Hemispheric dominance during the mental rotation task in patients with schizophrenia
Jiu CHEN, Laiqi YANG*, Jin ZHAO, Lanlan LI, Guangxiong LIU, Wentao MA, Yan ZHANG, Xingqu WU, Zihe DENG, Ran TUO
· Original article ·
Hemispheric dominance during the mental rotation task in patients with schizophrenia
Jiu CHEN, Laiqi YANG*, Jin ZHAO, Lanlan LI, Guangxiong LIU, Wentao MA, Yan ZHANG, Xingqu WU, Zihe DENG, Ran TUO
Background:Mental rotation is a spatial representation conversion capability using an imagined object and either object or self-rotation. This capability is impaired in schizophrenia.
Objective:To provide a more detailed assessment of impaired cognitive functioning in schizophrenia by comparing the electrophysiological profiles of patients with schizophrenia and controls while completing a mental rotation task using both normally-oriented images and mirror images.
Methods:This electroencephalographic study compared error rates, reaction times and the topographic map of eventrelated potentials in 32 participants with schizophrenia and 29 healthy controls during mental rotation tasks involving both normal images and mirror images.
Results:Among controls the mean error rate and the mean reaction time for normal images and mirror images were not significantly different but in the patient group the mean (sd) error rate was higher for mirror images than for normal images (42% [6%] vs. 32% [9%], t=2.64, p=0.031) and the mean reaction time was longer for mirror images than for normal images (587 [11] ms vs. 571 [18] ms, t=2.83, p=0.028). The amplitude of the P500 component at Pz (parietal area), Cz (central area), P3 (left parietal area) and P4 (right parietal area) were significantly lower in the patient group than in the control group for both normal images and mirror images. In both groups the P500 for both the normal and mirror images was significantly higher in the right parietal area (P4) compared with left parietal area (P3).
Conclusion:The mental rotation abilities of patients with schizophrenia for both normally-oriented images and mirror images are impaired. Patients with schizophrenia show a diminished left cerebral contribution to the mental rotation task, a more rapid response time, and a differential response to normal images versus mirror images not seen in healthy controls. Specific topographic characteristics of the EEG during mental rotation tasks are potential biomarkers for schizophrenia.
1. Introduction
Mental rotation (MR) is the ability to rotate mental representations of two dimensional and three dimensional objects. This usually involves creating a mental image of an object (or self), and then rotating the object mentally. Initially proposed by Shepard and his colleagues in the early 1970s,[1]most authors report that the cognitive activity for performing MR tasks predominantly occurs in the right cerebral hemisphere.[2]Patients with schizophrenia usually have impaired performance on MR tasks.[3]Most of these conclusions, however, were based on behavioral indicators. The current study aims to use event-related brain potential (ERP) topographic mapping techniques to compare the location and intensity of cognitive activity during MR tasks in patients with schizophrenia versus those in healthy controls and, thus, more precisely define the cognitive deficits in schizophrenia.
2. Participants and methods
2.1 Subjects
The enrollment of participants is shown in Figure 1. Inpatients at the Department of Psychiatry of the Third Hospital of the People’s Liberation Army in Baoji, Shaanxi from January 2011 to September 2011 with a diagnosis of schizophrenia based on the criteria in the third edition of the Chinese Classification of Mental Disorders[4](which only requires a one-month duration of psychotic symptoms) were screened for participation. Among the 106 patients screened 42 were excluded because they had taken antipsychoticor other psychotropic medications or received electroconvulsive treatment in the prior 2 weeks, had a history of major neurological or medical illness, were unable to adhere to the research protocol (i.e., 40 minutes of testing with ongoing electroencephalogram), were lefthanded, had poor vision, or refused to provide written informed consent. There were 64 eligible individuals; 32 participants were randomly selected for participation in the study. They included 16 men and 16 women aged 17-42 years; their mean (sd) age was 28.1 (6.3) years; their mean years of schooling was 12.8 (4.1) years; their mean duration of illness was 13 (6) weeks; and their mean score on the Brief Psychiatric Rating Scale[5](conducted immediately before the encephalographic examination) was 38.5 (3.4) (range 35 to 57). Only 12 of the patients had a duration of illness greater than six months and, thus, met DSM-IV[6]criteria of schizophrenia; the remaining 20 patients met DSMIV criteria of schizophreniform disorder. This army hospital serves both the military and the surrounding community; 18 of the 32 participants were active members of the military and the remaining 14 were community members.
A convenience control group of 29 medical students, trainees and interns at the Baiji Hospital who responded to a recruitment notice and who had no personal or family history of mental illness was identified. They included 15 men and 14 women aged 18-37 years; their mean (sd) age was 24.2 (5.2) years; and their mean years of schooling was 14.3 (2.1) years. There were no significant differences between cases and controls by gender (χ2=0.12, p=0.105), age (t=0.31, p=0.127), or years of education (t=0.25, p=0.121).
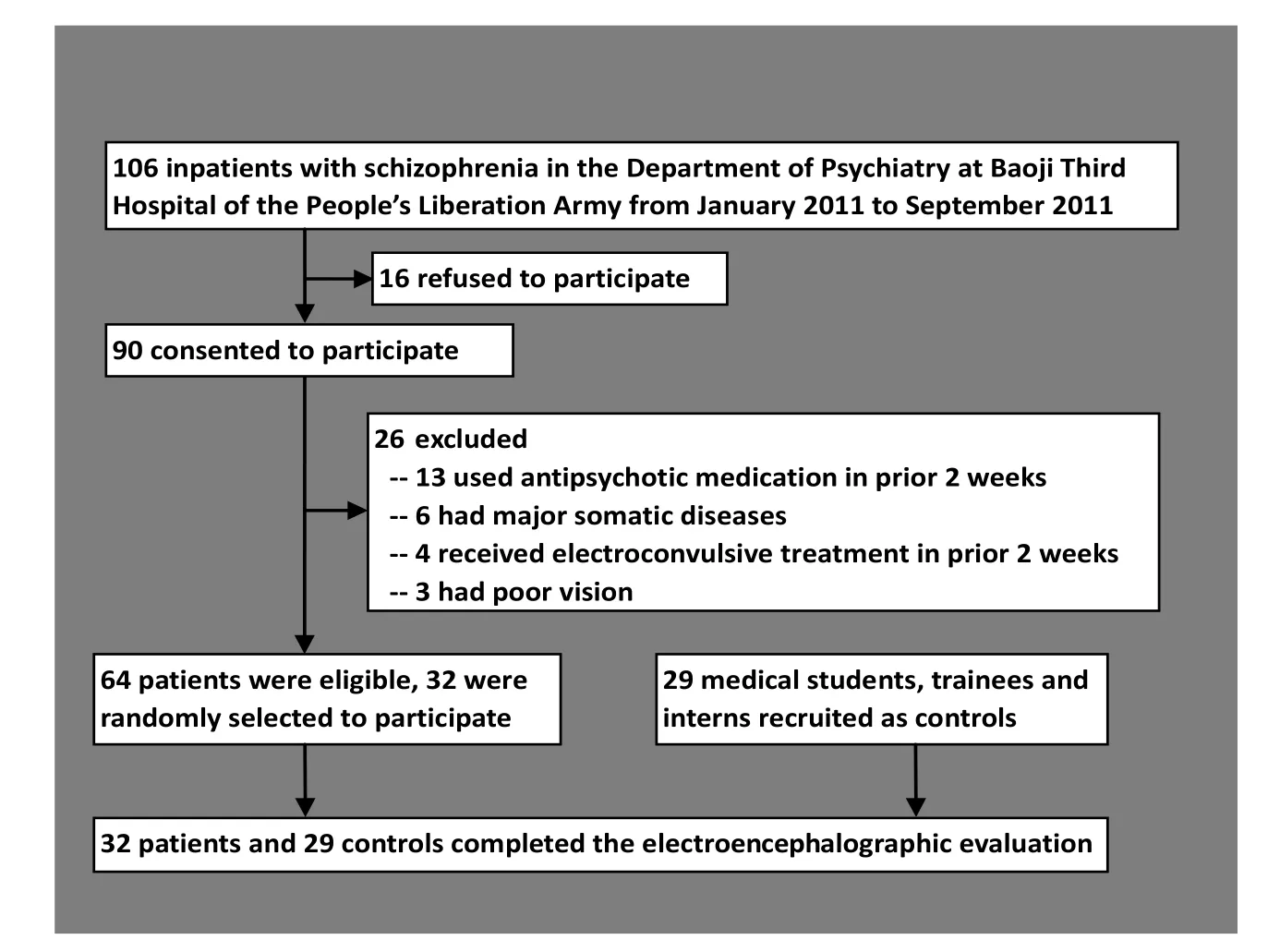
Figure 1. Flowchart of subject enrollment
This study was approved by the Medical Ethics Commission of the Baoji Third Hospital of the People’s Liberation Army.
2.2 Research Methods
2.2.1 Electrophysiological measurement
Using a BrainAmp MR 32 portable ERP system (Brain Products GmbH, Munich, Germany), two blinded investigators conducted the electrophysiological measurements for all participants in the morning. Both investigators had received training in the use of the apparatus and had good inter-rater reliability when independently assessing the wave amplitudes of ten patients (r=0.85-0.90). Participants were asked to wear a BrainCAP-MR 32-electrode cap to record EEG signals. The electrodes were located according to the International 10-20 system,[7]two ear electrodes served as reference electrodes, and the AFz electrode was used for grounding. The apparatus used a sampling rate of 500 Hz, scalp impedance of <5 kΩ, stimulation duration of 1200 ms, sensitivity of 5 μV, and a bandpass of 0.1-30 Hz. The EEG epochs were folded over 60 times. With a pre-stimulus baseline of 200 ms, the analysis process lasted until 1000 ms after stimulus presentation. Artifacts such as blinking were corrected offline. EEG signals with an amplitude greater than ±70 μV were interpreted as artifacts and rejected. P500 was measured in two completely independent analytic time windows via the triggering and recording systems; the response time and error count were automatically recorded.
2.2.2 Test procedures
The tests were performed with E-Prime 2.0 software (Psychology Software Tools). The participant was seated in a soft chair about 60 cm away from the monitor (a 17-inch color monitor, with a refresh rate of 75 Hz) in a quiet, dimly lit environment (relatively sound-proofed, with a temperature of about 24 °C). The participant was instructed to focus on the center of the screen throughout the test and trained in the test procedures until the rate of correct responses reached at least 60% prior to starting the formal testing period (all participants were able to achieve the 60% correct rate). During the test two stimulus pictures ('R' and 'F') were randomly presented. The pictures included normal and mirror images, each of which had 12 rotation angles: 0°, 30°, 60°, 90°, 120°, 150°, 180°, 210°, 240°, 270°, 300°, and 330°. The area of these pictures was no larger than 1.58 cm × 1.58 cm, with a visual angle of about 1.1° × 1.0°. During the procedures, a fixation point (the‘+’ symbol) appeared at the center of the screen for about 500 ms followed by the stimulus pictures. The participant was asked to indicate whether the pictures 'R' or 'F' were normally oriented or mirror images by clicking the right or left mouse.
During the formal test, each picture appeared 720 times (i.e., random presentation 30 times at each of the 12 angles). The participant was asked to make 1440 judgments on the rotations. Each task was divided in two sessions; 360 pictures were presented during each session. There was an interval between the two sessions, the duration of which was determined by the participant. The whole test lasted about 40 minutes. Electrophysiological measurements were performed simultaneously.
2.3 Statistical Analysis
The data were processed and analyzed using SPSS 17.0 software. Based on the literature,[4,5,7]P500 was chosen as the indicator for analyzing MR. Thirty-two observations with extreme reaction times (greater than three standard deviations beyond the mean) were excluded from the analysis. Differences between the groups in the mean error rate, mean reaction time, and the mean P500 at the Pz, Cz, P3 and P4 sites for both normally-oriented images and mirror images were compared using unpaired t-tests. Comparisons of specific measures for normally-orientated versus mirror images within each group were made using paired t-tests. All tests used a two-sided alpha of 0.05.
3. Results
3.1 Comparison of behavioral data
In the control group the error rates for normal images and mirror images were similar (29.1% v. 32.0%; paired-t=1.61, p=0.053) and the reaction times were also similar (602 ms v. 606 ms, paired-t=0.72, p=0.128). But in the patient group the error rate for mirror images was significantly higher than that for normally-oriented images (42.4% v. 32.2%, paired-t=3.46, p=0.029) and the reaction time was significantly longer (587 ms v. 571 ms, paired-t=4.01, p=0.023).
As shown in Table 1 the error rate for normal images was not significantly different between groups but the error rate for mirror images was significantly higher in the patient group than in the control group. The reaction time for both normal images and mirror images was significantly shorter in the patient group.
3.2 Comparison of ERP data
As shown in Table 2 the amplitudes of P500 at Pz, Cz, P3, and P4 were all significantly lower in the patient group than in the control group for both the normal images and the mirror images. In the control group the amplitudes of P500 at all four sites were significantly higher for normal images than for mirror images (Pz: paired-t=3.62, p=0.028; Cz: paired-t=3.81, p=0.024; P3: paired-t=3.41, p=0.034; P4: paired-t=4.26, p=0.019). However, in the patient group the difference between the P500 amplitude for normal images and mirror images was only statistically significant at the P4 electrode (Pz: paired-t=0.75, p=0.172; Cz: paired-t=1.02, p=0.163; P3: paired-t=0.62, p=0.201; P4: paired-t=2.31, p=0.043). The P500 amplitude at electrode P4 was significantly higher than that at P3 for all four assessments: normal images in the patient group (t=2.16, p=0.041), normal images in the control group (t=3.62, p=0.031), mirror images in the patient group (t=2.01, p=0.046), and mirror images in the control group (t=2.13, p=0.045).
3.3 Comparison of ERP topographic mapping results
The ERP topographic mapping results (Figure 2) of the normal and mirror images show differences at certain time ranges between the patient group and the control group. Strong positive components from 200 ms to 600 ms were observed at the parietal lobe, the occipital lobe, and between these two lobes in the control group and at the right parietal-occipital lobe in the patient group; but the activity was lower in the patient group than in the control group. In the patient group there was very little activity in the left parietal lobe or in the region between the left parietal lobe and the left occipital lobe; and there was a delay in the activation in these regions for the normally oriented image. In the control group the activation for the normal-orientation image and the mirror image was quite similar but in the patient group the activity was much stronger with the normal image than with the mirror image. When comparing the left hemisphere with the right hemisphere, the activation resulting from
the normal and mirror images in both patients and controls were greater in the right parietal-occipital lobe than in the left parietal-occipital lobe. In the patient group, at the -200 ms to 200 ms interval there was a prominent negative component for both normal and mirror images that was not seen in the control group.
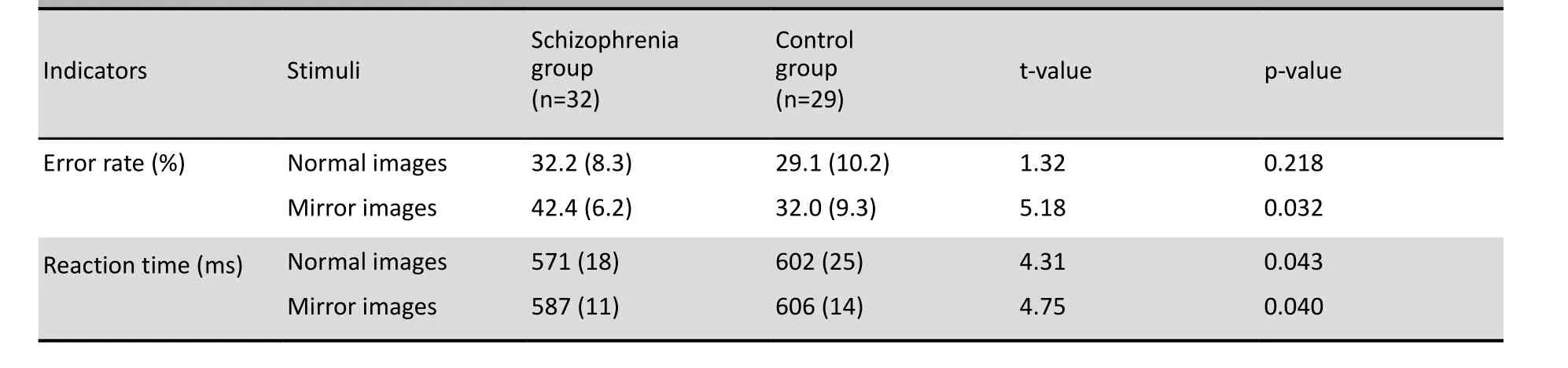
Table 1. Comparison of the mean (sd) error rate and the mean (sd) reaction time for the normal images and mirror images in the patient and control groups
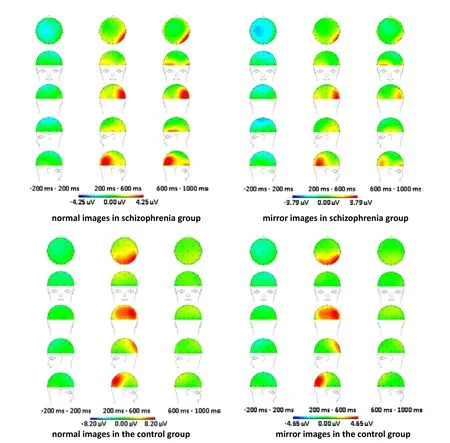
Figure 2. Comparison of ERP topographic mapping results between the two groups
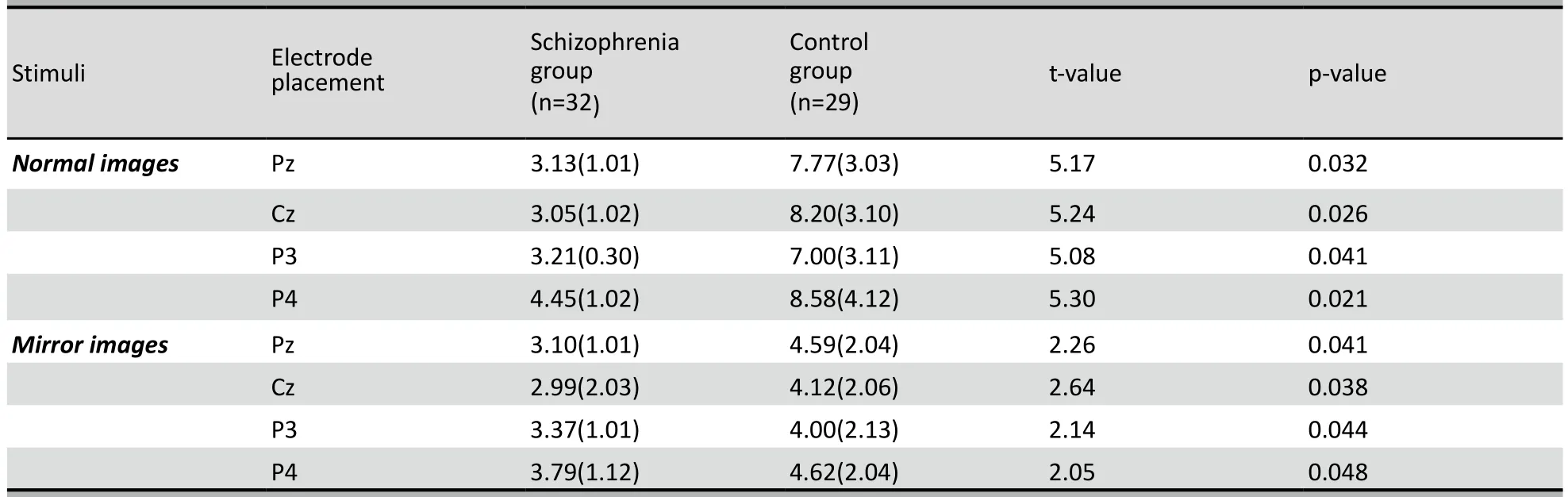
Table 2. Comparison of the mean (sd) amplitudes (μV) of P500 at electrodes Pz, Cz, P3, and P4 between the patient and control groups
4. Discussion
4.1 Main findings
The mental rotation (MR) task is an important tool for measuring spatial ability—one of the core cognitive capabilities of humans—and the P500 component of the ERP is a useful electrophysiological indicator of MR functionally.[8-11]Using this methodology we confirmed the findings of other studies[12-14]which report that the cortical regions activated during the MR task include the parietal lobe, parietal-occipital lobe, occipital lobe, and frontal lobe. We also identified several important differences in the electrophysiological responses to the MR task between patients with schizophrenia and control participants.
Consistent with several previous studies[2,15-17]in healthy controls we found strong evidence for right hemisphere dominance during the MR task but there was also activity in the left hemisphere indicating that both hemispheres participate during the task. Patients with schizophrenia also demonstrate right hemisphere dominance during the MR task, but the activity in the left hemisphere almost disappears and the amplitude of the P500 in the right hemisphere when presented both normal and mirror images is much lower than that seen in healthy controls. This suggests that MR abilities for both normal images and mirror images are impaired in patients with schizophrenia.
The MR ability of patients with schizophrenia is characterized by a significantly increased mirror imagerelated error rate and a significantly decreased reaction time for both normal and mirror images. These findings are consistent with the results of Vignemont and colleagues’[3]who suggest that patients’ MR deficits may be related to their tendency to forfeit accuracy for speed or to increased impulsivity when selecting responses. Our results suggest that the processing mechanisms for normal and mirror images during the MR task are different in patients than in controls, a conclusion that is supported by the findings of other researchers.[15,18]
As previously described by Band and colleagues[19]we found that a negative readiness potential precedes the actual execution of MR tasks. Patients with schizophrenia have higher negative readiness potentials than controls, suggesting that they consume more mental resources when preparing for their response to the stimuli. This may reflect a higher expectation that could result in a greater emphasis on the speed of response rather than on the accuracy of response.
4.2 Limitations
Our analysis of hemispheric dominance during the MR task was limited to a time-based analysis. We did not analyze changes in the ERP topographic mapping related to the angles of presenting the images and did not assess potential confounding factors such as age, gender and duration of illness. All the patients had a very short duration of illness (only 12 of the 32 patients met the 6-month duration of illness criteria used in the DSM-IV diagnostic system). This ensured that long term use of antipsychotic medication did not influence the results, but results may be different among patients with longer durations of illness. Further study with larger samples that include participants with varying lengths of illness, that follow changes in these parameters over time, and that assess these markers in first-degree family members of patients are needed to address these issues.
4.3 Implications
Our study confirms previous findings that there is a right hemisphere advantage or dominance for the MR task for both patients with schizophrenia and for controls and that the left hemisphere participation in the MR task is much weaker in patients with schizophrenia than in controls. We also found that the reaction times to stimuli in the MR task were faster in patients than in controls and that patients had a differential response to normally oriented versus mirror images that was not seen in controls. Further research is needed to confirm these findings and to determine whether or not the electrophysiological differences identified between patients and controls during the MR task can be used as biological markers for identifying individuals at high risk for schizophrenia.
Conflict of interest
The authors report no conflict of interest.
Fu nding
This study was supported by the special research fund for Traditional Chinese Medicine in Chinese Army (project number: 10ZYX108).
1. Shepard RN, Metzler J. Mental rotation of three-dimensional objects. Science 1971; 171(3972): 701-703.
2. Cohen G. Hemispheric differences in the utilization of advance information. In: RabbitI PMA, Dornic S. Attention and performance. London: Academic Press, 1975.
3. Vignemont F, ZallaT, Posada A, Louvegnez A, Koenig O, Georgieff N, et al. Mental rotation in schizophrenia. Conscious Cogn 2006; (15): 295-309.
4. Chinese Psychiatry Association. Chinese Classification of Mental Disorders. 3rd ed. Jinan: Shandong Science Press, 2000.
5. Zhang ZJ. Handbook of Scales for Behavioral Medicine. Beijing: Chinese Medical Multimedia Press, 2005: 332-335.
6. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. (DSM-IV). Washington, DC: American Psychiatric Association, 1994.
7. Jasper HH. The ten-twenty electrode system of the International Federation. Electroencephalogr Clin Neurophysiol 1958; 10(2): 371-375.
8. Wijers A, Otten L, Feenstra S, Mulder G, Mulder L. Brain potentials during selective attention, memory search, and mental rotation. Psychophysiology 1989; 26(4): 452-467.
9. Rosier F, Schumacher G, Soika B. What the brain reveals when it thinks. Event-related potentials during mental rotation and mental arithmetic. Ger J Psychol 1990; 14(2): 185-203.
10. Milivojevic B, Johnson BW, Hamm JP. Non-identical neural mechanisms for two types of mental transformation: eventrelated potentials during mental rotation and mental paper folding. Neuropsychologia 2003; 41(10): 1345-1356.
11. Liu LH, Wang ZB, Liu XF, Miao DM, Wang W. Influence of perceptional quality of stimulus on event-related potential P300 during mental rotation. J Fourth Mil Med Univ 2007; 28(7):663-665. (in Chinese)
12. Vingerhoets G, Lange FP, Vandemaele P, Deblaere K, Achten E. Motor Imagery in mental rotation: an fMRI study. Neuroimage 2002; 17(3): 1623-1633.
13. Harris IM, Miniussi C. Parietal lobe contribution to mental rotation demonstrated with rTMS. J Cogn Neurosci 2003; 15(3): 315-323.
14. Kawamichi H, Kikuchi Y, Ueno S. Spatio-temporal brain activity related to rotation method during a mental rotation task of three-dimensional objects: an MEG study. Neuroimage 2007; 37(3): 956-965.
15. Nú?ez-Pe?a M, Aznar-Casanova J. Mental rotation of mirrored letters: evidence from event-related brain potentials. Brain Cogn 2009; 69(1): 180-187.
16. Lamm C, Windischberger C, Leodolter U, Moser E, Bauer H. Evidence for premotor cortex activity during dynamic visuospatial imagery from single-trial functional magnetic resonance imaging and event-related slow cortical potentials. Neuroimage 2001; 14(2): 268-283.
17. Corballis MC, Sergent J. Hemispheric specialization for mental rotation. Coaex 1989; 25(1): 15-25.
18. Hamm JP, Johnson BW, Corballis MC. One good turn deserves another: an event-related brain potential study of rotated mirror-normal letter discriminations. Neuropsychologia 2004; 42(6): 810-820.
19. Band GPH, Miller J. Mental rotation interferes with response preparation. J Exp Psychol Hum Percept Perform 1997; 23(2): 319-338.
2011-10-06 , accepted: 2012-02-08)
10.3969/j.issn.1002-0829.2012.02.002
Center for Mental Disease Control and Prevention, Third Hospital of the People’s Liberation Army, Baoji , Shaanxi Province, China
*Correspondence: ericcst@yahoo.cn
- 上海精神醫學的其它文章
- Latent variable modeling
- Cross-sectional survey of prevalence and personality characteristics of college students with internet addiction in Wenzhou, China
- Correlation between insight and internalized stigma in patients with schizophrenia
- Symptom severity is more closely associated with social functioning status in inpatients with schizophrenia than cognitive deficits
- Case report on clozapine-associated neuroleptic malignant syndrome
- Clozapine is underutilized

