Regeneration of Spent Activated Carbon by Yeast and Chemical Method*
HE Wenhui (何文會), Lü Guocheng (呂國誠),**, CUI Jie (崔婕), WU Limei (吳麗梅) and LIAO Libing (廖立兵),**
1 School of Materials Science and Technology, China University of Geosciences, Beijing 100083, China
2 Sinoma Geological Engineering Exploration Academy, Beijing 100176, China
1 INTRODUCTION
Activated carbon (AC) has been widely used as sorbent due to its great adsorption capacity towards a variety of different solutes [1-3]. It is used in the fields of chemical environmental protection, food and pharmaceutical industry hydrometallurgy catalyst carrier,electrode material, military-chemical defense and so on [4, 5]. The AC has become essential in national economy and national defense construction.
However, because of its high material cost, it is necessary to reduce the overall cost for regeneration of spent AC. Current available methods for AC regeneration include chemical and biological methods, wet oxidative, ultrasound and solvent assisted, as well as heat and photo catalytic methods [6-10]. But these methods tend to have higher costs and are difficult to be industrialized. In order to achieve the aim, the costs must be minimized and the regenerated AC should be also ensured to be used in line with the requirements of national standards.
In this study, the spent AC was activated by yeast.The substance adsorbed in the spent AC was dissolved by NaOH to restore the adsorption capacity of AC.This method has the advantages such as less investment in equipment, simple process and sustainable regeneration, which ensures the quality of regeneration of AC to meet the national standards and achieve the economic and environmental requirements. The efficiency of AC regeneration was evaluated by adsorption of MB, and the surface area was calculated using the BET method from nitrogen adsorption at 77 K and the pore size distribution was characterized by the H-K equation. In this work, phenol was removed by AC from aqueous solution using simulative wastewater containing phenol and the adsorption isotherm and the adsorption characteristics of phenol on AC were investigated to illustrate the regeneration efficiency.
2 EXPERIMENTAL
2.1 Materials
The virgin and spent AC were supplied by Caramel Factory (Xiwang Co., Ltd., Shandong, China).The spent AC was saturated with caramel and contained significant amounts of impurities, such as sand, organic pigments, etc. Reagents used in the study include methylene blue (MB), sodium phosphate, monopotassium phosphate, anhydrous ethyl alcohol, sodium hydroxide and hydrochloric acid. They were purchased from a variety of manufactures and were analytical grade.
2.2 Pretreatment of spent AC
50 g spent AC was mixed with 100 ml water to make a pretreatment solution. The mixture was passed through a sieve with pore diameter of 0.1 mm to remove the sand. Then the mixture was rinsed with distilled water before being dried.
2.3 Treatment of spent AC by yeast and NaOH
After organic matter adsorbed in AC was broken
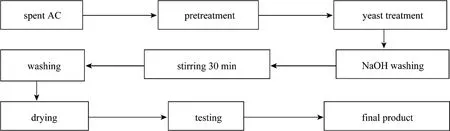
Figure 1 Technical process diagram of AC regeneration
down by yeast, the AC was washed using NaOH solution,and then part of the pigment including the majority of small organic molecules and proteins colloidal was brought to aqueous phase. Also, sugar, phenol, acids and other organic matters [11, 12] became soluble salts in water, and thus the adsorption capacity of AC was restored. In our study, single factor (such as the amount of yeast, yeast culture time, temperature, pH value, stirring temperature and NaOH concentration) was changed to regenerate AC. After regeneration, the samples were dried under ambient air condition for future study on efficiencies of AC regeneration. A detailed flow chart of the regeneration process was illustrated in Fig. 1.The efficiency of AC regeneration was evaluated by adsorption of MB, a standard method, to assess the performance of AC. 0.1 g AC was mixed with 10 ml of 1.5 g·L-1MB solution in a 50 ml flask. The mixture was oscillated for 20 min and centrifuged, and the absorbance was measured at the wavelength of 665 nm (GB/T12496.10-1999).
2.4 Methods of material characterization
Surface area (SA) and pore structure of AC were determined using V-Sorb 2800P surface area and pore size analyzer (Gold APP Instruments Corporation,China). The samples were degassed at 200 oC for 2.5 h.The SA was calculated using the BET method from nitrogen adsorption at 77 K [13-15] and the pore size distribution was characterized by the H-K equation [16].
The surface morphology of the new, spent and regenerated AC was characterized by a Scanning Electron Microscopy (JSM.54l0LV, Japan) to estimate the efficiency of regeneration.
The adsorption of phenol on AC was studied at certain temperature and pH value by batch experiments. The sorption isotherm is important for an experiment. In our study, the equilibrium amount of adsorbed phenol (Qe) by regenerated AC was calculated as follows:
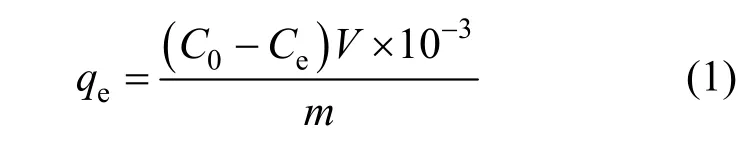
whereC0andCe(mg·L-1) represent the initial and equilibrium concentrations of phenol solution, respectively,Vis the volume of the solution (ml),Mis the quality of AC (g), andQeis saturated adsorption (mg·g-1).
碼組同步模塊的主要功能是監(jiān)測鏈路是否找到正確的字節(jié)邊界,也就是從串行的比特流中正確的對字節(jié)進行定界。根據(jù)碼組同步原理,當(dāng)接收端連續(xù)收到4個正確的/K/字節(jié)后,認(rèn)為達到碼組同步。
3 RESULTS AND DISCUSSION
3.1 Effect of different experimental conditions on the efficiency of AC regeneration
The amount of yeast is one of the important factors which had an impact on AC regeneration. When the amount of yeast varied from 0.025%-0.200%, the changes in regeneration could be found in Fig. 2 (a).MB adsorption of 0.075% yeast was about the same as 0.200% one. Taking into account the costs and economic benefits, 0.075% yeast was chosen to regenerate the spent AC.
One of the factors affecting the efficiency of regeneration is the time of yeast processing AC. Fig. 2 (b)shows that as the time increases from 0 to 8 h, the amount of MB adsorption enhances from 157.5 to 186 mg·g-1.Further increase in the time to 24 h does not improve MB adsorption significantly (186.3 mg·g-1). Thus, 8 h was chosen as the incubation time for further study.
In addition, the temperature of yeast processing AC also had an influence on the effect of AC regeneration. Maximum MB adsorption is achieved when the temperature is 35 °C (308 K) [Fig. 2 (c)]. Further increasing temperature results in a systemic decrease in MB adsorption, because yeast cannot survive at high temperature.
Figure 2 (d) shows the effect of the pH value of the yeast processing AC as indicated by MB adsorption.When the treatment solution is acidic, the MB adsorption is low. When a pH value of the treatment solution is 6, the best reproducibility of yeast to decompose organic matter adsorbed in AC is obtained with the maximum MB adsorption. It is known that the growth of yeast under acidic condition is not suitable.
Effect of the amount of NaOH on the efficiency of AC regeneration was evaluated under 0-8%.Maximum MB was obtained at 8% sodium hydroxide.However, adsorption of MB of 6% NaOH was about the same to 8% NaOH. Although the greater the amount of alkali was, the better regeneration of activated carbon was, taking into account the regeneration cost, 6%NaOH could achieve ideal regeneration [Fig. 2 (e)].
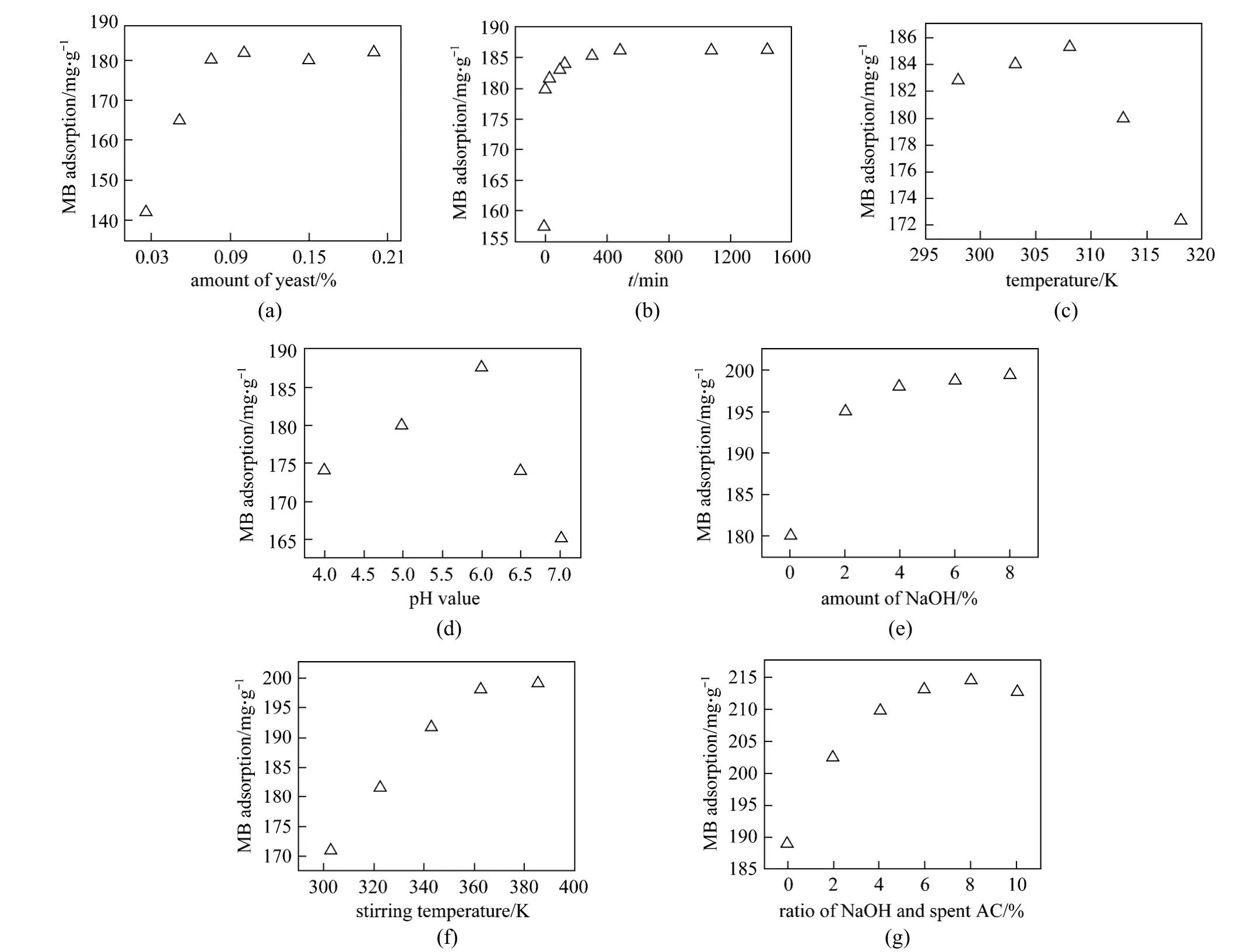
Figure 2 Effect of the amount of yeast (a) and incubation time (b), temperature (c), pH value on yeast processing AC (d),and the amount of NaOH (e), stirring temperature (f), the amount of NaOH after yeast processing AC (g) on the efficiency of AC adsorption of MB
Stirring temperature for regeneration was another factor that needed to be optimized. The MB adsorption quickly increases as the stirring temperature enhances from 30 °C (303 K) to 90 °C (363 K) [Fig. 2 (f)], suggesting that higher temperature favors organics desorption in NaOH. However, at excessive temperature beyond 90 °C, MB adsorption does not increase significantly. Because high temperature means high cost and risk, 90 °C of stirring temperature is chosen.
Figure 2 (g) shows the effect of AC washed by different concentrations of NaOH on the MB adsorption of the regeneration of AC under 0.075% yeast in 35 °C and pH 6 with processed AC 8 h. Fig. 2 (g) indicates that after washing the AC using 6%, 8% and 10% NaOH, the value of MB adsorption is all about 213 mg·g-1, showing that regeneration of AC is basically the same. Thus, the significant advantages of this study were less investment in equipment, simple process and low regeneration cost. In addition, the regeneration of AC could be used in line with the requirements of national standards.
3.2 Characterization of activated carbon
It is known that surface area (SA), pore volume and pore size distribution are important factors affecting the performance of AC. Larger surface area and pore volume can result in higher adsorption capacity.The nitrogen adsorption of virgin and regenerated AC are significantly higher than that of spent AC, suggesting that virgin and regenerated ACs have essentially the same pore structure (Fig. 3). The aperture of spent AC had been filled with a lot of material, resulting in a much lower N2adsorption capacity.
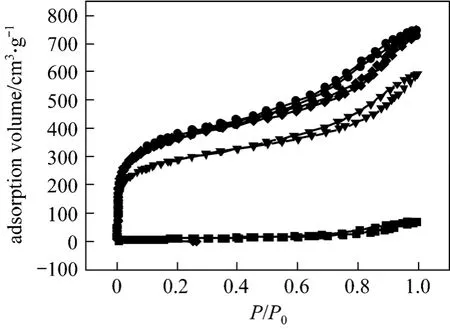
Figure 3 Adsorption isotherms of N2 at 77 K■ spent AC; ▼ virgin AC; ◆ 6% NaOH regenerated AC; ● 10%NaOH regenerated AC
Pore size distribution obtained by using the H-K equation is illustrated in Fig. 4 [17].
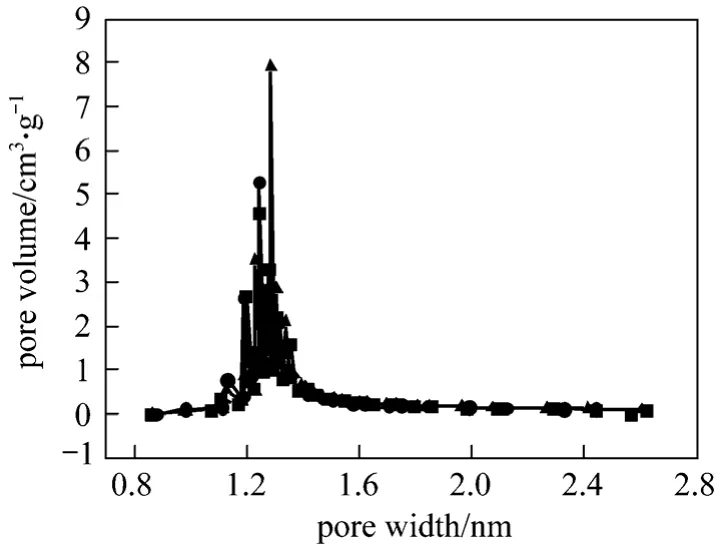
Figure 4 Pore size distribution obtained by the H-K■ 6% NaOH regenerated AC; ● 10% NaOH regenerated AC;▲ virgin AC
The pore diameters of virgin and regenerated AC are mainly in the range of 1.2-1.4 nm with a mean diameter at 1.28 nm and a pore volume of 1.13 cm3·g-1. Under good condition, yeast could quickly breed and effectively break down the organic matter adsorbed in the spent AC. In the NaOH solution,inorganics adsorbed in the spent AC were brought to charcoalin vitroand water-insoluble substances were turned into water-soluble salts, which restored the porous structure of the AC.
SEM was used to study the surface characteristics of AC. It is found that the macro pore space of the spent, virgin, and regenerated AC (6%NaOH) are different. It is obvious that there are many drum kits on the surface of spent AC particles. However, the particles surface of virgin and regenerated AC is relatively smooth. These drum kits are the materials adsorbed in the spent AC as indicated by arrows in Fig. 5 (a). The pores on the surface of the virgin AC are of various sizes and depths. In addition, the surface of AC is uneven. There are some small grains internally and on the surface that are thought to be metal oxide or carbon particles from the activating process [Fig. 5(b)]. It is clear that there is very little adsorption material in the surface area of regeneration of AC. These impurities reduce the absorption capacity [Fig. 5(c)]. But its rich pore structure enables it having large specific surface area and strong adsorption ability.
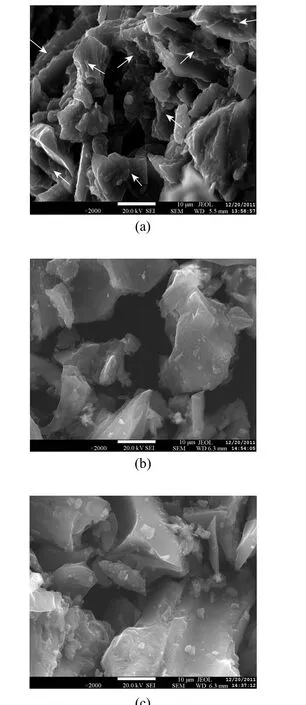
Figure 5 SEM micrograph of the spent (a), virgin (b),and regenerated (c) AC
3.3 Sorption isotherm of AC on phenol
3.3.1Effect of pH on phenol adsorption by regenerated AC
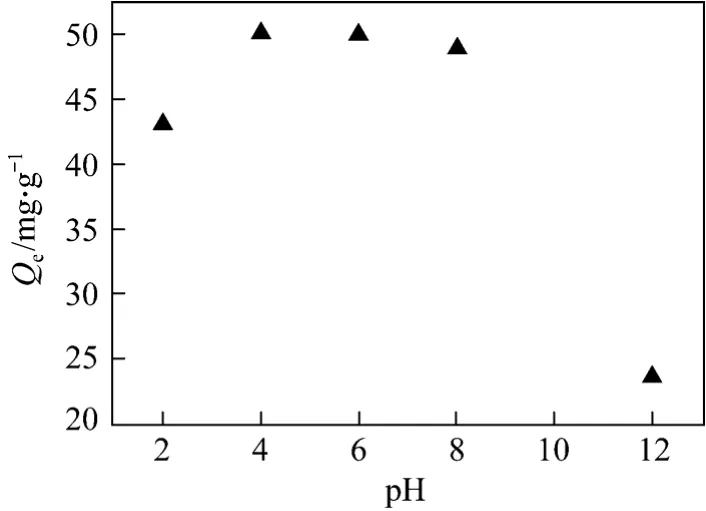
Figure 6 Effect of pH on phenol adsorption on regenerated AC
It is known that the pH of aqueous solutions is an important parameter that influences most of solid/liquid adsorption. Fig. 6 shows the effect of pH on phenol removal by regenerated AC. It is found that the best range of pH values was among 4-8. Considering the physical and chemical nature of phenol, its acidic and solubility are not high. In acidic and neutral conditions,the adsorption of phenol molecule on AC plays a dominant role (Fig. 6). However, in strong alkaline conditions, the phenol molecules turns into phenolate,which increases its solubility. Then, the desorption of phenol on AC is more obvious, leading to a sharp drop in the removal rate.
3.3.2Effect of contact time on phenol adsorption by regenerated AC
Figure 7 shows the kinetic data of regenerated AC on phenol adsorption at 298K [1] and pH of 4-8.With a certain amount of regenerated AC, the phenol removal increases with time and then attains equilibrium after 60 min. Thus, 60 min was chosen as one of our experimental conditions.
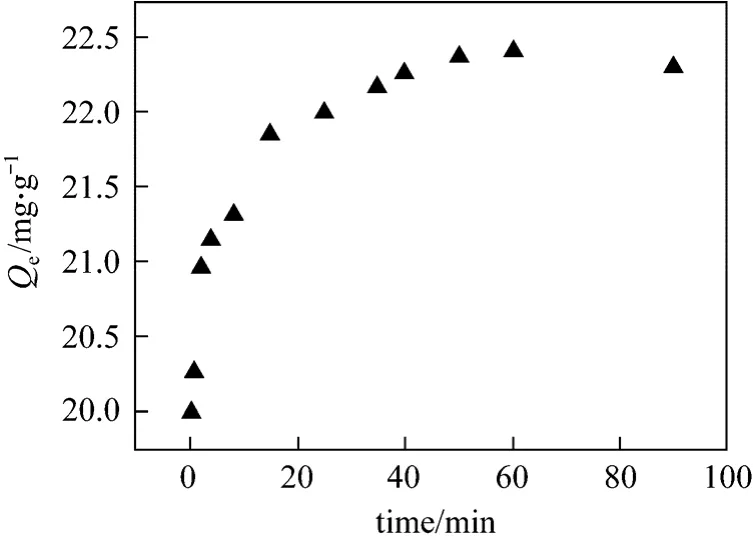
Figure 7 Effect of contact time on phenol adsorption on regenerated AC
3.3.3Establishment of sorption isotherms
In the same experimental conditions, the sorption isotherms of spent AC, regenerated AC and virgin AC are studied, respectively (Fig. 8). The adsorption amount is calculated according to Eq. (1). From Fig. 8 it is found that the regenerated AC and virgin AC have almost the same ability to remove the phenol, which far higher than that of spent AC.
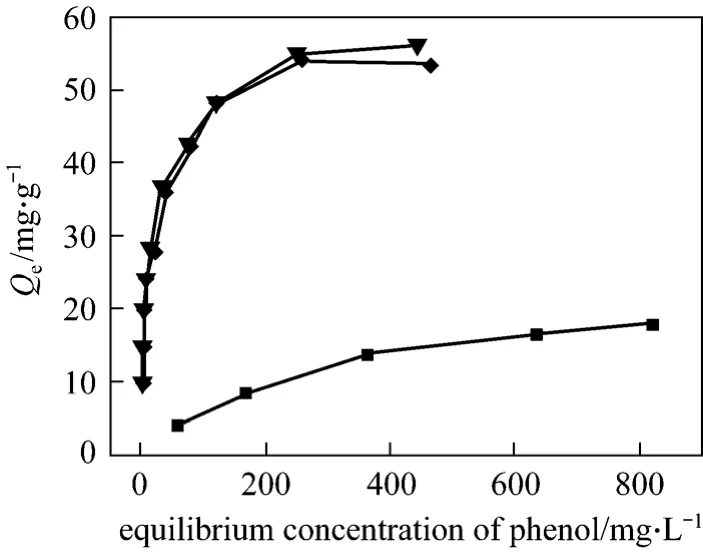
Figure 8 The sorption isotherms▼ virgin AC; ◆ regenerated AC; ■ spent AC
4 CONCLUSIONS
Using 0.075% yeast at 35 °C and a pH value of 6 for 8 h could effectively break down organic matter adsorbed in the spent AC. Adding 6% NaOH and stirring at 90 °C, the MB adsorption of the regenerated AC could reach 213 mg·g-1. The regenerated AC can be used in line with the requirements of national standards. The advantages of this study are less investment in equipment, simple process, sustainable regeneration, which makes it possible to industrialize the technics of AC regeneration with economic and environmental benefits.
1 Lü, G.C., Hao, J., Liu, L., Ma, H.W., Wei, M.Q., “The Adsorption of Phenol by Lignite Activated Carbon”,Chin.J.Chem.Eng., 19 (3),380-385 (2011).
2 Zhang, C.X., Long, D.H., Xing, B., Qiao, W., Zhang, R., Zhan, L.,Liang, X., Ling, L., “The superior electrochemical performance of oxygen rich activated carbons prepared from bituminous coal”,Electrochemistry Communications, 10, 1809-1811 (2008).
3 Lu, S.M., Ma, Y.G., Zhu, C.Y., Shen, S.H., “The enhancement of CO2chemical absorption by K2CO3aqueous solution in the presence of activated carbon particles”,Chin.J.Chem.Eng., 15 (6), 842-846(2007).
4 Yu, M.X., Wang, S.W., “Oxidative removal of dibenzothiophene by hydrogen peroxide catalyzed by activated carbon”,Journal of Chemical Industry and Engineering(China), 59 (6), 1425-1429(2008). (in Chinese)
5 Zhou, L.F., Yang, W.S., “Electrochemical decolorization of amaranth solution on ACF under potentiostatic mode1”,Journal of Chemical Industry and Engineering(China), 57 (10), 2420-2242 (2006). (in Chinese)
6 Jones, D.A., Lelyveld, T.P., Mavrofidis, S.D., “Microwave heating applications in environmental engineering a review”,Resources Conservation and Recycling, 34 (2), 75-90 (2002).
7 Salvador, F., Sanchez, J.C., “A new method for regeneration activated carbon by thermal desorption with liquid water under subcritical conditions”,Carbon, 34 (4), 511-516 (1996).
8 Chen, M.S., Wang, J.H., “Microwave regeneration of activated carbon containing toluene”,Environmental Pollution Control Technology and Equipment, 7 (6), 77-79 (2006).
9 Zhang, H.P., Ye, L.Y., “Electrochemical regeneration of activated carbon”, Chem. Eng. Progress, 20 (1), 17-20 (2001).
10 Mundale, V.D., Joglekar, H.S., “Regeneration of spent activated carbon by wet air oxidation”, Chem. Eng., 69, 1149-1159 (1991).
11 Liu, Y.F., Hou, J.L., “The regeneration of the activated carbon which was used for gourmat powder decolourisation”, 12, 21-23 (1996).
12 Xue, Y.X., Liu, J., “Yeast regenerate activated carbon and activated carbon used trinitrotoluene biofilm wastewater treatment”, Chemical Environmental, 3, 146-147 (1987).
13 Gómez, S.V., González-Garc?a, C.M., González-Mart?n, M.L., “Nitrogen adsorption isotherms on carbonaceous materials. Comparison of BET and Langmuir surface areas”, Powder Technology, 116,103-108 (2001).
14 Lippens, B.C., DeBoer, J.H., “Studies on pore systems in catalysts: V.The t method”, Journal of Catalysis, 4, 319-323 (1965).
15 Lu, A.H., Schmidt, W., Schüth, F., “Simplified novel synthesis of ordered mesoporous carbon with a bimodal pore system”, New Carbon Materials, 18, 181-185 (2003).
16 Horvath, G., Kawazoe, J., “Method for the calculation of effective pore size distribution in molecular sieve carbon”, J. Chem. Eng. Jpn.,16, 470-475 (1983).
17 Olivier, J.P., “Improving the models used for calculating the size distribution of micropore volume of activated carbons from adsorption data”, Carbon, 36, 1469-1472 (1998).
 Chinese Journal of Chemical Engineering2012年4期
Chinese Journal of Chemical Engineering2012年4期
- Chinese Journal of Chemical Engineering的其它文章
- HPLC Analysis of Egg Yolk Phosphatidylcholine by Evaporative Light Scattering Detector
- Removal of Organic Matter and Ammonia Nitrogen inAzodicarbonamide Wastewater by a Combination of Power Ultrasound Radiation and Hydrogen Peroxide*
- A Geometric Approach to Support Vector Regression and Its Application to Fermentation Process Fast Modeling*
- Three Dimensional Numerical Simulation of Convection-Condensation of Vapor with High Concentration Air in Tube with Inserts*
- Nanoparticle Migration in a Fully Developed Turbulent Pipe Flow Considering the Particle Coagulation*
- Optimization of Parameters for Melt Crystallization of p-Cresol*
