Direct Synthesis of Dimethyl Carbonate from CO2 and CH3OH Using 0.4 nm Molecular Sieve Supported Cu-Ni Bimetal Catalyst*
CHEN Huiling (陳惠玲), WANG Shuanjin (王栓緊), XIAO Min (肖敏), HAN Dongmei(韓冬梅), LU Yixin (盧一新) and MENG Yuezhong (孟躍中),**
1 The Key Laboratory of Low-carbon Chemistry & Energy Conservation of Guangdong Province; State Key Laboratory of Optoelectronic Materials and Technologies, Sun Yat-sen University, Guangzhou 510275, China
2 Department of Chemistry & Medicinal Chemistry Program, Office of Life Sciences, National University of Singapore, 3 Science Drive, Singapore 117543, Republic of Singapore
1 INTRODUCTION
Dimethyl carbonate (DMC) has attracted more attention as an environment friendly intermediate and a non-toxic substitute for poisonous and corrosive phosgene and dimethyl sulfate in the reaction of carbonylation and methylation [1, 2]. Moreover, DMC is considered to be an option for transportation fuels because of its high oxygen content (53.3%) and octane value [3]. Several commercial processes have been developed for the synthesis of DMC, including methanolysis of phosgene [4], ester exchange process[1], carbon monoxide-methyl nitrite process [1] and gas phase oxidative carbonylation of methanol [5].However, these traditional synthesis routes take use of toxic, corrosive, flammable and explosive gases such as phosgene, hydrogen, and carbon monoxide. Therefore, direct synthesis of DMC from CO2and CH3OH is a much more attractive method since such an approach is environmentally benign by nature [6, 7].
Up to now, a series of catalysts have been reported to be effective in the reaction, including organometallic cmpounds [8], metal tetra-alkoxides [9],alkali metals [10, 11], ZrO2[12, 13], [bmim]BF4/CH3ONa[14], (Cu-KF)/MgSiO [15], H3PW12O4-ZrO2[16], heterogeneous Mg/smectite [17], (Cu-Ni)/VSO [18] and Cu-(Ni, Mo, O)/SiO2[19]. However, due to the fact that CO2is highly stable and the deactivation of catalysts is easily induced by water formed in the reaction process, the yield of DMC obtained from one-step synthesis from CO2and CH3OH is still very low. Thus,the activation of CO2and the removal of water from reaction system are two mayor obstacles in the direct synthesis of DMC from CO2and CH3OH.
Many supported catalysts have been prepared and exhibited high catalytic effects on the synthesis of DMC [15-19]. To develop efficient catalytic systems,selecting a suitable supporting material is a crucial factor, because support is not merely a carrier but also it may significantly contribute to the activity of catalyst. 0.4 nm molecular sieve has many properties, including high specific surface area, strong adsorbability,good thermal stability and favorable hydrothermal stability, thereby it has been applied widely in catalytic field as water absorbent, catalyst and catalytic carrier[20-22]. In previous works [18, 23], the active component of Cu-Ni bimetal had exhibited high catalytic performances for the activation of CO2and CH3OH. Therefore,we utilize 0.4 nm molecular sieve as carrier in this work to prepare (Cu-Ni)/0.4 nm molecular sieve catalyst using the conventional wet impregnation method.The catalytic application in direct synthesis of DMC from CO2and CH3OH is systematically evaluated in the continuous flow fixed-bed reactor.
2 EXPERIMENTAL
2.1 Catalyst preparation
All the chemicals used in this work were analytical grade and commercially available. 0.4 nm molecular sieve was kindly purchased from Aladdin Reagent Co.,which had a specific surface area of 310 m2·g-1after pretreatment of grinding and sieving.
A series of different metal content from 10% to 30% (by mass) in the catalysts were prepared by impregnating 0.4 nm molecular sieve in solution from Cu(NO3)2·3H2O and Ni(NO3)2·6H2O. According to previous works [18, 23], we selected 6.8% (by mass)diluent ammonia solution and the Cu/Ni atom ratio of 2/1, besides, the molar ratio of NH3to (Cu + Ni) was 4 to 1. In the typical experiment, the pre-determined amounts of metal salts were dissolved in ammonia solution, and then the precursor solution was added to 6.0 g 0.4 nm molecular sieve. The resulting mixture was stirred at ambient temperature for 24 h, ultrasonicated for 3 h, aged for 24 h and then eliminated the solvent via rotary evaporation. The fully dried samples were crushed in an agate mortar, calcined at 773 K for 5 h and reduced in a H2(99.999% purity) flow (5 ml·min-1) at 773 K for 4 h with a heating rate of 275 K·min-1.
In order to characterize the TPR of samples,17.6% (by mass) CuO/0.4 nm molecular sieve and 9.13% (by mass) NiO/0.4 nm molecular sieve monometallic catalyst precursors were also prepared. In the preparation process, we adopted the above-mentioned similar steps, but the supporting component was single metal salt, and the molar ratio of NH3to Cu or NH3to Ni was 4.
2.2 Catalyst characterization
2.2.1 X-ray diffraction (XRD)
The phase structures of catalysts were recorded on a D/Max-IIIA power diffractometer (Shimadzu,Japan) using Cu Kα1(0.15406 nm) radiation source,operated at 35 kV and 25 mA. The intensity data were collected over a 2θ range of 5°-80° with a 0.02° step size and using a counting time of 1 s per point. The average crystallite sizes were estimated by XRD line-broadening with the help of the Debye-Scherrer equation:

where θ(hkl)is Bragg’s diffraction angle for the peak, K is Scherrer’s constant (K=0.89), λ is X-ray radiation wavelength (λ=0.15406 nm), and β (radians) is full-width at half-maximum intensity (FWHM) of the peak.
2.2.2 BET measurement and temperature programmed reduction (TPR)
Nitrogen adsorption/desorption isotherms were determined by N2physisorption in liquid N2(77 K)using ChemBET 3000 apparatus (Quantachrom, U.S.A).The Brunauer-Emmett-Teller approach was used to determine the surface area.
TPR experiments of calcined catalyst precursors were carried out using ChemBET 3000 apparatus equipped with a thermal conductivity detector (TCD).About 10 mg sample, which was placed in a quartz U-shaped tube, was undergone degassing pretreatment to dehydrate at 523 K for 3 h in a nitrogen flow (35 ml·min-1). After that, the pretreated sample was cooled to room temperature and heated to 1073 K at a heating rate of 281 K·min-1in a 5% H2/95% N2atmosphere. As a consequence of the temperature rising,the H2consumption signals of sample were continuously monitored by TCD.
2.2.3 Infra-red spectra (FTIR)
The Infrared spectra of CH3OH and CO2absorbed 1 h on the catalyst at 1.1 MPa and room temperature and the bare catalyst were recorded on an Analect RFX-65A type FTIR spectrophotometer. The spectra were recorded using the KBr pallet containing 0.1% (by mass) sample. Especially, the gaseous CH3OH was introduced by using a N2bubbling device in the adsorption process.
2.2.4 Temperature programmed desorption of ammonia (NH3-TPD) and carbon dioxide (CO2-TPD)
TPD experiments of different catalysts were performed on a U-shaped Pyrex tube furnace with helium as the carrier gas and NH3or CO2as adsorption gas using ChemBET 3000 apparatus. Typically, the 0.2 g sample was first degassed at 473 K for 2 h under a flow of helium (35 ml·min-1) to remove water and physisorbed organic molecules. When the temperature of sample was lowered to 323 K, 30% NH3/70% He or 30% CO2/70% N2mixing flow (60 ml·min-1) was introduced into the sample cell to allow the sample to absorb NH3or CO2for 1 h. After the adsorption, the mixing gas was switched to helium flow (30 ml·min-1)again to sweep the sample for 1 h. Upon completion,the furnace with sample was heated up from 323 K to 873 K at a rate of 281 K·min-1under a flow of helium(30 ml·min-1). The NH3or CO2released from the sample, as the consequence of temperature rising, was continuously monitored by TCD.
2.3 Direct synthesis of DMC from CH3OH and CO2
The catalytic activity of the synthesized (Cu-Ni)/0.4 nm molecular sieve catalysts for the synthesis of DMC from CH3OH and CO2were evaluated by using a continuous tubular fixed-bed micro-gaseous reactor. In the synthesis reaction, water was a mainly disadvantageous factor to the DMC generation, the fluxion of reaction system can take away water vapor nicely and the reaction products were detected momentarily online. The device structure used for DMC synthesis was illustrated in our previous works [19, 23] as shown schematically in Fig. 1. The total length of reactor module is 50 cm and the inside diameter is 15 mm. Moreover,the length of constant temperature zone reaches a maximum of 10 cm in the midst of the reactor. The heater temperature is measured by thermocouple with an accuracy of ±1 °C and the system pressure is determined by pressure sensor and controlled by back-pressure regulator with an accuracy of ±0.01 MPa. 4.5 g catalysts were loaded in the constant temperature zone, and to avoid gas flow blowing catalysts into the detection system and increase the contact probability of reactants, glass wool and quartz sand were filled in the both sides of catalysts. In experiment,the gaseous CH3OH was introduced into reactorviaCO2bubbling device. By careful control of temperature and gas flow rate, the molar ratio of CO2to CH3OH was quite constant at about 10 and the space velocity of total gas was controlled to be 510 h-1.

Figure 1 Schematic diagram of reactor module
The catalytic reaction was carried out at different temperatures (363-403 K) and different pressures(0.6-1.3 MPa). The resulting mixture was introduced into an on-line GC (GC7890F) equipped with a thermal conductivity detector (TCD) and a flame ionization detector (FID) to analyze the composition and concentration. The condensed liquid from the cut-off valve was collected and analyzed by the gas chromatograph mass spectrometer (GCMS-QP2010 plus) (Rigaku,Japan) to confirm the DMC formation in the reaction system. Catalyst activity was mainly indicated by CH3OH conversion and DMC selectivity. These parameters were calculated according to the following formulae:
where by-products could be dimethyl ether (DME),CO, CH2O and H2O.
3 RESULTS AND DISCUSSION
3.1 Catalytic synthesis of DMC from CO2 and CH3OH

Figure 2 Dependence of CH3OH conversion and DMC selectivity on the bimetal loading in catalysts (reaction conditions: 4.5 g catalyst, 1.1 MPa, 393 K, 5 h, n(CO2/CH3OH)=10,gas space velocity of 510 h-1)● CH3OH conversion; ■ DMC yield; ▲ DMC selectivity
3.1.1Effect of bimetal loading on the DMC synthesisFigure 2 shows that the effect of bimetal loading on catalytic performances is apparent. In the prepared catalysts, the metal content varies from 10 to 30% (by mass). The catalytic activity was found to be obviously improved with the increasing bimetal loading,and the catalyst had the highest DMC yield of 5.0%when the bimetal content reached 20% (by mass).However, once it exceeded 20% (by mass), the DMC yield declined slightly due to the integrated effect of diminishing CH3OH conversion and increasing DMC selectivity. Presumably, this is owing to the synergetic effects of Cu-Ni alloy on the activation of CH3OH and CO2. The excessive metal particles generate aggregation in sintering process, and the decrease of surface area limits the movement of metal electrons, where electrons play an important role in the activation CH3OH and CO2. In addition, metal electrons intensify the basicity of catalyst but reduce the acidity of catalyst. From the TPD analysis, proper basic and acid sites conduce to DMC formation. Therefore, the optimal bimetal content in catalytic system should be 20% (by mass).
3.1.2Effect of reaction conditions on the DMC synthesis
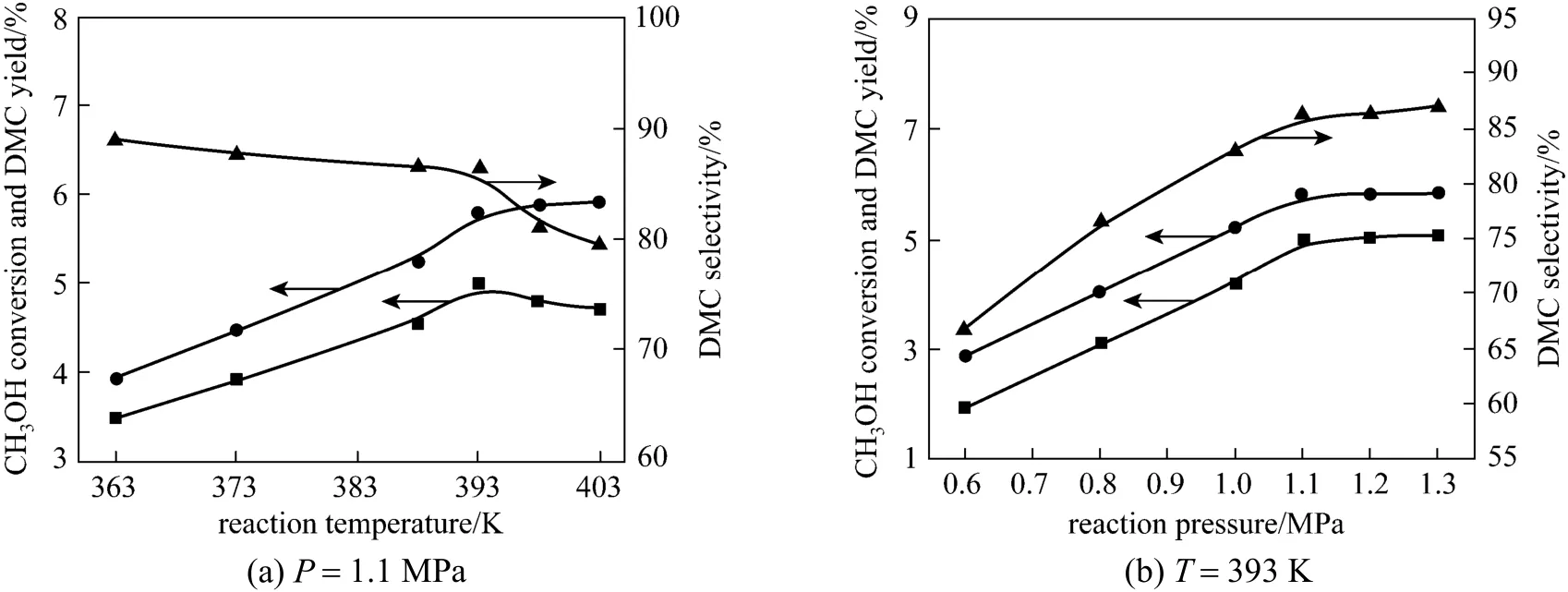
Figure 3 Dependence of CH3OH conversion and DMC selectivity on (a) reaction temperature and (b) reaction pressure[reaction conditions: 20% (by mass) (Cu-Ni), 4.5 g catalyst, 5 h, n(CO2/CH3OH)=10, gas space velocity of 510 h-1]● CH3OH conversion; ■ DMC yield; ▲ DMC selectivity
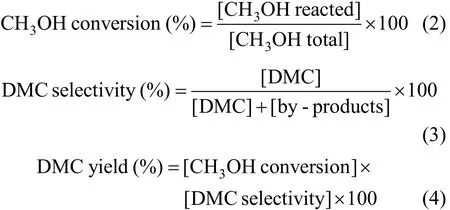
The catalytic reaction was carried out at different temperatures of 363-403 K and under different pressures ranging from 0.6 to 1.3 MPa. The results are summarized and shown in Fig. 3. Fig. 3 (a) shows the dependence of catalytic activity on reaction temperature under the constant pressure of 1.1 MPa. With the increasing temperature, the CH3OH conversion enhanced obviously, and the DMC selectivity dropped slowly. The DMC yield reached a peak value of 5.0%at 393 K, and then started to decrease due to the sharp decline in DMC selectivity above the temperature of 393 K. The reason may be that increasing temperature limited the DMC formation and leaded to the fall in DMC selectivity because of the appearance of side reactions. From the Gibbs-Helmholtz equation and relative literature [6, 24, 25], the DMC synthesis from CO2and CH3OH is an equilibrium reaction and high temperature is unfavorable to the reaction in the viewpoint of thermodynamics. Moreover, in order to promote the reaction spontaneously, increasing temperature demands higher pressure. On the other hand,the activation of CO2and CH3OH needs proper high temperature, and the particularity of reaction equipment promotes reaction because the gaseous resultants were taken away from reaction system continuously.In view of kinetics, the removal of H2O and DMC facilitates the reaction. Thus, we choose the reaction temperature of 393 K.
Pressure also plays an important role in the reaction. Fig. 3 (b) shows the pressure dependence of DMC synthesis at the constant temperature of 393 K.The CH3OH conversion, DMC yield and DMC selectivity enhanced remarkably from 0.6 to 1.1 MPa, and leveled off above the pressure of 1.1 MPa. The increasing pressure contributes to the synthesis of DMC, but in order to keep gas phase reaction state, vaporization temperatures of CH3OH and H2O increase under the higher pressure. Therefore, considering that the higher pressure has no significant effect on the synthesis reaction, the optimized reaction temperature is 1.1 MPa.
3.1.3Stability of catalyst

Figure 4 Stability of 20% (by mass) (Cu-Ni)/0.4 nm molecular sieve catalyst (reaction conditions: 4.5 g catalyst, 1.1 MPa, 393 K, n(CO2/CH3OH)=10, gas space velocity of 510 h-1)● CH3OH conversion; ■ DMC yield; ▲ DMC selectivity
Based on above results, the evaluation test was carried out at 393 K and under 1.1 MPa for 7 h to examine the stability of catalyst. Fig. 4 shows the CH3OH conversion, DMC yield and DMC selectivity as a function of reaction time. The CH3OH conversion and DMC yield increased obviously in 0.5-2 h, and then leveled off from 2 to 4.5 h, finally declined slowly after 4.5 h. In the initial reaction time of 0.5-2 h, CH3OH and CO2were adsorbed and activated on the catalyst to form active species, where active species were OCH3and2CO-according to IR analysis and references [26, 27]. After 2 h, DMC formed and desorbed from the surface of catalyst continuously,and with the reaction time increasing, the catalyst was deactivated gradually due to the change of phase state and the water generated by synthetic reaction system.On the other hand, the increasing DMC selectivity indicates that the appearance of side reaction decreased. The catalyst developed in this study exhibits quite good stability and activity in direct synthesis of DMC under given experimental conditions.
3.2 Characterization of catalysts
A quantitative comparison of the BET surfacearea of 0.4 nm molecular sieve and catalysts with different bimetal content from 10% to 30% (by mass) are given in Table 1. Evidently, the surface area of catalysts dropped sharply after loading two metals. The main reason is that metal particles cover the carrier and block the holes of carrier. In addition, the surface area of catalysts decreased slowly with metal contents because excessive metal particles easily led to agglomeration during calcination and reduction.

Table 1 BET surface area of catalysts① All the samples were determined by BET measurement after grinding and sifting by 149 μm (100 mesh) sieve.

Figure 5 XRD patterns of (a) 0.4 nm molecular sieve, (b)fresh 20% (by mass) (Cu-Ni)/0.4 nm molecular sieve and (c)used 20% (by mass) (Cu-Ni)/0.4 nm molecular sieve* Cu-Ni alloy; ○ CuO
The XRD patterns of 0.4 nm molecular sieve,fresh 20% (by mass) (Cu-Ni)/0.4 nm molecular sieve and used 20% (by mass) (Cu-Ni)/0.4 nm molecular sieve are illustrated in Fig. 5. The XRD pattern of 0.4 nm molecular sieve in Fig. 5 (a) has a series of characteristic diffraction peaks concentration in the angle range of 6°-35°. While for the catalyst, in addition to those of the carrier in Fig. 5 (a), three typical peaks at 43.89°, 51.14° and 74.12° are observed in Fig. 5 (b)corresponding to (111), (200) and (220) surface respectively, which are also assigned as the diffraction of metal phases of Cu-Ni alloy [28, 29]. The crystallite sizes of Cu-Ni alloy calculated from the Debye-Scherrer equation using (111), (200) and (220) diffraction lines are 32.70 nm, 33.62 nm and 41.89 nm, respectively.From Fig. 5 (c), it can be seen that two peaks appear at 35.76° and 58.38°, which are corresponding to CuO(110) and CuO (202) surface respectively. Therefore,the XRD results indicate that copper was oxidized and the valence state of copper changed from 0 to +2 after evaluation.

Figure 6 TPR profiles of calcined catalyst precursors(a) 0.4 nm molecular sieve; (b) NiO/0.4 nm molecular sieve;(c) CuO/0.4 nm molecular sieve; (d) (CuO-NiO)/0.4 nm molecular sieve
The TPR profiles of different samples are presented in Fig. 6. For comparative purpose, the TPR of CuO/0.4 nm molecular sieve and NiO/0.4 nm molecular sieve monometallic catalyst precursors were also characterized. As shown in Fig. 6, the carrier doesn’t show any reduction peak [Fig. 6 (a)] and the NiO/0.4 nm molecular sieve shows only one distinct peak at 700 K [Fig. 6 (b)], which is related to the reduction of NiO to Ni. This indicates that the sample has a single and well-dispersed NiO species on the surface. For the CuO/0.4 nm molecular sieve, there are two reduction peaks at 565 K and 585 K, respectively [Fig. 6 (c)]. The peaks are attributed to the stepwise reduction of surface dispersed CuO species,i.e.,CuO→Cu2O and Cu2O→Cu [30]. The TPR profile of(CuO-NiO)/0.4 nm molecular sieve shows three reduction peaks [Fig. 6 (d)], the peaks at 546 K and 580 K are related to the reduction of CuO species, and the broad peak observed at 669 K is ascribed to the reduction of NiO species and CuO-NiO compound. In addition, the reduction temperatures of CuO and NiO species on the (CuO-NiO)/0.4 nm molecular sieve are lower than those of monometallic catalyst precursors of CuO/0.4 nm molecular sieve and NiO/0.4 nm molecular sieve due to the interaction between metal oxides and carrier.
Figure 7 shows the IR spectra of the bare catalyst and adsorbed catalysts. As shown in Fig. 7 (a), 3436 cm-1, 1644 cm-1, 1007 cm-1, 556 cm-1and 464 cm-1are present in IR adsorption peaks of 20% (by mass)(Cu-Ni)/0.4 nm molecular sieve. After absorbing CH3OH on the catalyst, the IR spectrum in Fig. 7 (b)shows two absorption peaks of C H bond (2936 cm-1and 2855 cm-1) and C O bond (1043 cm-1), which indicated the formation of methoxyl species [31]. From Fig. 7 (c), CO2absorbed on the catalyst generated bidentate carbonate species at 1565 cm-1[32]. For easier comparison, co-adsorption of CH3OH and CO2experiment was also conducted, and we can see that the IR spectrum combines the absorption species of CH3OH and CO2in Fig. 7 (d).
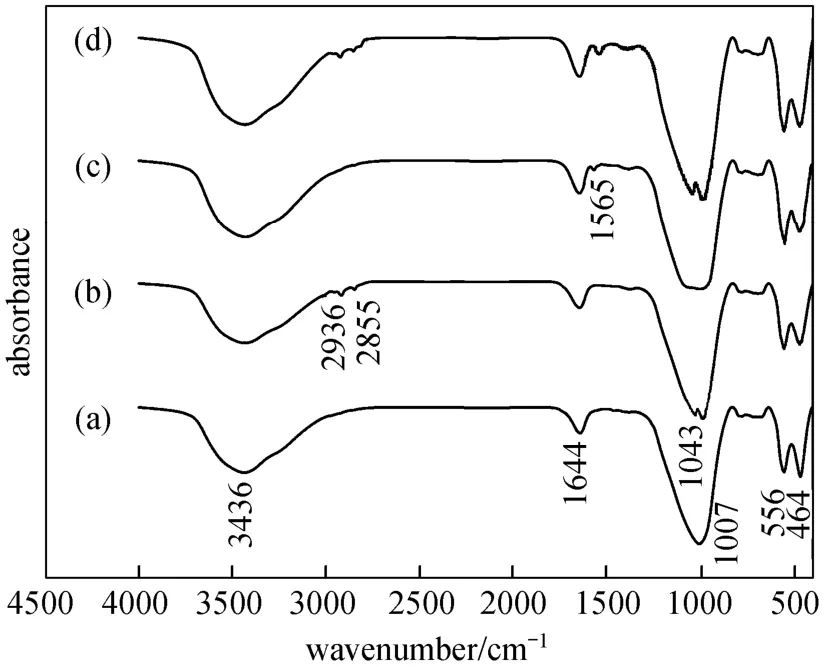
Figure 7 IR spectra of CH3OH and CO2 absorbed on 20%(by mass) (Cu-Ni)/0.4 nm molecular sieve system(a) catalyst; (b) catalyst + CH3OH; (c) catalyst + CO2; (d) catalyst + CH3OH + CO2
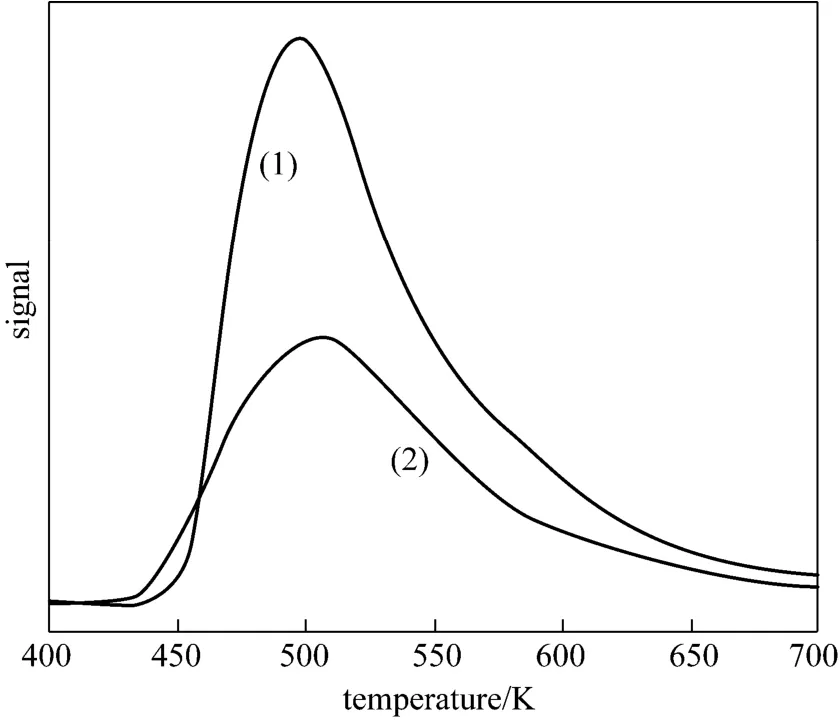
Figure 8 NH3-TPD patterns of (1) 0.4 nm molecular sieve and (2) 20% (by mass) (Cu-Ni)/0.4 nm molecular sieve
The acid property of 0.4 nm molecular sieve and 20% (by mass) (Cu-Ni)/0.4 nm molecular sieve were measured by NH3-TPD experiments. As shown in Fig. 8,the NH3-TPD profiles show a broad and asymmetric desorption feature, it could be assigned to the desorption of physisorbed NH3from Lewis acid sites. After NH3was adsorbed 1 h on the samples at 323 K, the desorption temperatures of 0.4 nm molecular sieve and 20% (by mass) (Cu-Ni)/0.4 nm molecular sieve were monitored at 443-660 K and 432-648 K, respectively. Moreover, 0.4 nm molecular sieve exhibited a larger acidity (acid amount, peak area) than 20% (by mass) (Cu-Ni)/0.4 nm molecular sieve because the electrons of metal particles have Lewis basic sites and they can neutralize the acidity of carrier. According to the mechanism of DMC formation [33, 34], CO2is activated to2CO-species on the effect of metal electrons and acid sites because CO2belongs to electron acceptor and can receive electron to form activated species. On the other hand, CH3OH is activated to methyl species on the acid sites, and the species is negative to the DMC selectivity. Therefore, proper acid sites are favorable for the facile activation of CO2to2CO-species and the improvement of DMC selectivity in the synthesis of DMC from CO2and CH3OH.
To investigate the basic property of 0.4 nm molecular sieve and 20% (by mass) (Cu-Ni)/0.4 nm molecular sieve, CO2-TPD experiments were carried out.From the profiles of Fig. 9, CO2desorption amount of 0.4 nm molecular sieve and 20% (by mass) (Cu-Ni)/0.4 nm molecular sieve were monitored at 374-566 K and 387-603 K respectively, and CO2-TPD peak of 0.4 nm molecular sieve appeared at a lower temperature than that of 20% (by mass) (Cu-Ni)/0.4 nm molecular sieve.However, the basicity (basic amount, peak area) of 0.4 nm molecular sieve was smaller than that of 20% (by mass) (Cu-Ni)/0.4 nm molecular sieve with respect to the basicity superimposition of metal electrons and carrier. The first reaction step involved the formation of methoxyl species from methanol on the basic sites,and then the methoxyl carbonate anion was formed by the reaction of methoxyl species with activated carbon dioxide on the basic sites [16, 34]. Thus, the basicity of catalysts plays an important role in determining the catalytic performance on the direct synthesis of DMC from CH3OH and CO2.
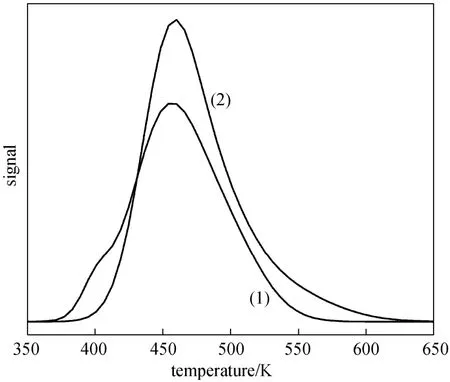
Figure 9 CO2-TPD profiles of (1) 0.4 nm molecular sieve and (2) 20% (by mass) (Cu-Ni)/0.4 nm molecular sieve
4 CONCLUSIONS
Different bimetal contents of (Cu-Ni)/0.4 nm molecular sieve catalysts were prepared and applied in the direct synthesis of DMC from CH3OH and CO2.Under the optimal reaction conditions (393 K, 1.1 MPa, 5 h), the catalyst of 20% (by mass) (Cu-Ni)/0.4 nm molecular sieve showed that the highest selectivity and yield of DMC were higher than 86.0% and 5.0%,respectively. The evaluation results are generally larger than those of references (DMC yield of 2.0%-4.8%)[16-24]. The synergetic effect of metal Cu-Ni alloy played an important role in the activation of CH3OH and CO2. It was believed that the large amount of basic sites can facilitate the activation of CH3OH to methoxyl species and their subsequent reaction withspecies activated on acid sites.
ACKNOWLEDGEMENTS
LU Yixin thanks National University of Singapore for financial support.
1 Aresta, M., Quaranta, E., “Carbon dioxide: A substitute for phosgene”, Chemtech., 27 (3), 32-40 (1997).
2 Shaikh, A.A.G., Sivaram, S., “Organic carbonate”, Chem. Rev., 96(3), 951-976 (1996).
3 Ono, Y., “Dimethyl carbonate for environmentally benign reactions”,Pure Appl. Chem., 68 (2), 367-375 (1996).
4 Tundo, P., Moraglio, G., Trotta, F., “Gas-liquid phase-transfer catalysis: A new continuous-flow method in organic synthesis”, Ind. Eng.Chem. Res., 28 (7), 881-890 (1989).
5 Molzahn, D., Jones, M.E., Hartwell, G. E., Puga, J., “Production of dialkyl carbonates using copper catalysts”, US Pat., 5387708 (1995).
6 Fujita, S., Bhanage, B. M., Ikushima, Y., Arai, M., “Synthesis of dimethyl carbonate from carbon dioxide and methanol in the presence of methyl iodide and base catalysts under mild conditions: Effect of reaction conditions and reaction mechanism”, Green Chem., 3,87-91 (2001).
7 Choi, J.C., He, L.N., Yasuda, H., Sakakura, T., “Selective and high yield synthesis of dimethyl carbonate directly from carbon dioxide and methanol”, Geen Chem., 4, 230-234 (2002).
8 Kizlink, J., Pastucha, I., “Synthesis of dimethyl carbonate from carbon dioxide and methanol in the presence of organotin compounds”,Collect. Czech. Chem. Comm., 58, 1399-1402 (1993).
9 Kizlink, J., Pastucha, I., “Preparation of dimethyl carbonate from methanol and carbon dioxide in the presence of Sn (IV) and Ti (IV)alkoxides and metal acetates”, Collect. Czech. Chem. Comm., 60,687-692 (1995).
10 Zhang, D., Wang, Q., Yao, J., Wang, Y., Zeng, Y., Wang, G.,“Simultaneous synthesis of dimethyl carbonate and poly (ethylene terephthalate) using alkali metals as catalysts”, Chin. J. Chem. Eng.,15 (5), 772-774 (2007).
11 Fang, S., Fujimoto, K., “Direct synthesis of dimethyl carbonate from carbon dioxide and methanol catalyzed by base”, Appl. Catal. A Gen., 142, L1-L3 (1996).
12 Tomishige, K., Sakaihiro, T., Ikeda, Y., Fujimoto, K., “A novel method of direct synthesis of dimethyl carbonate from methanol and carbon dioxide catalyzed by zirconia”, Catal. Lett., 58 (4), 225-229(1999).
13 Tomishige, K., Furusawa, Y., Ikeda, Y., Asadullah, M., Fujimoto, K.,“CeO2-ZrO2solid solution catalyst for selective synthesis of dimethyl carbonate from methanol and carbon dioxide”, Catal. Lett.,76 (1-2), 71-74 (2001).
14 Chen, X.Z., Hu, C.W., Su, J.H., Yu, T., Gao, Z.M., “One-pot synthesis of dimethyl carbonate catalyzed by [bmim]BF4/CH3ONa”, Chin.J Catal., 27 (6), 485-488 (2006).
15 Li, C., Zhong, S., “Study on application of membrane reactor in direct synthesis DMC from CO2and CH3OH over Cu-KF/MgSiO catalyst”, Catal. Today, 82 (1-4), 83-90 (2003).
16 Jiang, C.J., Guo, Y.H., Wang, C.G., Hu, C.W., Wu, Y., Wang, E.B.,“Synthesis of dimethyl carbonate from methanol and carbon dioxide in the presence of polyoxometalates under mild conditions”, Appl.Catal. A Gen., 256 (1-2), 203-212 (2003).
17 Bhanage, B.M., Fujita, S., Ikushima, Y., Torii, K., Arai, M., “Synthesis of dimethyl carbonate and glycols from carbon dioxide, epoxies and methanol using heterogeneous Mg containing smectite catalysts:Effect of reaction variables on activity and selectivity performance”,Green Chem., 5 (1), 71-75 (2003).
18 Wu, X.L., Meng, Y.Z., Xiao, M., Lu, Y.X., “Direct synthesis of dimethyl carbonate (DMC) using Cu-Ni/VSO as catalyst”, J. Mol.Catal. A Chem., 249 (1-2), 93-97 (2006).
19 Wang, X.J., Xiao, M., Wang, S.J., Lu, Y.X., Meng, Y.Z., “Direct synthesis of dimethyl carbonate from carbon dioxide and methanol using supported copper (Ni, V, O) catalyst with photo-assistance”, J.Mol. Catal. A Chem., 278, 92-96 (2007).
20 Zhu, W., Gora, L., van den berg, A.W.C., Kapteijn, F., Jansen, J.C.,Moulijn, J.A., “Water vapour separation from permanent gases by a zeolite-4A membrane”, J. Membr. Sci., 253 (1-2), 57-66 (2005).
21 Liu, Y.H., Cao, L.H., “An expedient one-step synthesis of polysubstituted guanidinoglucosides using HgO-4 ? molecular sieves as catalyst”, Carbohydr. Res., 343 (14), 2376-2383 (2008).
22 Li, Y., Zhao, X.Q., Wang, Y.J., “Synthesis of dimethyl carbonate from methanol, propylene oxide and carbon dioxide over KOH/4A molecular sieve”, Appl. Catal. A Gen., 279 (1-2), 205-208 (2005).
23 Bian, J., Xiao, M., Wang, S.J., Lu, Y.X., Meng, Y.Z., “Carbon nanotubes supported Cu-Ni bimetallic catalysts and their properties for the direct synthesis of dimethyl carbonate from methanol and carbon dioxide”, Appl. Sur. Sci., 255, 7188-7196 (2009).
24 Tomishige, K., Kuninori, K., “Catalytic and direct synthesis of dimethyl carbonate starting from carbon dioxide using CeO2-ZrO2solid solution heterogeneous catalyst effect of H2O removal from the reaction system”, Appl. Catal. A Gen., 237 (1-2), 103-109 (2002).
25 Benson, S.W., Thermochemical Kinetics: Methods for the Estimation of Thermochemical Data and Rate Parameters, Wiley, New York,18-51 (1968).
26 Xie, S., Bell, A.T., “An in situ Raman study of dimethyl carbonate synthesis from carbon dioxide and methanol over zirconia”, Catal.Lett., 70 (3-4), 137-143 (2000).
27 Tomishige, K., Ikeda, Y., Sakaihori, T., Fujimoto, K., “Catalytic properties and structure of zirconia catalysts for direct synthesis of dimethyl carbonate from methanol and carbon dioxide”, J. Catal.,192 (2), 355-362 (2000).
28 Niu, H.L., Chen, Q.W., Lin, Y.S., Zhu, H.F., Ning, M., “Hydrothermal formation of magnetic Ni-Cu alloy nanocrystallites at low temperatures”, Nanotechnol., 15, 1054-1058 (2004).
29 Naghash, A.R., Etsell, T.H., Xu, S., “XRD and XPS study of Cu-Ni interactions on reduced copper-nickel-aluminum oxide solid solution catalysts”, Chem. Mater., 18, 2480-2488 (2006).
30 Chen, H., Zhu, H., Wu, Y., Gao, F., Dong, L., Zhu, J., “Dispersion,reduction and catalytic properties of copper oxide supported on Ce0.5Zr0.5O2solid solution”, J. Mol. Catal. A Chem., 255, 254-259(2006).
31 Ouyang, F., Nakayama, A., Tabada, K., Suzuki, E., “Infrared study of a novel acid-base site on ZrO2by adsorbed probe molecules (I)Pyridine, carbon dioxide, and formic acid adsorption”, J. Phys.Chem. B, 104, 2012-2018 (2000).
32 Buscaa, G., Lorenzellia, V., “Infrared spectroscopic identification of species arising from reactive adsorption of carbon oxides on metal oxide surfaces”, Mater. Chem., 7 (1), 89-126 (1982).
33 Jung, K.T., Bell, A.T., “Effects of catalyst phase structure on the elementary processes involved in the synthesis of dimethyl carbonate from methanol and carbon dioxide over zirconia”, Top Catal., 20(1-4), 97-105 (2002).
34 La, K.W., Jung, J.C., Kim, H., Baeck, S.H., Song, I.K., “Effect of acid-base properties of H3PW12O40/CexTi1-xO2catalysts on the direct synthesis of dimethyl carbonate from methanol and carbon dioxide:A TPD study of H3PW12O40/CexTi1-xO2catalysts”, J. Mol. Catal. A Chem., 269 (1-2), 41-45 (2007).
 Chinese Journal of Chemical Engineering2012年5期
Chinese Journal of Chemical Engineering2012年5期
- Chinese Journal of Chemical Engineering的其它文章
- Adsorption and Desorption of Praseodymium (III) from Aqueous Solution Using D72 Resin*
- Reactive Distillation for Producing n-Butyl Acetate: Experiment and Simulation
- One Step Bioleaching of Sulphide Ore with Low Concentration of Arsenic by Aspergillus niger and Taguchi Orthogonal Array Optimization*
- Adsorption of Chlortetracycline from Water by Rectories*
- Optimization of Fermentation Media for Enhancing Nitrite-oxidizing Activity by Artificial Neural Network Coupling Genetic Algorithm*
- Effect of Propanoic Acid on Ethanol Fermentation by Saccharomyces cerevisiae in an Ethanol-Methane Coupled Fermentation Process*
