Isothermal Crystallization Behavior of Poly(ethylene terephthalate)/Carbon Black Masterbatch
JIANG Zhao-hui(姜兆輝),JIN Jian(金 劍),XIAO Chang-fa(肖長發),LI Xin(李 鑫)
1 Key Laboratory of Fiber Modification and Functional Fiber,Tianjin Polytechnic University,Tianjin 300160,China
2 State Key Laboratory of Biobased Fiber Manufacture Technology,China Textile Academy,Beijing 100025,China
Introduction
Poly(ethylene terephthalate)(PET)is a semi-crystalline polymer and has been widely used in the production of fibers,films,bottles,and engineering plastics,due to its excellent spinnability,mechanical properties,low cost,heat and chemical resistances,etc.As is known,crystallization properties seriously affect the morphology and properties of end products.Currently,some literatures have reported the crystallization behaviors of PET, PET/inorganic particle composites, and its copolyester[1-7].Carbon black(CB)has been widely used as polymer additives,such as reinforcing fillers,pigments,and electricity conductive additives.In the study of Li et al.[8],PET/CB composite containing 3% (weight ratio)CB was prepared and non-isothermal crystallization kinetics ofthe masterbatch was analyzed.And CB can act as nucleating agent in the process of polymer crystallization,which is confirmed in the studies of poly (lactic acid) /CB composite[9],polypropylene/CB composite[10,11], polyamide/CB composites[12].
As far as we are concerned,there has been few literature that emphasizes the effect of high CB content(more than 20%,weight ratio) on the isothermal crystallization behavior of polymer.In this paper,masterbatch was prepared by melt blending using a separate feeding technique and differential scanning calorimetry(DSC),an effective technique to evaluate the crystallization process,was employed to investigate the crystallization behavior of PET/CB masterbatch.
1 Experimental
1.1 Materials and equipment
PET,semi-delustring,Heng Li Chemical Fiber Co.,Ltd.,Jiangsu,China,was received in pellet form.CB,N220,primary particle size of 23 nm,was purchased from Tianjin Lihua Jin Chemical Co.,Ltd.,China.Dispersant was provided by Beijing University of Chemical Technology joint venture in Rushan chemical plant.
High speed grinder was purchased from Zhongxing Weiye Co.,Ltd.,Beijing,China.Co-rotate twin-screw extruder,ZSK-25WLE with a screw diameter of 25 mm(L/d=25/1 where L/d meant the ratio of length to diameter for the screw),was made by WP corporation in Germany.DSC,Perkin Elmer Pyris1(Perkin Elmer,USA)was chosen to measure thermal properties of PET/CB masterbatch.
1.2 Preparation of PET/CB masterbatch
The dried CB and PET pellets were accurately delivered into the twin-screw extruder using a separate feeding technique.The temperature and rotate speed were 270 ℃ and 200 r/min,respectively.Aftermeltblending,extrusion,cooling and pelletizing,the cylindricalPET/CB blending masterbatch containing 20% CB was obtained with the size of 2.5 mm(diameter) × 3.5 mm(length).
1.3 Transmission electron microscope(TEM)observation
Samples for TEM analysis weretaken randomlyfrom different locations.Ultra thin sections with a thickness of 100 nm were prepared with ultra-sonic diamond knife(Ultrotome 2088,LKB,Sweden).The TEM investigations were performed on an H-800(HITACHI,Japan)microscope,which was adjusted with an acceleration voltage of 200 kV.
1.4 DSC measurement
DSC scans of all the samples had been taken at different cooling rates using Perkin Elmer Pyris1 DSC.The samples were heated over a temperature ranging from room temperature to 290℃ at the heating rate 20℃/min,held for 5 min to eliminate thermal history,and then cooled to crystallization temperature 210,215,220,225℃ for PET and 215,220,225,230℃ for PET/CB composites at the cooling rate 80℃/min.All the measurements were carried out under a nitrogen atmosphere.
2 Results and Discussion
2.1 Observation of CB dispersion in PET matrix
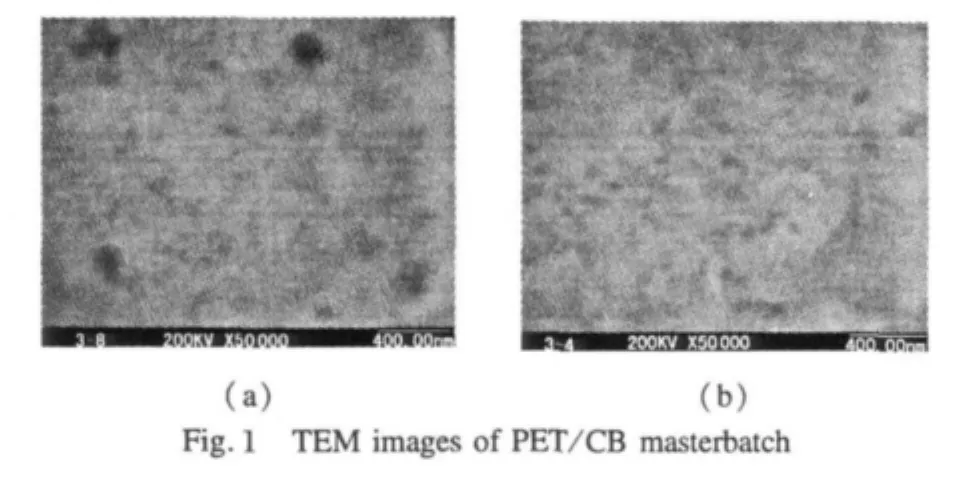
Figure 1 displays TEM images of cross-sections of PET/CB masterbatch.As shown in the images,CB particles are well dispersed and uniformly distributed in PET matrix,like the islands in the sea without connection.From TEM images,we can estimate that the mean size of CB particles is much less than 1 μm,which is desired.Besides,in PET/CB masterbatch PET forms a continuous phase and CB particles disperse uniformly.Therefore,although the weight of samples(5 mg)used in DSC measurement is extremely little,the results shouldn't differ greatly because of sampling location.
2.2 Isothermal crystallization characteristics
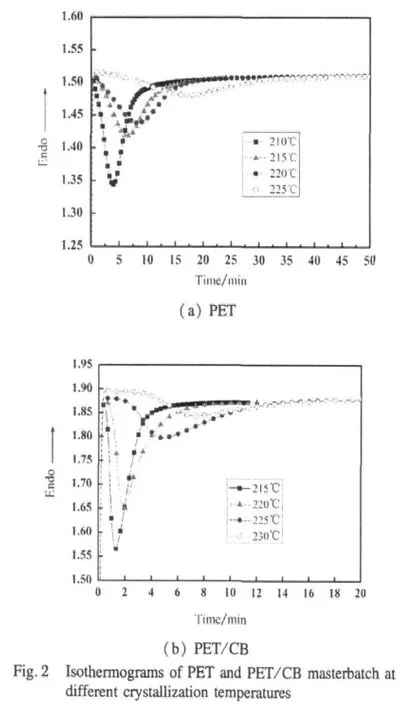
The isothermal crystallization exotherms of PET and PET/CB masterbatch are shown in Fig.2.It can be seen that the exothermal peak becomes broader with the isothermal crystallization temperature increasing,which means the crystallization rate becomes slower.Furthermore,under the same crystallization temperature the crystallization time of PET/CB masterbatch is much shorter than that of PET,which informs the heterogeneous nucleation of CB during crystallization process.
2.3 Isothermal crystallization kinetics based on Avrami equation
Based on the assumption that the evolution of crystallinity is linearly proportional to the evolution of heat released during the crystallization,the so-called Avrami equation can be applied to analyzetheisothermalcrystallization ofPET and PET/CB masterbatch:

where n is the Avrami exponent,which is a function of the nucleation process;and K is the growth function,which is dependent on nucleation and crystal growth.Xc(t)is the relative crystallinity,which can be obtained according to Eq.(2):
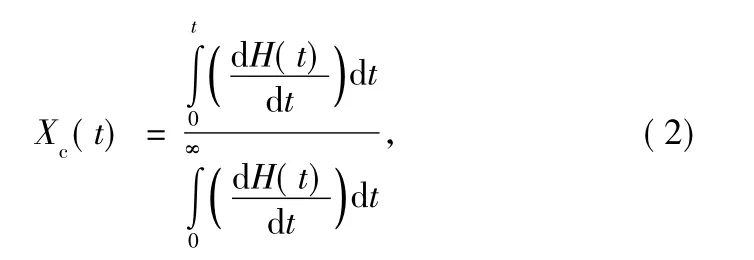
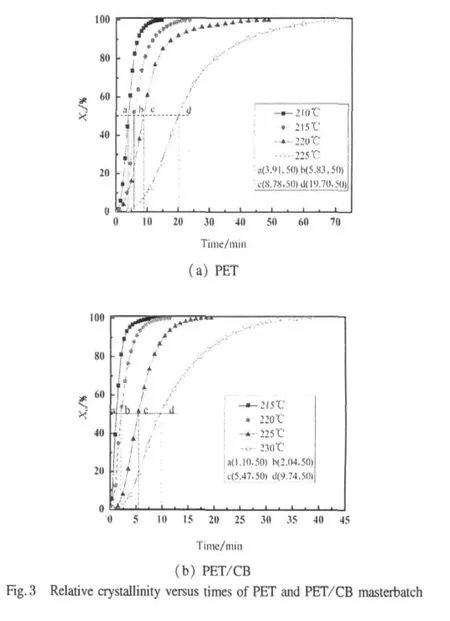
where dH(t)denotes the measured enthalpy of crystallization during an inflnitesimal time interval dt.The limits t and ∞ are used to denote the elapsed time during the course of crystallization and at the end of the crystallization process,respectively.The Xc(t)versus t is plotted in Fig.3.All curves exhibit a sigmoid dependence with time.From these curves,the half-time of crystallization,t1/2,defined as the time required for half of the ultimate crystallinity to develop,can be achieved and denoted as graphic value.
The double logarithmic form of Eq.(1)can be written as follows:
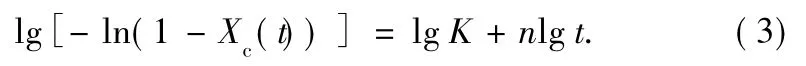
By plotting of lg[-ln(1-Xc(t))]versus lg t at different crystallization temperatures,proximate straight lines are shown in Fig.4.From the slope and intercept in Fig.4,the Avrami exponent n and the overall rate constant K are obtained in Table 1.The half-time of crystallization,t1/2,can be obtained from graphic method(Fig.3)and the measured kinetic parameters[13,14],and we denoted it as calculated value:
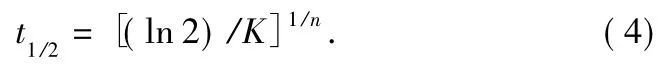
Besides,the ultimate crystallinity(Xc)listed in Table 1 can be calculated by Ref.[15]:
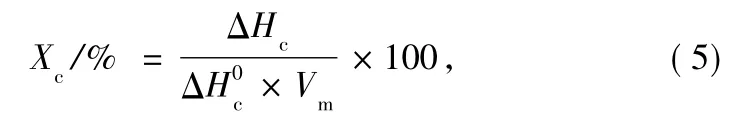
where ΔHcis the crystallization enthalpy,Δrefers to enthalpy of 100%crystalline PET,considered as 135.8 J/g[16],and Vmis the volume fraction of PET.
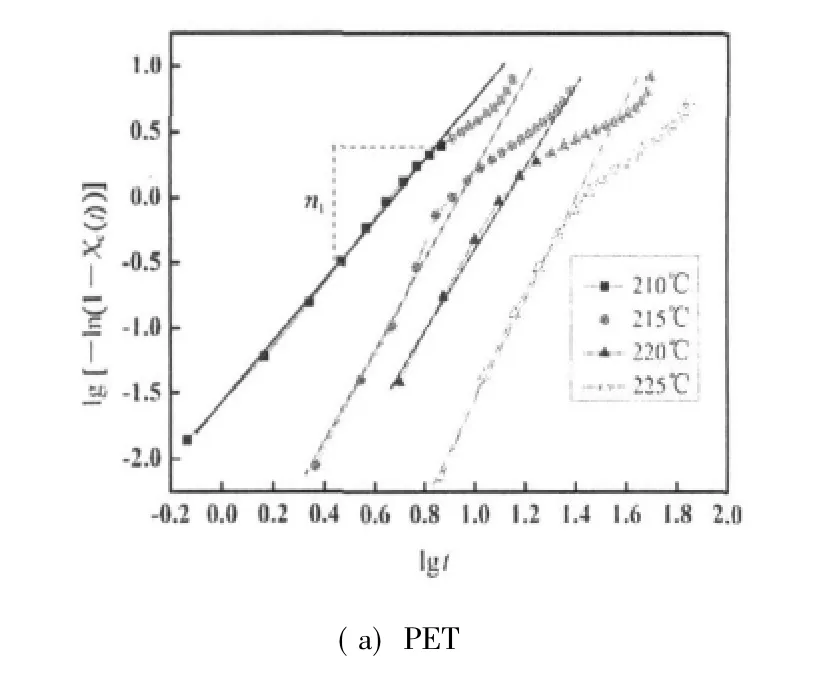
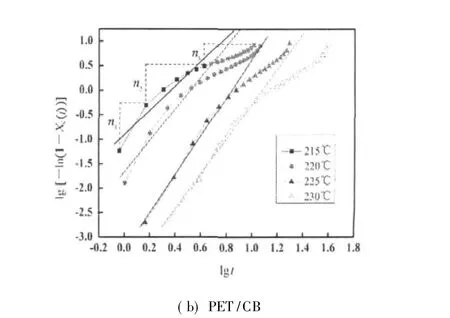
Fig.4 Plots of lg[-ln(1-Xc(t))]versus lg t
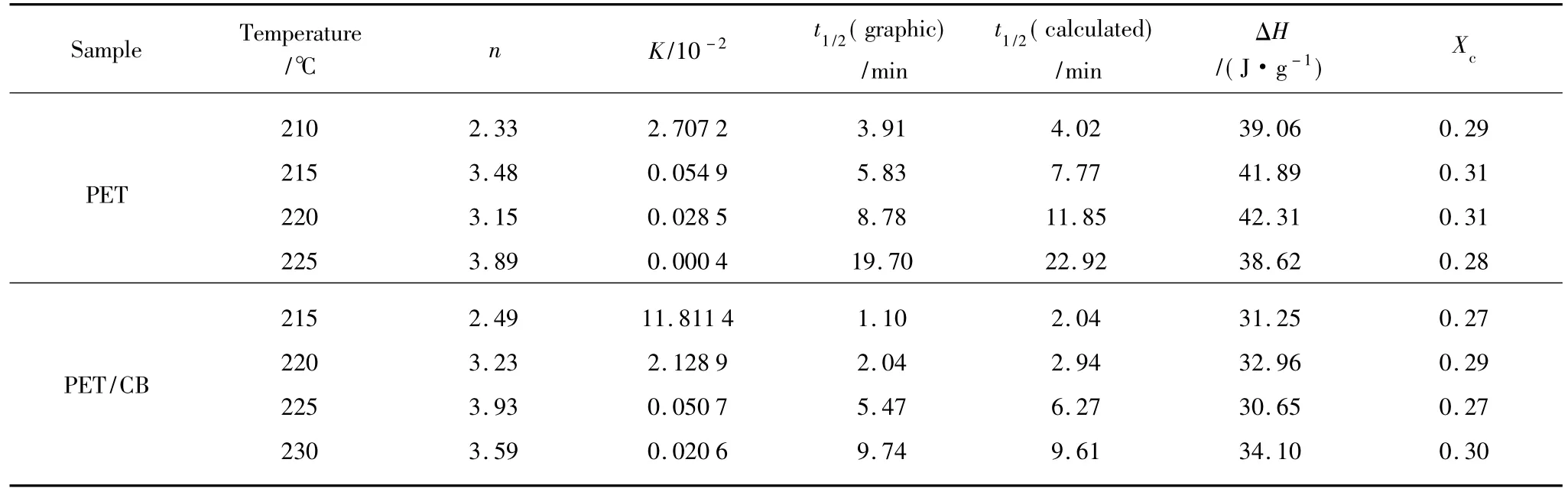
Table 1 Isothermal crystallization parameters of PET and PET/CB masterbatch
Figure 4 shows the plots of lg[-ln(1-Xc(t))]versus lg t for PET and PET/CB composites.The kinetic parameters of Avrami equation,such as n and K,can be determined with fltting the initial stage of the curves.Furthermore,compared with virgin PET,PET/CB composite shows the multi-exponents,named n1,n2,and n3(transfer region)during crystallization.When the crystallization temperatures of PET and PET/CB composites exceed 215 and 220℃ respectively,the n values are all greater than 3.This indicates a thermal nucleation process followed by a three-dimensional crystal growth.While the crystallization temperatures of PET and PET/CB composites are 210 and 215 ℃ respectively,the n values are close to 2.5,which informs a thermal nucleation process followed by mixing two-dimensional and three-dimensional crystal growths.
From Table 1,t1/2increases exponentially with increasing crystallization temperature,indicating that the rate of crystallization is slower when the supercooling is smaller.The overall rate constant K is extremely sensitive to temperature,which determines both the nucleation and the growth processes.Moreover,the overallrate constantis greaterwhen the crystallization temperature decreases.The average n value of PET determined in each crystallization temperature is 3.21,while that of PET/CB masterbatch is 3.31,indicating that both the nucleation and the growth mechanism are the same in the crystallization temperature range investigated.Both values are slightly greater than the theoretical value of 3.0 predicted for instantaneous nucleation with spherulitic growth geometry.By theory,n value is an integer between 1 and 4 depending on different crystallization mechanisms.The non-integral n value may be due to the secondary crystallization or the crystal perfection.Under the same crystallization temperature,t1/2of PET/CB is much smaller than that of PET,which means that CB particles act as heterogeneous nucleating agents.
2.4 Equilibrium melting temperature
Figure 5 presents the melting behaviors of isothermally crystallized PET and PET/CB masterbatch at different crystallization temperatures.In Fig.5,both endotherms of PET and PET/CB masterbatch show a single melting endotherm peak,indicating that the addition of CB doesn't change crystallization mechanism of PET.It is clearly indicated that increasing Tcpromotes remelting temperature due to more stable and perfect crystals under higher crystallization temperature.
Estimation of the correct equilibrium melting temperature()is an important task since the analysis of the growth kinetics is very sensitive to.The relationship among the observed melting temperature Tm,the crystallization temperature Tc,and the extrapolation of these data tois expressed by Hoffman-Weeks equation:
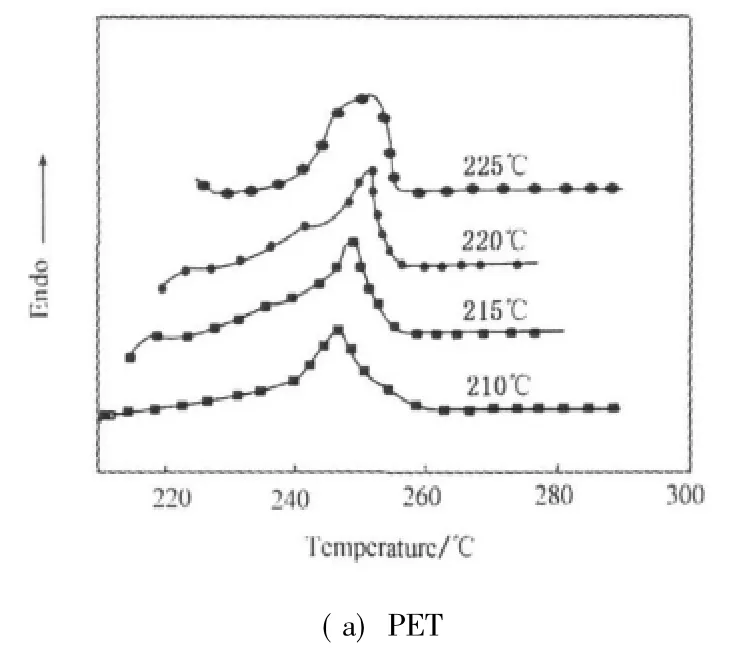
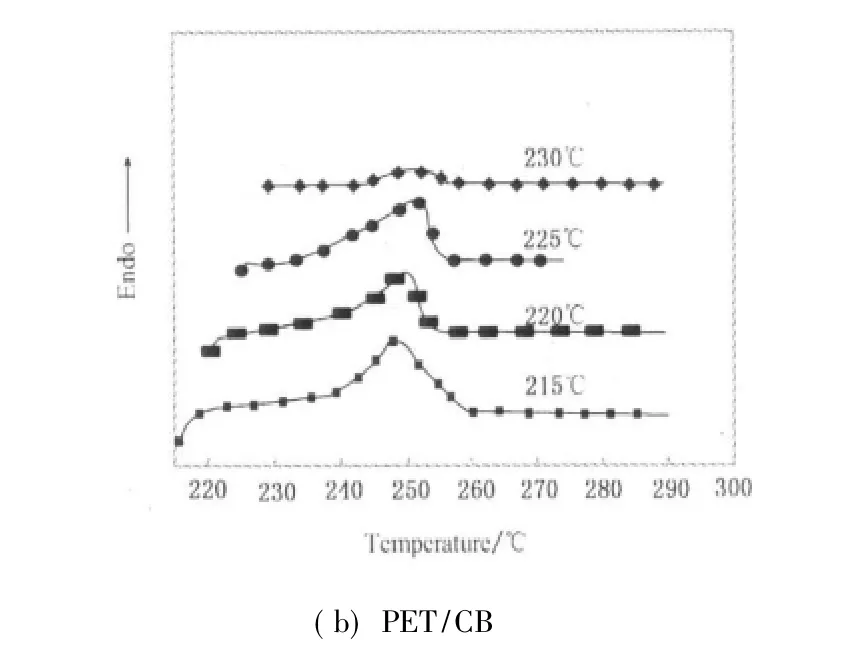
Fig.5 Melting endotherms of PET and PET/CB masterbatch after isothermal crystallization
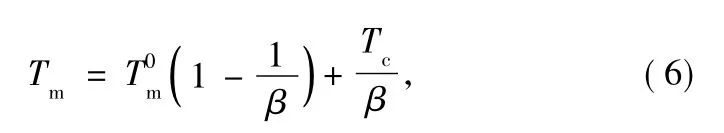
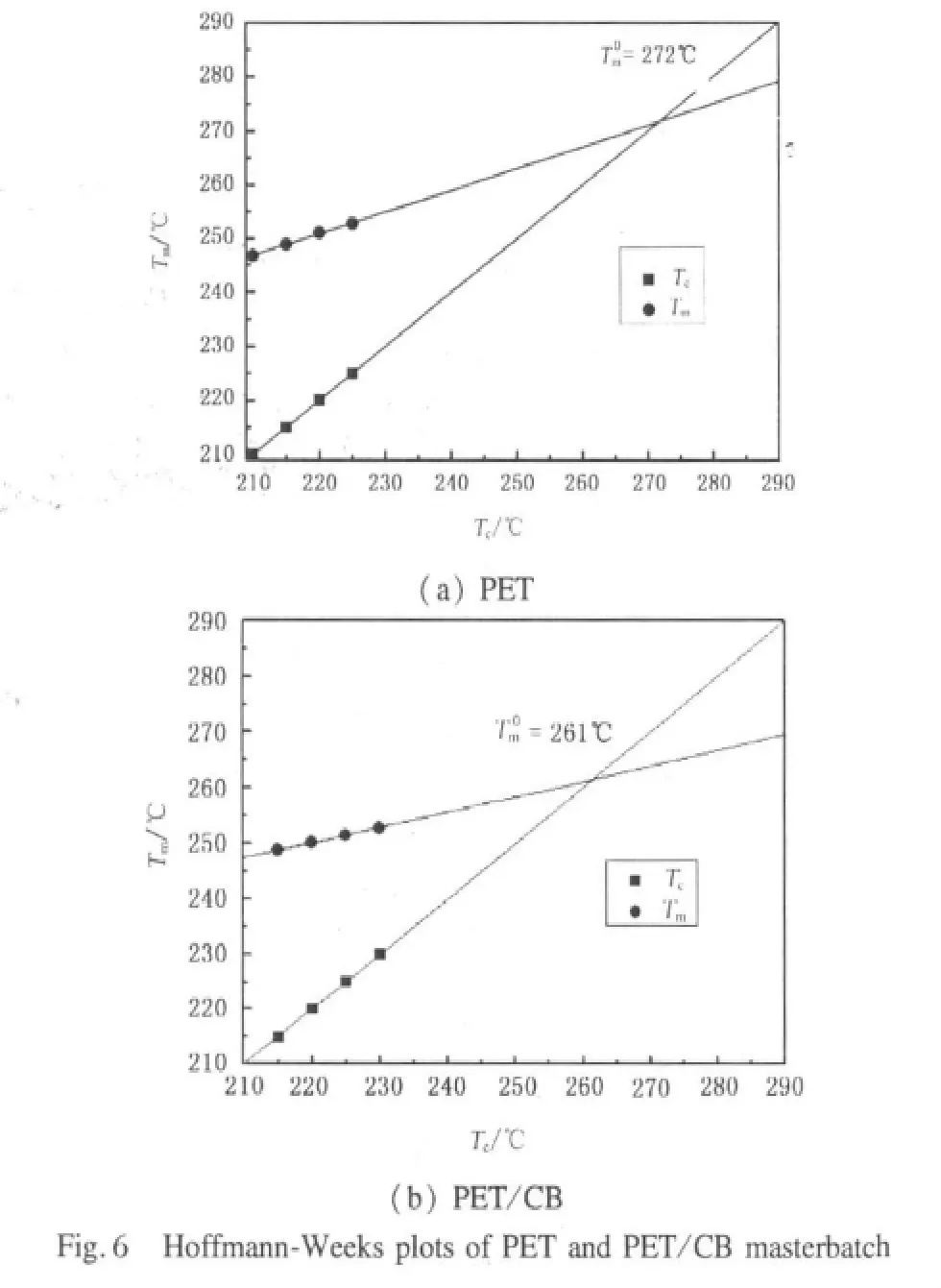
where Tmis the melting temperature,Tcis the isothermal crystallization temperature.β is a ratio factor depending on the thickness of the crystallite and the critical crystalline nucleus,and it is assumed to be a constant in narrow range of crystallization temperature.Figure 6 shows typical Hoffman-Weeks plots of PET and PET/CB masterbatch.The intersection temperature of Tcversus Tmwith Tc=Tmis considered as T0m.The equilibrium melting temperatures of PET and PET/CB masterbatch are 272 and 261℃,respectively.of PET is higher than that of PET/CB masterbatch,which implies that CB particles act as an effective nucleating agent for PET and promote the crystallization rate of PET,so thedecreases due to more imperfect PET crystals.
2.5 The fold surface free energy based on Hoffman-Lauritzen relationship
Usually,the rate of crystallization G is described as the reciprocal of t1/2,that is,G=(t1/2)-1.Figure 7 shows the temperature dependence of the rate of isothermal crystallization for PET and PET/CB composites.In Fig.7,in range of the investigated isothermal crystallization temperature,crystallization rate decreases with increasing crystallization temperature for both PET and PET/CB composites.Besides,the addition of CB significantly accelerates the crystallization rate,which implies that CB has acted as a heterogeneous nucleation agent for PET.It is worth noting that,the slope of the curve for PET crystallization rate over crystallization temperature is smaller than that for PET/CB composites,which indicates that the crystallization of the latter is more sensitive to temperature.
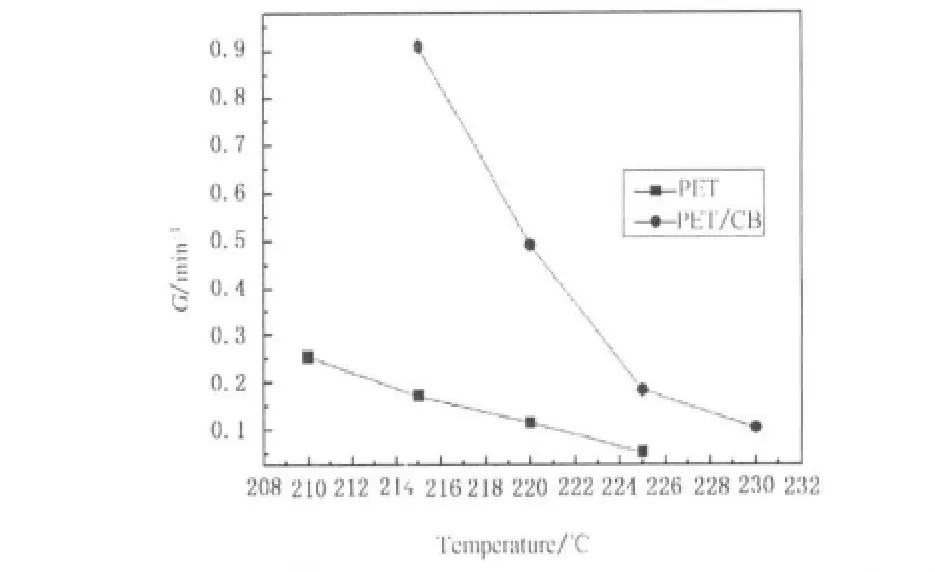
Fig.7 Temperature dependence of the rate of isothermal crystallization for PET and PET/CB masterbatch
Hoffman-Lauritzen secondary nucleation theory can be described as follows[17]:

where G is the radial growth rate and G0is an overall constant factor that depends on the molecular weight.The first exponential term in Eq.(7)accounts for the process of transport of molecular segments to the crystalline surface(transport term),where U*is the activation energy.R is the gas constant,and T∞=Tg-30K is the temperature at which the transport of segments across the liquid-solid interface becomes infinitely slow.The second exponential term(nucleation term)is a measure of the probability ofthe formation ofa thermodynamically stable secondary surface nucleus,where ΔT is supercooling(-Tc)and f is a correction factor for the effect of the temperature on ΔHf(taken to be 2Tc/+Tc).The nucleation constant(Kg)is obtained as follows:
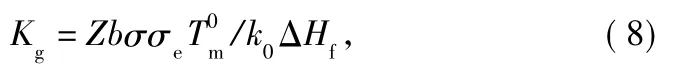
where Z is 4 for regimesⅠandⅢ and 2 for regimeⅡ.b is the monomolecular layer thickness,taken to be the perpendicular separation of(010)planes,and this is 0.553 nm[18].σeis the fold surface free energy.ΔHfis the enthalpy of fusion per unit volume(2.1×108J/m3)and k0is the Boltzmann constant equaling to 1.38 ×10-23J/K.σ is the side surface free energy of the polymer crystal,which can be estimated by the Thomas-Stavely relationship:
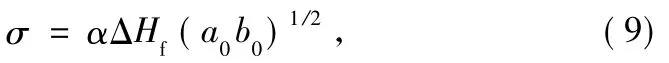
where α is derived empirically to be 0.11 by analogy with the known behaviour of hydrocarbons[19].The unit cell dimensions,a0and b0for PET are 0.457 and 0.595 nm,respectively[20].So,the value of σ for PET is calculated to be 1.2 × 10-2J/m2.
Usually Eq.(7)is written in a logarithmic form:
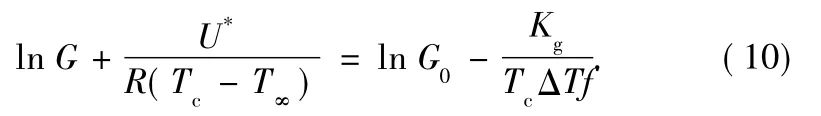
By plotting the left side of Eq.(10)versus 1/(TcΔTf),a straight line must be obtained having a slope equal to Kgand an intercept equal to ln G0.
The activation energy(U*)and the equilibrium melting temperature() are two importantfactors to investigate crystallization kinetics with Hoffman-Lauritzen secondary nucleation theory.Theof PET and PET/CB composites have already been determined to be 272 and 261℃according to linear Hoffman-Weeks plots.The value of U*is much more important to the secondary nucleation analysis than that ofand the U*value is hard to determine experimentally[20].Therefore,a suitable U*value should be selected.A series of U*values used for PET in previous literatures are summarized in Table 2[21,22].
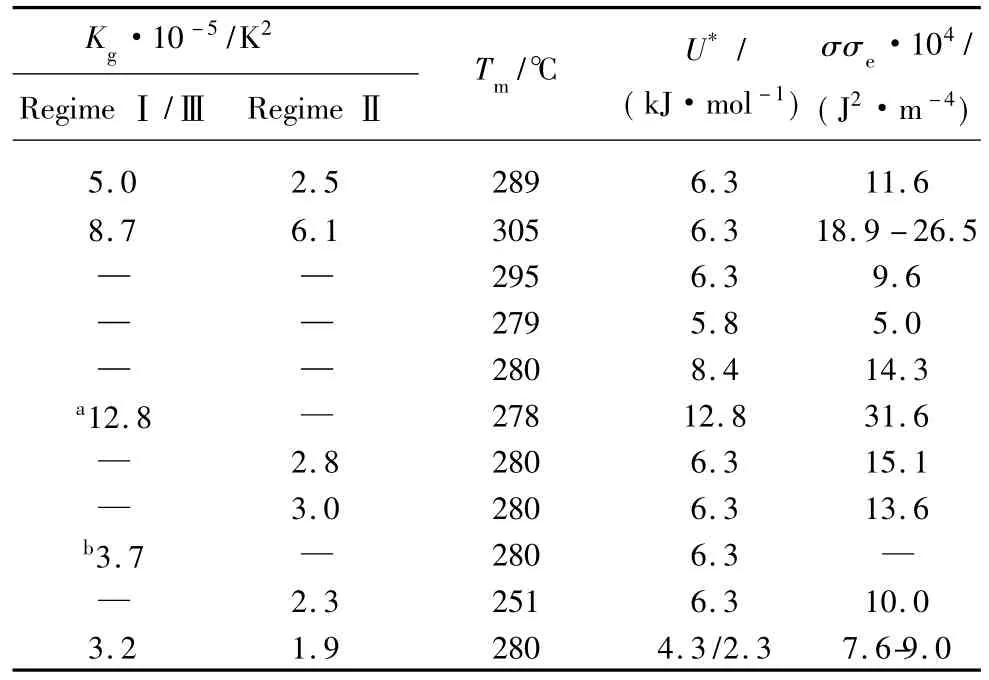
Table 2 Crystallization parameters for PET
It can be clearly seen that there is no uniform U*value for PET.In this paper,we calculated Kgand G0with four different sets of U*values,namely 4 300,6 300,8 400,and 12 800 J·mol-1.In Fig.8,plots of ln G+U*/R(Tc- T∞)versus 1/(TcΔTf)are shown for PET and PET/CB masterbatch.The values thus calculated for Kgand G0are listed in Table 3.
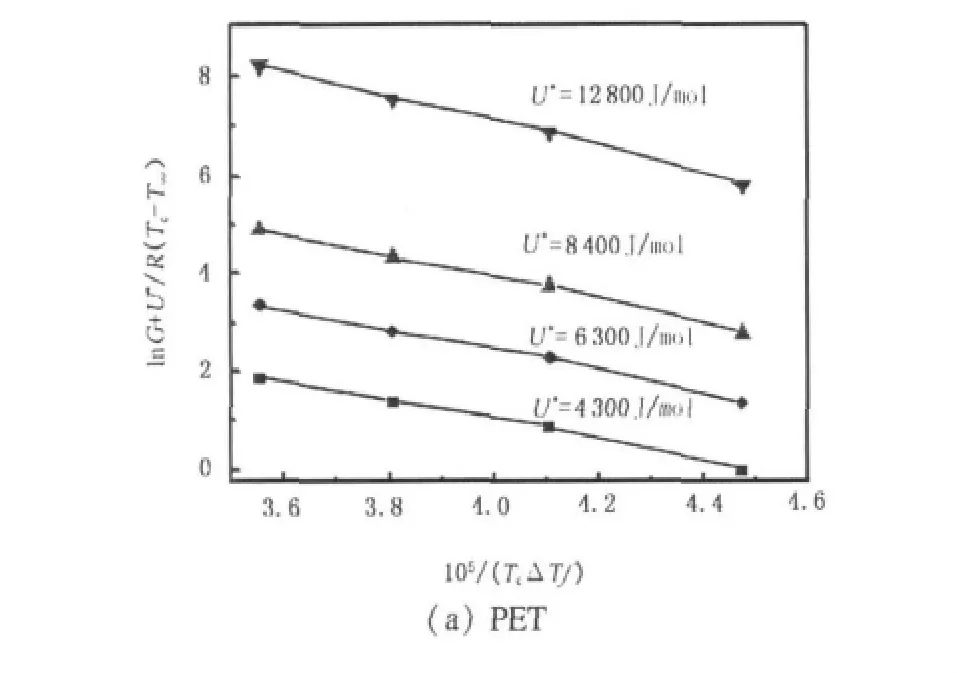
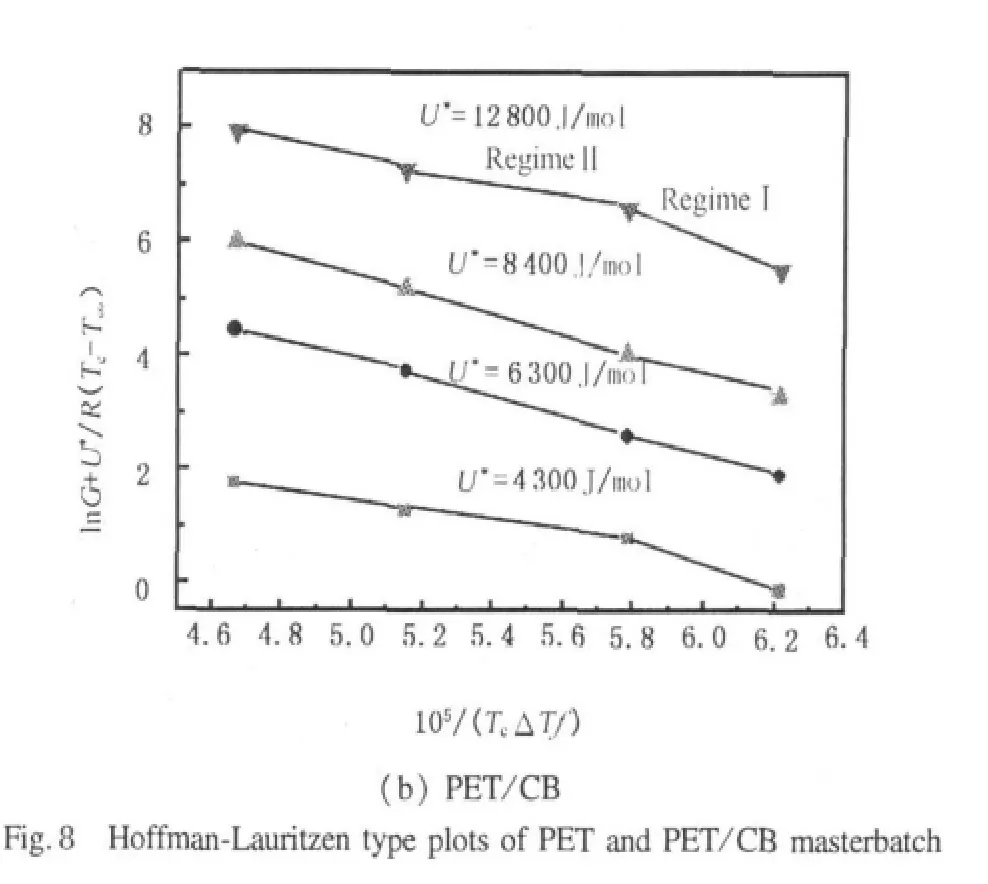
As shown in Fig.8,for PET,the plots of ln G +[U*/R(Tc- T∞)]versus 1/(TcΔTf) show good linear relationship in range of crystallization temperatures under every U*,which is also in agreement with the results of plot G versus Tcwithout obvious regime transition,though a regime transition is reported by other authors at 217 and 236 ℃[19,20].Beisides,the U*=12 800 J/mol is appropriate for PET because of the best linear relationship in Fig.8(a)and the consistency of the nucleation constant Kgwith the result based on regimeⅡcrystallization[19],which seems to indicate that PET crystallizationbelongstoregimeⅡ intheinvestigated temperature range.Nevertheless,for PET/CB masterbatch,a transition from regimeⅠto regimeⅡoccurs at around 225℃,which is consistent with a transition point in plot of G versus Tc.That is,at temperature above 225℃,PET/CB masterbatch crystallization follows regimeⅠ kinetics,while below 225℃,regimeⅡ is operative.The ratio of slopes in Fig.8(b)between Kg(Ⅰ)and Kg(Ⅱ)is 2.11,when U*is taken to be 12 800 J/mol.This is very close to the theoretical value of 2 predicted by Hoffman-Lauritzen secondary nucleation theory.Therefore,for PET crystallization,using U*=12 800 J/mol,Kg=2.56 ×105,and Z=2,the value of σeis estimated as 100.3 mJ/m2,which is in agreement with the resultby Lu[19].ForPET/CB masterbatch,using U*=12 800 J/mol,Kg=2.47 ×105,and Z=4,the value of σeis obtained to be 48.3 mJ/m2.The crystallization rate of PET/CB is much faster than that of PET,owing to the smaller value of fold surface free energy σeand the faster crystallization rate[22],which is consistent with the analysis by Avrami equation.
3 Conclusions
The isothermal crystallization kinetics of PET and PET/CB masterbatch were analyzed using Avrami equation and Hoffman-Lauritzen secondary nucleation theory.The Avrami analysis indicated that the primary crystallizations of PET and PET/CB masterbatch followed the mechanism of three-dimension spherical growth on heterogeneous nuclei,while the crystals grew linearly in the secondary crystallization process.
According to Hoffman-Weeks equation,the equilibrium melting temperatures of PET and PET/CB masterbatch were obtained to be 272 and 261℃,respectively.This implied that CB particles acted as an effective nucleating agent for PET and promoted the crystallization rate of PET,so theof PET/CB masterbatch decreased due to more imperfect PET crystals.
Analysis based on Hoffman-Lauritzen relationship equation showed that no significant evidence for regime transition of PET wasfound,though such observations had been reported previously in the literature.ForPET/CB masterbatch,a transition from regimeⅠto regimeⅡoccurs around 225℃.At temperature above 225℃,PET/CB masterbatch crystallization followed regimeⅠ kinetics,while below 225℃,regimeⅡ was operative.The fold surface free energy σe(100.3 mJ/m2)of PET was much greater than that of PET/CB masterbatch(48.3 mJ/m2) indicating heterogeneous nucleation effectofCB particles.
[1]Verhpyen O,Dupret F,Legras R.Isothermal and Non-isothermal Crystallization Kinetics of Polyethylene Terephthalate:Mathematical Modeling and Experimental Measurement[J].Polymer Engineering& Science,1999,38(9):1594-1610.
[2]Anand K A,Agarwal U S,Joseph R.Carbon Nanotubes Induced Crystallization of Poly(ethylene terephthalate) [J].Polymer,2006,47(11):3976-3980.
[3]Anand K A,Agarwal U S,Joseph R.Carbon Nanotubes-Reinforced PET Nanocomposite by Melting-Compounding[J].Journal of Applied Polymer Science,2007,104(5):3090-3095.
[4]Kim J K,Park H S,Kim S H.Multiwall-Carbon-Nanotube-Reinforced Poly(ethylene terephthalate)Nanocomposites by Melt Compounding[J].Journal of Applied Polymer Science,2007,103(3):1450-1457.
[5]Cai D,Zhang Y,Chen Y M.Effect of Organic Modification of SiO2on Non-isothermal Crystallization of PET in PET/SiO2Nanocomposites[J].Iranian Polymer Journal,2007,16(12):851-859.
[6]Xanthos M,Baltzis B C,Hsu P P.Effects of Carbonate Salts on Crystallization Kinetics and Properties of Recycled Poly(ethylene terephthalate)[J].Journal of Applied Polymer Science,1997,64(7):1423-1435.
[7]Ge C H,ShiL Y,YangH,etal.NonisothermalMelt Crystallization Kinetics of Poly(ethylene terephthalate)/Barite Nanocomposites[J].Polymer Composites,2010,31(9):1504-1514.
[8]Li X H,Guo W H,Zhou Q L,et al.Non-isothermal Crystallization Kinetics of Poly(ethylene terephthalate)/Grafted Carbon Black Composite[J].Polymer Bulletin,2007,59(5):685-697.
[9]Su Z Z,Guo W H,Liu Y J,et al.Non-isothermal Crystallization Kinetics of Poly(lactic acid)/Modified Carbon Black Composite[J].Polymer Bulletin,2009,62(5):629-642.
[10]MuchaM,KrolikowskiZ.Application ofDSC to Study Crystallization Kinetics of Polypropylene Containing Fillers[J].Journal of Thermal Analysis and Calorimetry,2003,74(2):549-557.
[11]Wiemann K,Kaminsky W,Gojny F H,et al.Synthesis and Properties of Syndiotactic Poly(propylene)/Carbon Nanofiber and Nanotube Composites Prepared by in situ Polymerization with Metallocene/MAO Catalysts[J].Macromolecular Chemistry Physics,2005,206(15):1472-1478.
[12]del Río C,Ojeda M C,Acosta J L.Carbon Black Effect on the Microstructure of Incompatible Polymer Blends[J].European Polymer Journal,2000,36(8):1687-1695.
[13]Li J,Zhou C X,Wang G,et al.Isothermal and Nonisothermal Crystallization Kinetics of Elastomeric Polypropylene[J].Polymer Testing,2002,21(5):583-589.
[14]Arroyo M,Lopez-Manchado M A,Avalos F.Crystallization Kinetics of Polypropylene:Ⅱ.Effect of the Addition of Short Glass Fibres[J].Polymer,1997,38(22):5587-5593.
[15]Feng N,Huang R,Xu D Z,et al.Study on Crystallization Behaviors of Nano-CaCO3Filled PA6 Composites[J].Engineering Plastics Application,2006,34(10):45-48.(in Chinese)
[16]Dong W,Zhao J,Li C X,et al.Study of the Amorphous Phase in Semicrystalline Poly(ethylene terephthalate) via Dynamic Mechanical Thermal Analysis[J].Polymer Bulletin,2002,49(2/3):197-203.
[17]Chen X Q,Xu J J,Lu H B,et al.Isothermal Crystallization Kinetics of Poly (butylene terephthalate)/Attapulgite Nanocomposites[J].Journal of Polymer Science:Part B Polymer Physics,2006,44(15):2112-2121.
[18]Rahman M H,Nandi A K.On the Crystallization Mechanism of Poly(ethylene terepthalate)in Its Blends with Poly(vinylidene fluoride)[J].Polymer,2002,43(25):6863-6870.
[19]Lu X F,Hay J N.Isothermal Crystallization Kinetics and Melting Behaviour of Poly(ethylene terephthalate)[J].Polymer,2001,42(23):9423-9431.
[20]Medellin-Rodriguez F J,Phillips P J,Lin J S.Application of Secondary Nucleation Theory to Semirigid Macromolecules:PEEK,PET,and PEN[J].Macromolecules,1995,28(23):7744-7755.
[21]Vyazovkin S,Stone J,Sbirrazzuoli N.Hoffman-Lauritzen Parameters for Non-isothermal Crystallization of Poly(ethylene terephthalate)and Poly(ethylene oxide)Melts[J].Journal of Thermal Analysis and Calorimetry,2005,80(1):177-180.
[22]Jiang X L,Luo S J,Sun K,et al.Effect of Nucleating Agents on Crystallization Kinetics of PET[J].Express Polymer Letters,2007,1(4):245-251.
 Journal of Donghua University(English Edition)
2012年2期
Journal of Donghua University(English Edition)
2012年2期
- Journal of Donghua University(English Edition)的其它文章
- A New Design Method for Variable Digital Filter Based on Field Programmable Gate Array(FPGA)
- Clogging Process Caused by Organic Particle Accumulation and Biofilm Growth in Subsurface Wastewater Infiltration System
- The Usability of Polyoxyethylene Stearate as Lubricant for Sizing Cotton Warp Yarns
- A Stochastic Study on the Wicking Phenomena
- Reverse Solution and Parametric Design of the Conjugate Cam Weft Insertion Mechanism Based on VB.NET and UG
