Solvothermal Synthesis and Optical Performance of One-dimensional Strontium Hydroxyapatite Nanorod*
NING Zhanglei (寧張磊), CHANG Zhidong (常志東),**, LI Wenjun (李文軍),**, SUN Changyan (孫長艷), ZHANG Jinghua (張經華)and LIU Yang (劉洋)
1School of Chemical and Biological Engineering, University of Science and Technology Beijing, Beijing 100083, China
2Beijing Centre for Physical & Chemical Analysis, Beijing 100089, China
Solvothermal Synthesis and Optical Performance of One-dimensional Strontium Hydroxyapatite Nanorod*
NING Zhanglei (寧張磊)1, CHANG Zhidong (常志東)1,**, LI Wenjun (李文軍)1,**, SUN Changyan (孫長艷)1, ZHANG Jinghua (張經華)2and LIU Yang (劉洋)2
1School of Chemical and Biological Engineering, University of Science and Technology Beijing, Beijing 100083, China
2Beijing Centre for Physical & Chemical Analysis, Beijing 100089, China
One-dimensional strontium hydroxyapatite (Sr-HAp) nanorods were successfully synthesized by a simple solvothermal method. The products were characterized via X-ray diffraction (XRD), Fourier transform infrared (FT-IR), cold field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM), photoluminescence (PL) excitation and emission spectra. The experimental results indicated that oleic acid as a surfactant played a key role in confining the growth of the Sr-HAp powders. A possible formation mechanism of the one-dimensional nanorod was proposed and elaborated. Moreover, the as-obtained Sr-HAp samples showed an intense and bright emission band centered at 460 nm under long-wavelength UV light excitation and the contents of NaOH used in the synthetic process had an obvious impact on the optical performance of Sr-HAp powders. The possible luminescent mechanism of the Sr-HAp samples was discussed.
strontium hydroxyapatite, biomaterial, solvothermal synthesis, optical performance
Chinese Journal of Chemical Engineering,20(1) 89—94 (2012)
1 INTRODUCTION
The chemical and physical properties of functional materials are strongly related to their size, shape and dimensionality [1]. One-dimensional nanostructures, including nanorods, nanotubes, and nanowires have attracted extensive interests over the past few decades due to their numerous potential applications in the fabrication of electronic, optical, optoelectronic, and magnetic devices [2, 3]. Up to date, much effort has been made in pursuing effective methods of synthesizing the size and shape controlled nanocrystals with excellent properties or new functional materials. For this purpose, the liquid-solid-solution (LSS) method is a general strategy for nanocrystal synthesis, which based on phase transfer and separation mechanism occurring at the interfaces among the liquid, solid and solution phases during the synthesis process. This strategy can generate many kinds of metal, oxide, or composite nanocrystals with a variety of properties such as semiconducting, fluorescent and magnetic ones [4].
Hydroxyapatite (denoted as HAp) has attracted great interest in modern materials chemistry due to its outstanding biocompatibility and bioactivity [5-7]. One of the main structural characteristics of HAp is its ability to accept strontium and other divalent cations to substitute calcium in the lattice of HAp. In the past few decades, some research works on the shapecontrolled synthesis of strontium hydroxyapatite (Sr-HAp) have been reported, including thin films [8], microspheres [9] and nanowires [2]. HAp and Sr-doped HAp materials can be extensively used in many industrial or technological fields as chromatographic columns, catalysts, adsorbents and optical materials. As the optical performance is concerned, previous studies are focused mainly on hydroxyapatite analogue doped with rare earth metal (RE) ions [10-14]. Utilizing the change of the luminescence intensity, RE-doped HAp can be used as a drug delivery carrier to trace and monitor the drug storage/release properties [15]. However, the high price of rare earth metal (such as Eu3+or Tb3+) limits greatly the extensive application of HAp as an efficient phosphor. So a class of efficient, stable and inexpensive luminescent materials which can be used in biomedicine or biotechnology is strongly desired.
To the best of our knowledge, there is no report on the one-dimensional strontium hydroxyapatite (Sr-HAp) nanorods with excellent optical performance prepared by the LSS method. Herein, we report the synthesis of one-dimensional strontium hydroxyapatite nanorods by using oleic acid, ethanol, NaOH, Sr(NO3)2and (NH4)2HPO4based on the LSS strategy. Interestingly, the samples show an intense emission under UV light irradiation without using rare earth elements as activators. The photoluminescence (PL) intensities of the samples with different content of NaOH are also studied and the possible mechanism of the optical properties has been proposed briefly.
2 MATERIALS AND EXPERIMENTAL
2.1 Materials
All chemical reagents of analytical grade and were produced from Beijing Chemical Factory and used directly without further purification. Deionized water was used throughout.
2.2 Experimental procedures
In this work, 3.5 ml oleic acid, 15 ml ethanol and 0.15 g sodium hydroxide (default condition, unless otherwise specified in Fig. 7) were agitated and mixed together in a 50 ml beaker to form a homogeneous milky emulsion. Then 8 ml aqueous solution containing 1.5 mmol Sr(NO3)2was added to the above emulsion. After stirring for 10 min, 8 ml aqueous solution containing 1.0 mmol (NH4)2HPO4was added to the mixture under stirring. After additional agitation for 15 min, the as-obtained mixture emulsion was transferred into a 50 ml Teflon bottle held in a stainless steel autoclave, sealed and maintained at 180 °C for 24 h. After the autoclave cooled to room temperature naturally, cyclohexane was used to clear the redundant oleic acid on the surface of the solid products and then ethanol was added into the cyclohexane solution to deposit the products. The precipitates were separated through centrifugation at 3000 r·min?1for 10 min. The obtained solid was washed by deionized water and absolute alcohol several times respectively, and then dried at 60 °C to obtain the final samples. Based on the amount of P, the yield of Sr-HAp was about 85%. Different preparation conditions in the absence of oleic acid and with different contents of NaOH were tested to investigate the morphology and optical property.
X-ray powder diffraction (XRD) was recorded on D/MAX-RB diffractometer (Rigaku, Japan) with Cu Kαsource (λ=0.15405) in the 2θ range from 10° to 70°. The Fourier transform infrared (FT-IR) spectrum was obtained on NEXUS 670 (Thermo Nicolet, USA). The
samples were mixed with KBr powder and pressed into tablets and the spectrum was recorded in the region of 400-4000 cm?1with the resolution of 4 cm?1. The morphology was scanned using S4800 field emission scanning electron microscope (Hitachi, Japan) and F-20 transmission electron microscopy (TEM) with a field emission gun operating at 200 kV (FEI, USA). The energy-dispersive X-ray spectrum (EDX) was measured with XFlash 5010 Detector (Bruker, Germany). The photoluminescence (PL) emission and excitation spectra were determined on F-4500 fluorescence spectrophotometer (Hitachi, Japan) equipped with a 150 W xenon lamp as the excitation source.
3 RESULTS AND DISCUSSION
3.1 Structural characterization
The composition and phase purity of the as-obtained product were examined by XRD pattern. Fig. 1 (a) shows the XRD pattern of the Sr-HAp powders synthesized by the solvothermal method. The peaks are strong and narrow, indicating that the as-prepared samples are well crystallized in spite of the moderate reaction conditions (at relatively low temperature 180 °C). All the diffraction peaks of the sample are characteristic of a pure hexagonal phase, which are consistent with the values in the standard card (JCPDS No. 33-1348), as shown in Fig. 1 (b). Moreover, no peak shifting and other phases are detected in the XRD pattern, which indicate that the pure Sr-HAp crystals have been obtained by this method.

Figure 1 XRD patterns of as-obtained Sr-HAp powder (a) and the standard data of Sr-Hap (b)
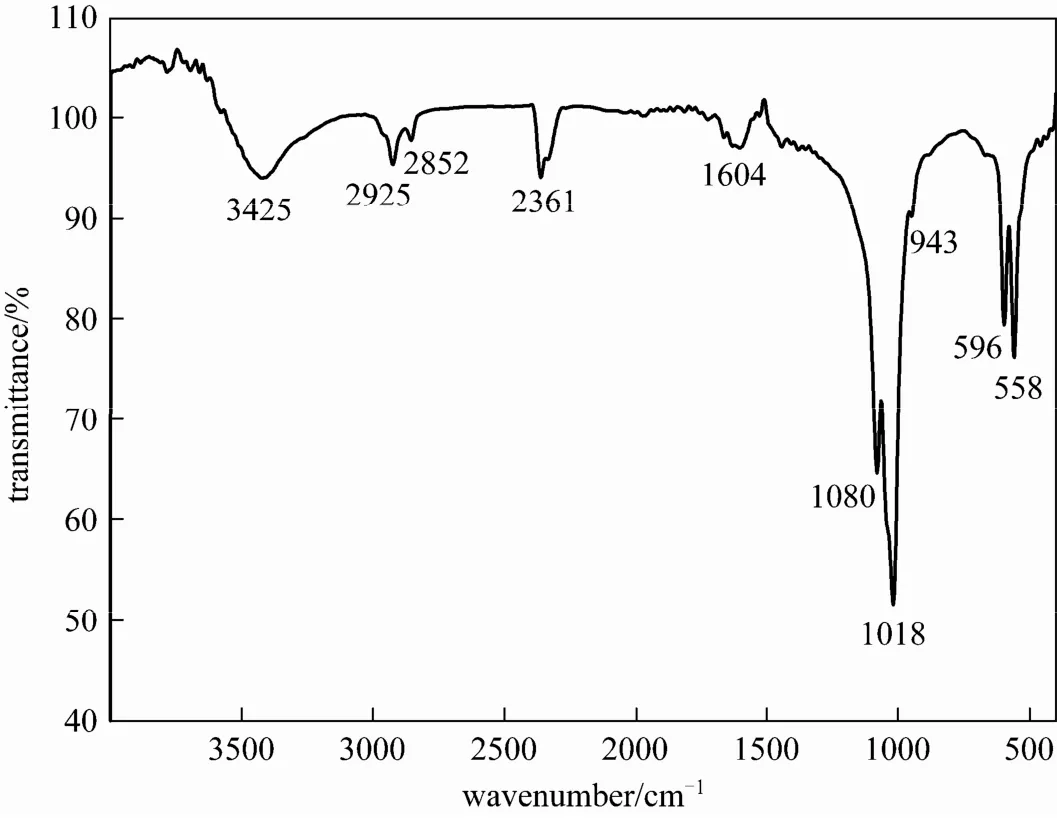
Figure 2 FT-IR spectra of the as-prepared Sr-HAp powder

Figure 3 EDX spectrum of as-obtained Sr-HAp powder
Figure 2 shows the Fourier transform infrared spectrum (FT-IR) of the Sr-HAp powder. The typical bands of apatite can be seen in this figure. Absorption peaks at 1080 cm?1, 1018 cm?1, 596 cm?1and 558 cm?1are the characteristic bands for[16, 17]. The band at 3425 cm?1is ascribed to the OH?vibration of H2O absorbed in the sample [18]. In addition, the characteristic OH bands of HAp at 3570 cm?1, 1260 cm?1are not clearly visible from Fig. 2, possibly because of an over high content of carbon related substance [16]. The additional bands about 3000 cm?1are assigned to the symmetric and antisymmetric stretch vibration of theCH2groups, indicating the existence of oleic acid within the Sr-HAp particle which did not wash completely. The ν(C O) stretch of the pure oleic acid appears at 1710 cm?1[19], but this peak is not found in the FT-IR spectrum of the as-obtained Sr-HAp powders. The peaks appear between 1400-1600 cm?1which correspond to the ν(C O) stretch, similar to the results of the literature [20]. From the FT-IR spectrum, we can conclude that a certain level of carbon-related impurities or defects has taken place in the products [17, 18].
The energy-dispersive X-ray spectrum (EDX) analysis was utilized to determine the chemical composition of the as-obtained samples. In Fig. 3, the EDX spectrum shows that the Sr-HAp samples are composed of only phosphorus, strontium, carbon, and oxygen. The detected carbon from the sample prepared by the hydrothermal process, in which some organic additives were employed, indicating that some carbon impurities have been introduced into the Sr-HAp host lattices or on the surface. The average atomic ratio of Sr/P is about 1.695, which is in good agreement with 1.667 of the chemical formula of Sr10(PO4)6(OH)2.
3.2 Morphology and growth mechanism of the as-obtained Sr-HAp samples
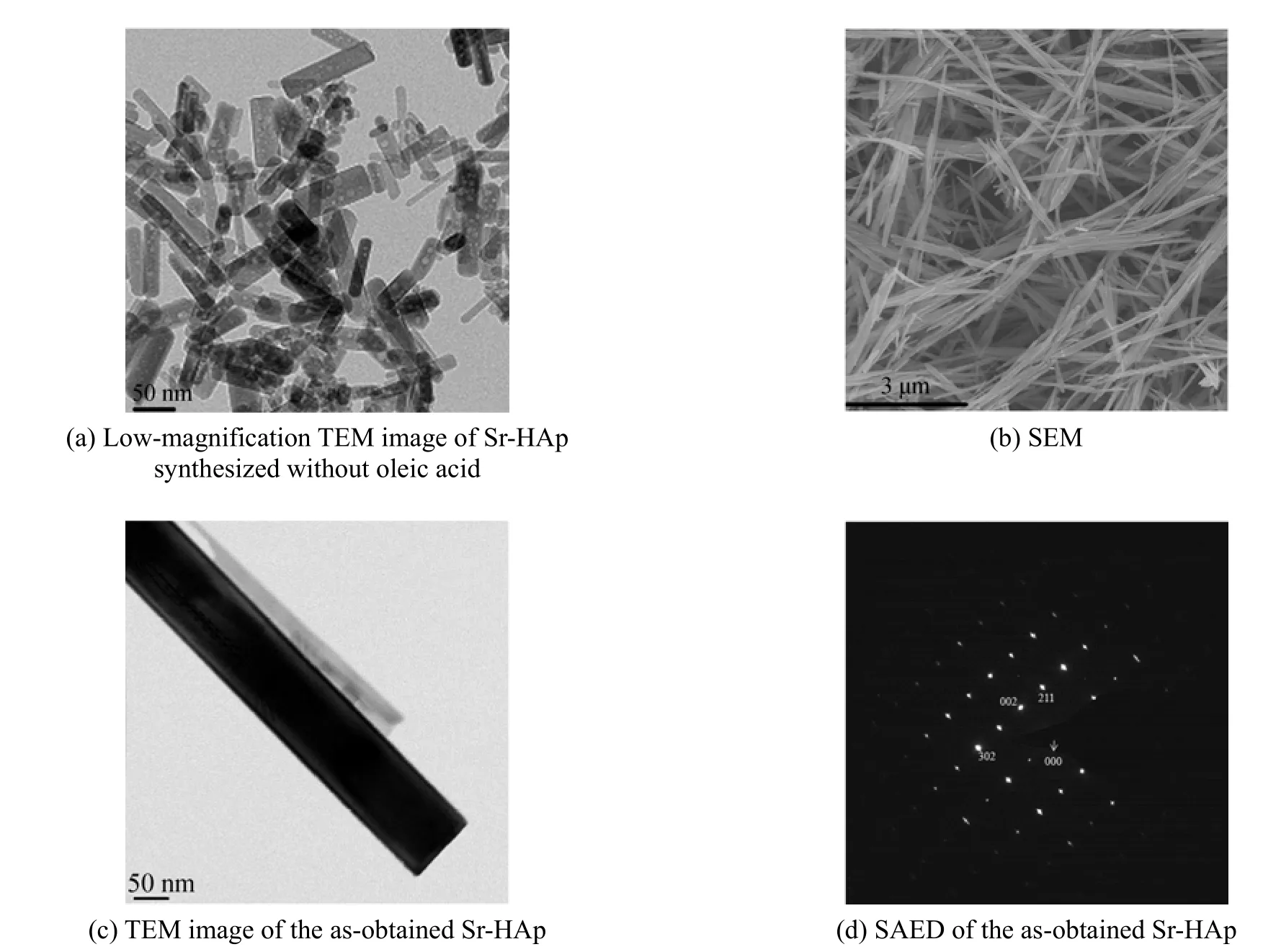
Figure 4 TEM and SEM images of as-prepared samples
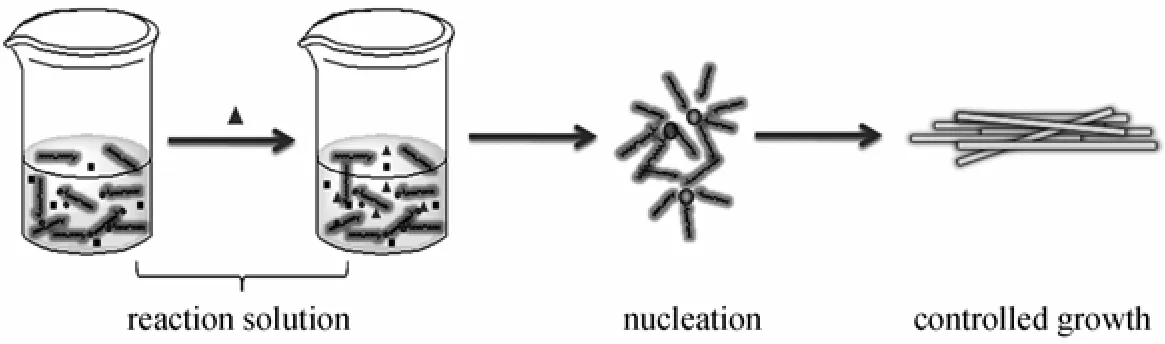
Figure 5 Schematic diagram showing the growth mechanism of the one-dimensional Sr-HAp nanorod
To investigate the influence of organic additive (oleic acid), on the morphology in this work, a controlled experiment was carried out in the absence of oleic acid. Fig. 4 (a) shows a typical TEM image of the samples obtained without oleic acid. The sample is obviously composed of abundant flake nanoparticles with the size ranging from 5 to 100 nm. As shown in the Figs. 4 (b) and 4 (c), synthesized with the oleic acid, nanorods with diameters of ~50 nm and lengths of ~5 μm are obtained. All single nanorods have nearly the same diameters and lengths, showing the effectiveness of this method in confining the size and dimension of the Sr-HAp powders. To get more information about the structure, the crystalline structures of the Sr-HAp samples were further confirmed by selectedarea electron diffraction (SAED). The discrete SAED spots [Fig. 4 (d)] demonstrate that the synthetic samples are well-crystallized as hexagonal single crystal.
The synthesis was carried out based on the description of liquid-solid-solution (LSS) strategy [4]. Firstly, in our experiments, oleic acid, sodium hydroxide and ethanol were agitated to form sodium oleate. In this procedure, two phases have been formed: the liquid phase consisted of the excess oleic acid and ethanol, and the solid phase containing sodium oleate. Then the aqueous solution containing 1.5 mmol Sr(NO3)2was added into the system under vigorous stirring, in which a third phase was formed (the solution phase). When the mixture is stirred intensely, a phase transfer process of the Sr2+ions occurred spontaneously across the interface of the sodium oleate and the water/ethanol solution based on ion exchange, which led to the formation of (ROO)nSr and the entry of sodium ions into the aqueous phase. With the addition of34PO?ions into the system, the reaction between metal oleate and34PO?formed a complex mixture. Oleic acid, as a selective adsorption surfactant, has been widely used as efficient protecting reagents for the growth of crystals in organic-solvent-based synthetic routes [20]. At the designated temperature, the in situ generated Sr-HAp microcrystals, whose surface was covered by adsorbed oleic acid molecules,were further confined to grow into well crystallized and certain shaped nanorods. Based on the steps stated above, we have a basic understanding of the growth mechanism of the one-dimensional Sr-HAp nano-material as shown schematically in Fig. 5.
3.3 Optical performance
The optical performance of the as-prepared Sr-HAp sample was examined. Fig. 6 shows the emission spectrum (EM) and the excitation spectrum (EX) of the Sr-HAp samples. The emission spectrum displays a strong band from 430 to 600 nm with a maximum at 460 nm. The corresponding excitation spectrum display a maximum about 400 nm. Comparing with the typical narrow spectrum of RE (such as Eu3+,Tb3+) doped HAp, the emission spectrum of this kind of Sr-HAp materials is much wider than the emission spectrum of the former. And this broad band spectrum implies the optical performance may be resulted by the surface defects or electronic centers rather than rare earth activator. Moreover, most of RE-doped HAp luminescent materials excitated under 300 nm UV light [12, 14, 15], while this Sr-HAp materials just using an excitation wavelength of 400 nm, which need lower energy than the previous ones.
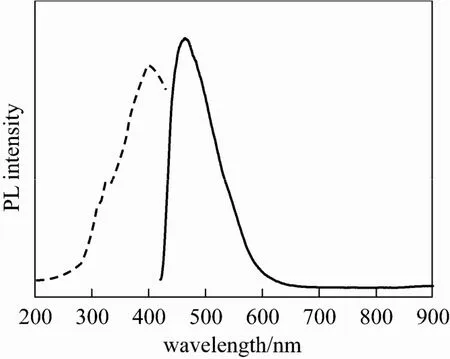
Figure 6 PL excitation spectrum (dash lines), emission spectrum (in lines) of the Sr-HAp
Figure 7 shows the PL emission spectra of the Sr-HAp samples synthesized with different contents of NaOH (other conditions remained as described in Section 2.2). It is found that the shape and profile for the emission spectra vary little with the NaOH addition, but the emission intensity varies largely. The Sr-HAp sample prepared with 0.15 g NaOH shows the strongest emission intensity and that with 0.40 g NaOH has the weakest emission intensity. The reason might be that the content of NaOH affects the quality of the luminescent centers under the solvothermal method. The exact mechanism of the change of PL intensity and maximum emission wavelength of the as-obtained samples needs further study due to the complex reaction process.

Figure 7 PL emission spectra of the Sr-HApsamples synthesized with different contents of NaOHmNaOH/g:0.10; 0.12;0.15; 0.20; 0.30;0.40
Considering neither Sr2+nor34PO?is able to show luminescence property, the observed luminescence from the as-obtained powders may be relate to some defects or electronic centers in the host lattice. There are some reports on the synthesis of self-activated luminescent HAp [21] and Sr-HAp [9] samples resulted from the CO?2radical impurities in the crystal lattice due to the additive trisodium citrate in the reaction solution. However, it has not been reported that luminescent Sr-HAp synthesized by the LSS strategy in the solvothermal method. Since in the paper, no other activating ions were introduced in the synthesized process, the optical property is ascribed to the presence of oleic acid. In the formation process of the apatite, the adsorbed oleic acid molecules can introduce some carbon, oxygen or negative charges on the surface of the samples, and these will possibly cause the defects or electronic centers in the crystals and may be result in some trap states. Further experiments are underway to elucidate the exact luminescence mechanism and explore the biocompatibility of the luminescence Sr-HAp nanorods.
4 CONCLUSIONS
Using oleic acid as a surfactant, we have successfully synthesized the one-dimensional Sr-HAp nanorods with a simple solvothermal method. The as-obtained sample exhibits a strong emission peak at about 460 nm at room temperature, which is maybe due to the defects or electronic center in the process of synthesis. Considering the Sr-HAp has excellent optical performance without using expensive rare earth ions, it may be potentially used as a new kind of green and low-cost luminescence biomaterial.
ACKNOWLEDGEMENTS
The authors are grateful to Prof. Che Ping and Prof. Liu Shixiang from University of Science and Technology Beijing for their helpful discussion and advice; Dr. Luo Wenli and Lin Qingxia at PetroChina Exploration & Development Research Institute fortheir keen interest and encouragement during the course of this work.
REFERENCES
1 Neira, I.S., Kolen’ ko, Y.V., Lebedev, O.I., van Tendeloo, G., Gupta, H.S., Guitian, F., Yoshimura, M., “An effective morphology control of hydroxyapatite crystals via hydrothermal synthesis”, Cryst. Growth Des.,9(1), 466-474 (2009).
2 Kim, T.G., Park, B., “Synthesis and growth mechanisms of one-dimensional strontium hydroxyapatite nanostructures”, Inorg. Chem.,44(26), 9895-9901 (2005).
3 Lieber, C.M., “One-dimensional nanostructures: Chemistry, physics & applications”, Solid State Commun.,107(11), 607-616 (1998).
4 Wang, X., Zhuang, J., Peng, Q., Li, Y.D., “A general strategy for nanocrystal synthesis”, Nature,437(7055), 121-124 (2005).
5 Landi, E., Sprio, S., Sandri, M., Celotti, G., Tampieri, A., “Development of Sr and CO3co-substituted hydroxyapatites for biomedical applications”, Acta Biomaterialia,4(3), 656-663 (2008).
6 Landi, E., Tampieri, A., Celotti, G., Sprio, S., Sandri, M., Logroscino, G., “Sr-substituted hydroxyapatites for osteoporotic bone replacement”, Acta Biomaterialia,3(6), 961-969 (2007).
7 Zhao, X.H., Yang, L.F., Zuo, Y., Xiong, J.P., “Hydroxyapatite coatings on titanium prepared by electrodeposition in a modified simulated body fluid”, Chin. J. Chem. Eng.,17(4), 667-671 (2009).
8 Capuccini, C., Torricelli, P., Sima, F., Boanini, E., Ristoscu, C., Bracci, B., Socol, G., Fini, M., Mihailescu, I.N., Bigi, A., “Strontium-substituted hydroxyapatite coatings synthesized by pulsed-laser deposition: In vitro osteoblast and osteoclast response”, Acta Biomaterialia,4(6), 1885-1893 (2008).
9 Zhang, C.M., Cheng, Z.Y., Yang, P.P., Xu, Z.H., Peng, C., Li, G.G., Lin, J., “Architectures of strontium hydroxyapatite microspheres: Solvothermal synthesis and luminescence properties”, Langmuir,25(23), 13591-13598 (2009).
10 Graeve, O.A., Kanakala, R., Madadi, A., Williams, B. C., Glass, K. C., “Luminescence variations in hydroxyapatites doped with Eu2+and Eu3+ions”, Biomaterials, 31 (15), 4259-4267 (2010).
11 Lebugle, A., Pelle, F., Charvillat, C., Rousselot, I., Chane-Ching, J.Y.,“Colloidal and monocrystalline Ln(3+) doped apatite calcium phosphate as biocompatible fluorescent probes”, Chem. Commun., (6), 606-608 (2006).
12 Yang, C., Yang, P.P., Wang, W.X., Wang, J., Zhang, M.L., Lin, J.,“Solvothermal synthesis and characterization of Ln (Eu3+, Tb3+) doped hydroxyapatite”, J. Colloid Interface Sci.,328(1), 203-210 (2008).
13 Han, Y.C., Wang, X.Y., Li, S.O., Ma, X.H., “Synthesis of terbium doped calcium phosphate nanocrystalline powders by citric acid sol-gel combustion method”, J. Sol-Gel Sci. Technol.,49(1), 125-129 (2009).
14 Yang, C.C., Chen, S.Y., Liu, D.M., “Phase characterization and tunable photoluminescence of Eu-doped strontium-substituted nanohalophosphate”, J. Cryst. Growth,293(1), 113-117 (2006).
15 Yang, P.P., Quan, Z.W., Li, C.X., Kang, X.J., Lian, H.Z., Lin, J.,“Bioactive, luminescent and mesoporous europium-doped hydroxyapatite as a drug carrier”, Biomaterials,29(32), 4341-4347 (2008).
16 Lin, K.L., Chang, J., Zhu, Y.J., Wu, W., Cheng, G.F., Zeng, Y., Ruan, M.L., “A facile one-step surfactant-free and low-temperature hydrothermal method to prepare uniform 3D structured carbonated apatite flowers”, Cryst. Growth Des.,9(1), 177-181 (2009).
17 Salarian, M., Solati-Hashjin, M., Shafiei, S.S., Salarian, R., Nemati, Z.A., “Template-directed hydrothermal synthesis of dandelion-like hydroxyapatite in the presence of cetyltrimethylammonium bromide and polyethylene glycol”, Ceram. Int.,35(7), 2563-2569 (2009).
18 He, Q.J., Huang, Z.L., Liu, Y., Chen, W., Xu, T., “Template-directed one-step synthesis of flowerlike porous carbonated hydroxyapatite spheres”, Mater. Lett.,61(1), 141-143 (2007).
19 Bronstein, L.M., Huang, X.L., Retrum, J., Schmucker, A., Pink, M., Stein, B.D., Dragnea, B., “Influence of iron oleate complex structure on iron oxide nanoparticle formation”, Chem. Mater.,19(15), 3624-3632 (2007).
20 Zhang, X., Quan, Z., Yang, J., Yang, P., Lian, H., Lin, J., “Solvothermal synthesis of well-dispersed NaMgF3nanocrystals and their optical properties”, J. Colloid Interface Sci.,329(1), 103-106 (2009).
21 Zhang, C.M., Yang, J., Quan, Z.W., Yang, P.P., Li, C.X., Hou, Z.Y., Lin, J., “Hydroxyapatite nano- and microcrystals with multiform morphologies: Controllable synthesis and luminescence properties”, Cryst. Growth Des.,9(6), 2725-2733 (2009).
2010-05-30, accepted 2010-11-09.
* Supported by the National Natural Science Foundation of China (20876157) and the Fundamental Research Funds for the Central Universities (FRF-BR-10-002A).
** To whom correspondence should be addressed. E-mail: zdchang@ustb.edu.cn; wjli@sas.ustb.edu.cn
 Chinese Journal of Chemical Engineering2012年1期
Chinese Journal of Chemical Engineering2012年1期
- Chinese Journal of Chemical Engineering的其它文章
- Festschrift in Honor of the 90thBirthday of Prof. Chen Jiayong
- Ternary System of Fe-based Ionic Liquid, Ethanol and Water for Wet Flue Gas Desulfurization*
- The Research Progress of CO2Capture with Ionic Liquids*
- Synthesis of PGMA Microspheres with Amino Groups for High-capacity Adsorption of Cr(VI) by Cerium Initiated Graft Polymerization*
- Effects of Additives and Coagulant Temperature on Fabrication of High Performance PVDF/Pluronic F127 Blend Hollow Fiber Membranes via Nonsolvent Induced Phase Separation
- Polymer/Ceramic Composite Membranes and Their Application in Pervaporation Process
