fig.5(b),the width of a molecular“strand”in the ridge-like image is about 5.0 nm,similar to the size of an assembled VOPc nanoparticle,but much larger than the size of a VOPc molecule. Therefore,the strand can not be a VOPc molecular row.It is well known that the STM imaging can be greatly affected by the STM tip state[51-52],which may explain the appearance of the molecular ridge-like structure.The STM tip effect hypothesis receives strong support from the result in Fig.5(c)where the VOPc molecular structure suddenly changed from the ridge-like image to the normalimage(the same asthatin Fig.5(a))when the tip scanned from the bottom to the top without changing the scanning parameters.Thus,the only possibility for the sudden change of the imaged VOPc molecular structure comes from the variation of the STM tip state.We also did the experiments by applying an electric pulse on to the STM tip,leading to that the ridge-like image changed back into the normal one shown in Fig.2(c)and Fig.5(a).This implied that the ridge-like structure might appear upon the sticking of some VOPc molecular species or nanoparticles to the STM tip.The image in Fig.5(d)displays mixed normal and ridge-like VOPc structures where the individual VOPc molecule is still distinguishable,clearly indicating the complexity and diversity of the tip state.

Fig.4 Assemblies of the VOPc molecules and nanoparticles from the colloidal solutions of various VOPc contents(a)c=2.5×10-2g·L-1,Vsample=-0.48 V,I=0.31 nA,scan range:100 nm×100 nm;(b)c=2.5×10-1g·L-1,Vsample=-0.75 V,I=0.38 nA,scan range:200 nm×200 nm; (c)c=6.3×10-1g·L-1,Vsample=-0.71 V,I=0.34 nA,scan range:100 nm×100 nm

Fig.5 Normal and ridge-like STM images of the VOPc SAM on HOPG (a)normal STM image of the VOPc SAM(Vsample=-0.74 V,I=0.10 nA;70 nm×70 nm);(b)ridge-like STM image of the VOPc SAM(Vsample=-0.74 V,I=0.10 nA; 100 nm×100 nm);(c)the sudden change of the ridge-like STM image into the normal one during the scanning from bottom to top(Vsample=-0.50 V,I=0.37 nA;80 nm×80 nm); (d)coexistence of the normal and ridge-like structures in which the individual VOPc molecules can still be visible(Vsample=-0.80 V,I=0.40 nA;108 nm×108 nm)
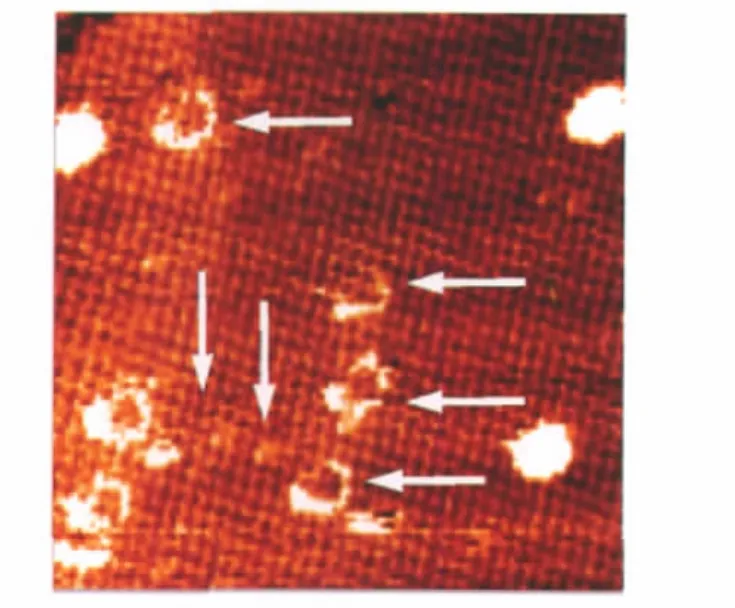
Fig.6 STM image of the VOPc SAM and nanoparticles after thermal treatment at 450 K for 30 minVsample=-0.80 V,I=0.37 nA;scan range:70 nm×70 nm.The arrows indicate the vestiges of the leaving VOPc nanoparticles during the thermal treatment.
To study the stabilities of the VOPc SAM and nanoparticles on HOPG,we treated the assembly of the VOPc molecules/ nanoparticles shown in Fig.4(c)at 450 K for about 30 min.The result is shown in Fig.6.After the thermal treatment,most of the VOPc nanoparticles were removed.With the removal of the VOPc nanoparticles,exposed underlying was the perfect VOPc SAM rather than bare HOPG substrate.In Fig.6,some irregular looped vestiges left upon removing VOPc nanoparticles on top of the self-assembled molecular layer still can be seen,suggesting that the interaction between the VOPc SAM and HOPG is much stronger than that of the VOPc molecules in the nanocrystals.
3 Conclusions
In summary,we have demonstrated that VOPc molecules dissolved with a low concentration in a VOPc/C2H4Cl2colloidal solution could self-assemble on HOPG to form a VOPc SAM.It was found that the VOPc nanoparticles size-selectively assembled on the VOPc SAM rather than directly at the bare HOPG substrate surface.The assembled organic semiconductor nanoparticles possessed a very narrow size-distribution,(4.60±0.47)nm, with a standard deviation of ca 10%.These VOPc nanoparticle assemblies and SAMs will be beneficial to the study on the intrinsic properties of the organic semiconductor at nanoscale and molecular levels and to the fabrication of promising photoelectronic devices such as photoelectronic sensors,imagers or photoreceptors in a controlled manner.
1 Murray,C.B.;Kagan,C.R.;Bawendi,M.G.Science,1995,270: 1335
2 Springholz,G.;Holy,V.;Pinczolits,M.;Bauer,G.Science,1998, 282:734
3 Collier,C.P.;Vossmeyer,T.;Heath,J.R.Annu.Rev.Phys.Chem., 1998,49:371
4 Mirkin,C.A.Inorg.Chem.,2000,39:2258
5 Hayward,R.C.;Saville,D.A.;Aksay,I.A.Nature,2000,404:56
6 Li,M.;Schnablegger,H.;Mann,S.Nature,1999,402:393
7 Alchalabi,K.;Zimin,D.;Kostorz,G.;Zogg,H.Phys.Rev.Lett., 2003,90:026104
8 Fu,X.Y.;Wang,Y.;Huang,L.X.;Sha,Y.L.;Gui,L.L.;Lai,L. H.;Tang,Y.Q.Adv.Mater.,2003,15:902
9 Petritsch,K.;Dittmer,J.J.;Marseglia,E.A.;Friend,R.H.;Lux, A.;Rozenberg,G.G.;Moratti,S.C.;Holmes,A.B.Sol.Energy Mater.Sol.Cells,2000,61:63
10 Ginger,D.S.;Greenham,N.C.Phys.Rev.B,1999,59:10622
11 Huynh,W.U.;Peng,X.G.;Alivisatos,A.P.Adv.Mater.,1999, 11:923
12 Law,K.Y.Chem.Rev.,1993,93:449
13 Wei,X.F.;Di,Z.W.;Yan,J.M.;Jiang,K.J.;Wang,Y.Q.;Yang, L.M.Photographic Science and Photochemistry,2002,20:191 [魏先福,邸振文,閻建民,蔣克健,王艷喬,楊聯明.感光科學與光化學,2002,20:191]
14 Li,Y.F.;Li,S.L.;Shao,K.F.;Yang,L.M.Information Recording Materials,2005,6:21 [李英鋒,李紹路,邵科峰,楊聯明.信息記錄材料,2005,6:21]
15 Flora,W.H.;Hall,H.K.;Armstrong,N.R.J.Phys.Chem.B, 2003,107:1142
16 Nuesch,F.;Carrara,M.;Romero,D.B.;Zuppiroli,L.Chem.Phys. Lett.,2001,347:311
17 Jenekhe,S.A.;Yi,S.J.Adv.Mater.,2000,12:1274
18 Street,R.A.;Graham,J.;Popovic,Z.D.;Hor,A.;Ready,S.;Ho,J. J.Non-Cryst.Solids,2002,299:1240
19 Trombach,N.;Tada,H.;Hiller,S.;Schlettwein,D.;W?hrle,D. Thin Solid Films,2001,396:109
20 Pannemann,C.;Dyakonov,V.;Parisi,J.;Hild,O.;W?hrle,D. Synth.Met.,2001,121:1585
21 Rella,R.;Serra,A.;Siciliano,P.;Tepore,A.;Valli,L.;Zocco,A. Langmuir,1997,13:6562
22 Bouvet,M.;Leroy,A.;Simon,J.;Tournilhac,F.;Guillaud,G.; Lessnick,P.;Maillard,A.;Spirkovitch,S.;Debliquy,M.;de Haan, A.;Decroly,A.Sens.Actuators B,2001,72:86
23 Gu,D.H.;Chen,Q.Y.;Tang,X.D.;Gan,F.X.;Shen,S.Y.;Liu, K.;Xu,H.J.Opt.Commun.,1995,121:125
24 Gu,D.H.;Chen,Q.Y.;Shu,J.P.;Tang,X.D.;Gan,F.X.;Shen,S. Y.;Liu,K.;Xu,H.J.Thin Solid Films,1995,257:88
25 Fang,S.L.;Hoshi,H.J.;Kohama,K.C.;Maruyama,Y.J.Phys. Chem.,1996,100:4104
26 Yamashita,M.;Inui,F.;Irokawa,K.;Morinaga,A.;Tako,T.;Mito, A.;Moriwaki,H.Appl.Surf.Sci.,1998,130:883
27 Wang,Y.;Deng,K.;Gui,L.L.;Tang,Y.Q.;Zhou,J.W.;Cai,L. Y.;Qiu,J.B.;Ren,D.;Wang,Y.Q.J.Colloid Interface Sci.,1999, 213:270
28 Liu,W.J.;Wang,Y.;Gui,L.L.;Tang,Y.Q.Langmuir,1999,15: 2130
29 Zhang,X.R.;Wang,Y.F.;Ma,Y.;Ye,Y.C.;Wang,Y.;Wu,K. Langmuir,2006,22:344
30 Chao,W.;Zhang,X.R.;Xiao,C.;Liang,D.J.;Wang,Y.J.Colloid Interface Sci.,2008,325:198
31 Wang,Y.;Liang,D.J.Adv.Mater.,2010,in press
32 Gimzewski,J.K.;Stoll,E.;Schlittler,R.R.Surf.Sci.,1987,181:267
33 Lippel,P.H.;Wilson,R.J.;Miller,M.D.;Woll,C.;Chiang,S. Phys.Rev.Lett.,1989,62:171
34 Lu,X.;Hipps,K.W.;Wang,X.D.;Mazur,U.J.Am.Chem.Soc., 1996,118:7197
35 Hipps,K.W.;Lu,X.;Wang,X.D.;Mazur,U.J.Phys.Chem., 1996,100:11207
36 Lu,X.;Hipps,K.W.J.Phys.Chem.B,1997,101:5391
37 Qiu,X.H.;Wang,C.;Zeng,Q.D.;Xu,B.;Yin,S.X.;Wang,H. N.;Xu,S.D.;Bai,C.L.J.Am.Chem.Soc.,2000,122:5550
38 Xu,B.;Yin,S.X.;Wang,C.;Qiu,X.H.;Zeng,Q.D.;Bai,C.L. J.Phys.Chem.B,2000,104:10502
39 Lei,S.B.;Wang,C.;Wan,L.J.;Bai,C.L.J.Phys.Chem.B,2004, 108:1173
40 Sashikata,K.;Sugata,T.;Sugimasa,M.;Itaya,K.Langmuir,1998, 14:2896
41 Wan,L.J.;Shundo,S.;Inukai,J.;Itaya,K.Langmuir,2000,16: 2164
42 Yoshimoto,S.;Tada,A.;Suto,K.;Narita,R.;Itaya,K.Langmuir, 2003,19:672
43 Yoshimoto,S.;Tada,A.;Suto,K.;Itaya,K.J.Phys.Chem.B, 2003,107:5836
44 Nazin,G.V.;Qiu,X.H.;Ho,W.Science,2003,302:77
45 Qiu,X.H.;Nazin,G.V.;Ho,W.Phys.Rev.Lett.,2004,92: 206102
46 Gopakumar,T.G.;Lackinger,M.;Hackert,M.;Müller,F.; Hietschold,M.J.Phys.Chem.B,2004,108:7839
47 Griffiths,C.H.;Walker,M.S.;Goldstein,P.Mol.Cryst.Liq. Cryst.,1976,33:149
48 Wang,Y.F.;Ye,Y.C.;Wu,K.Surf.Sci.,2006,600:729
49 Hipps,K.W.;Barlow,D.E.;Mazur,U.J.Phys.Chem.B,2000, 104:2444
50 Barlow,D.E.;Hipps,K.W.J.Phys.Chem.B,2000,104:5993
51 Xu,Q.M.;Wan,L.J.;Yin,S.X.;Wang,C.;Bai,C.L.J.Phys. Chem.B,2001,105:10465
52 Olson,J.A.;Bühlmann,P.Anal.Chem.,2003,75:1089
December 4,2009;Revised:January 21,2010;Published on Web:February 25,2010.
Self-Assemblies of Oxovanadium Phthalocyanine Molecules and Nanoclusters on Highly Oriented Pyrolytic Graphite
WANG Yong-Feng ZHANG Xin-Ran YE Ying-Chun LIANG De-Jian WANG Yuan*WU Kai*
(Beijing National Laboratory for Molecular Sciences,State Key Laboratory for Structural Chemistry of Unstable and Stable Species, College of Chemistry&Molecular Engineering,Peking University,Beijing 100871,P.R.China)
Self-assembled monolayer(SAM)of oxovanadium phthalocyanine(VOPc),an organic semiconductor compound,was prepared at room temperature on a highly oriented pyrolytic graphite(HOPG)substrate by immersing the substrate in a 1,2-dichloroethane(C2H4Cl2)colloidal solution containing both VOPc nanoparticles(2-20 nm)and dissolvedVOPcmolecules(ca10-3g·L-1).TheVOPcnanoparticlesfurtherassembledonthe VOPc SAM.The nanoparticleassembling in the experimental conditions exhibited a high size-selectivity to VOPc nanoparticles of(4.60±0.47)nm with a standard deviation of 10%.As revealed by scanning tunneling microscopy(STM)measurements,with increasing VOPc concentration of the colloidal solution from 2.5×10-2to 2.5×10-1g·L-1,the amount of VOPc nanoparticles assembled on the molecular layer increased gradually,and a dense VOPc nanoparticle monolayer formed in the end. The found assembly structures and method are promising for the development of photoelectric or electric functional systems.
Self-assembly;Oxovanadium phthalocyanine;Nanoparticles;HOPG;Scanning tunneling microscopy;VOPc
*Corresponding authors.Email:wangy@pku.edu.cn,kaiwu@pku.edu.cn;Tel:+86-10-62757497,+86-10-62754005.
The project was supported by the National Natural Science Foundation of China(20773001,20973004,20973003,50821061)and National Key Basic Research Program of China(973)(2006CB806102,2009CB929403).
國家自然科學基金(20773001,20973004,20973003,50821061)及國家重點基礎研究發展規劃項目(973)(2006CB806102,2009CB929403)資助
O647

