肋果茶中的萜類化學成分研究
張蘭春,吳興德,何 雋,彭麗艷,張榮平,趙勤實*
1昆明醫科大學藥學院,昆明650500;2中國科學院昆明植物研究所植物化學與西部植物資源持續利用國家重點實驗室,昆明650204
Introduction
Sladenia celastrifolia Kurz(Sladeniaceae)is a monotypic species widely distributed in Burma,northern Thailand,and the southern China[1].Terpenoids have been isolated from this plant[2].To discover more terpenoids,we initiated a phytochemical study on the stems and leaves of S.celastrifolia.As a result,a new seco-iridoid,sladeniafolin A(1)was isolated from this plant,together with fourteen known terpenoids,grasshopper ketone(2)[3],(3S,5R,6S,7E,9R)-7-megastigmene-3,6,9-triol(3)[4],hedytriol(4)[5],(3S,5R,6R,7E,9R)-3,5,6,9-tetrahydroxy-7-megastigmene(5)[6],1'S*,4'R*-8-(4'-hydroxy-2',6',6'-trimethylcyclohex-2-enyl)-6-methyloct-3E,5E,7E-trien-2-one(6)[7],2α,3α,19α,23-tetrahydroxyurs-12-en-28-oic acid(7)[8],2α,3β,19α,23-tetrahydroxyurs-12-en-28-oicacid(8)[8],pomolic acid(9)[9],3-O-acetyl pomolic acid(10)[10],ursaldehyde(11)[11],camarolide(12)[12],3β-hydroxyurs-11-en-13β(28)-ol-ide(13)[13],3β-hydroxy-11α,12α-epoxy-urs-13β,28-olide(14)[14],and 28-O-β-D-glucopyranosyl euscaphic acid(15)[15].Herein,we report the isolations and structure elucidations of these compounds.
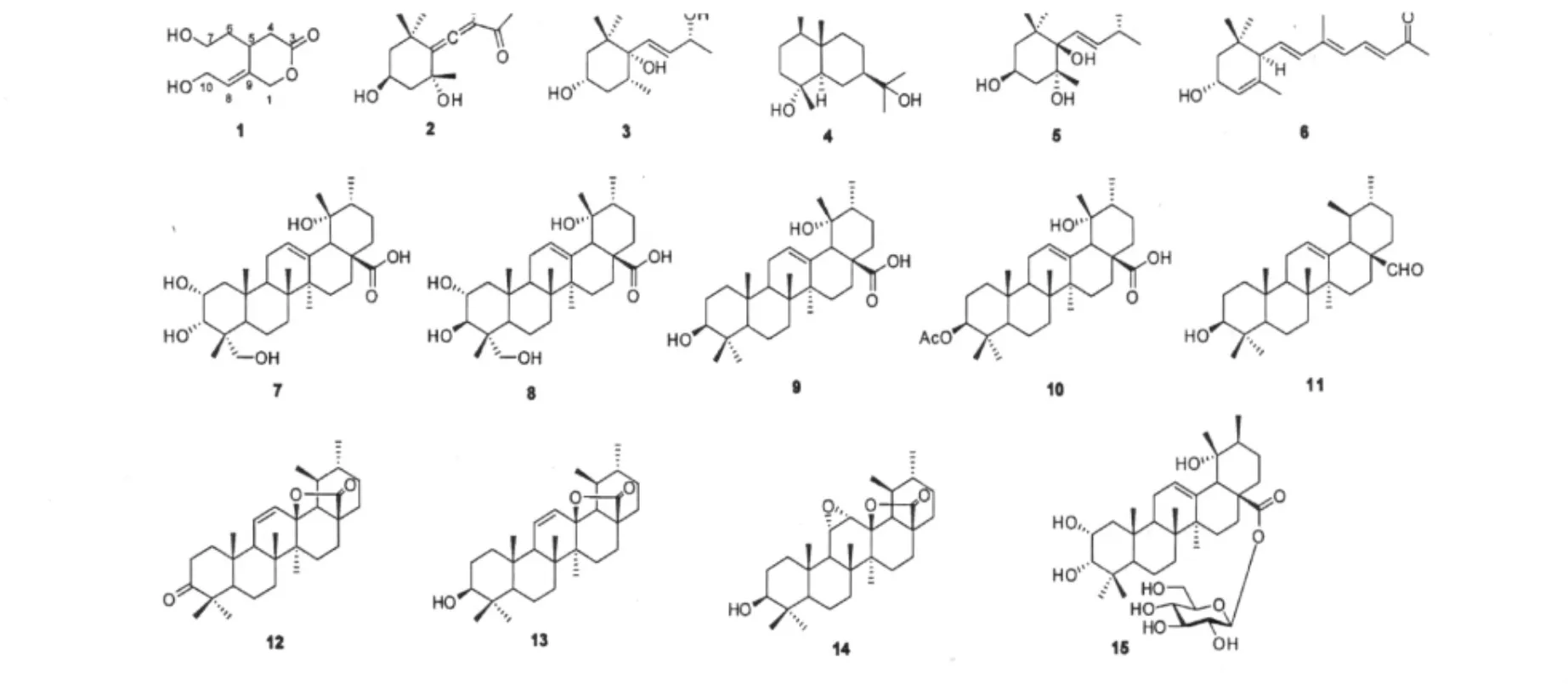
Fig.1 Compounds 1-15 from S.celastrifolia
Experimental
General procedures
Optical rotation was measured on a Jasco P-1020 polarimeter.The IR spectrum was obtained with a Bruker Tensor 27 spectrometer KBr disc.NMR spectra were recorded on Bruker AM-400,DRX-500,and Avance III 600 spectrometers(δ in ppm,J in Hz).ESI-MS spectra were carried out on a Bruker HCT/Esquire spectrometer,HR-ESI-MS spectra on an API QSTAR Pulsar spectrometer.Column chromatography(GC)was performed using silica gel(100-200 and 200-300 mesh,Qingdao Haiyang Chemical Co.Ltd,Qingdao,People’s Republic of China),Lichropre RP-18 gel(40-63 μm,Merck,Darmstadt,Germany)and MCI gel(75-150 μm,Mitsubishi Chemical Corporation,Japan),Fractions were visualized by heating silica gel plates sprayed with 10%H2SO4in ethanol.
Plant Material
Stems and leaves of S.celastrifolia were collected in Kumming Botanical Gardens of Yannan Province,P.R. China,in July 2010.The plant was indentified by Prof. X.Cheng at Kunming Institute of Botany.A voucher specimen(No.20100715)was deposited at the State Key Laboratory of Photochemistry and Resources in West China,Kunming Institute of Botany,Chinese A-cademy of Sciences,R.P.China.
Extraction and Isolation
The air-dried plant material(28 kg)was percolated with 95%EtOH(3×75 L,48 h)at room temperature.The extract was evaporated to dryness under reduced pressure.The obtained residue was suspended in H2O and partitioned successively with EtOAc to afford an EtOAc extract 1.6 kg.The EtOAc extract was subjected to column silica gel,which eluted gradiently with petroleum ether:Me2CO(1∶0→0∶1)to afford five fractions(1-5).
Fraction 1(10 g)was separated over silical gel column using petroleum ether:Me2CO(9∶1→3∶2)as solvents to give 13(50 mg)and 14(10 mg).Fraction 2(20 g)was divided in to 2 subfractions(2a-2b)over silica gel column with petroleum ether:EtOAc(1∶0→0∶1)as solvents.Subfraction 2a was further purified on silica gel column using petroleum ether:Me2CO (8∶2→7∶3)and combined with sephadax LH-20 eluted with CHCl3-MeOH(1∶1)to afford 11(10 mg)and 12(2 mg).Subfraction 2b with was chromatographed further over MCI(85%MeOH-H2O to 100%MeOH) give fraction 2b1 and 2b2.Subfraction 2b1 was purified on a silica gel column eluted with petroleum ether: Me2CO(8∶2)and than conbined with sephadax LH-20 eluted with MeOH to afford 10(10 mg),9(9 mg) and 6(5 mg).Subfraction 2b2 was applied to an RP-18 gel eluted with MeOH-H2O(7∶3→1∶0),followed by chromatographed over repeated silica gel CC and finally purified by sephadax LH-20 eluted with MeOH to 5(30 mg)and 8(10 mg).Fraction 3(10 g)was separated over silica gel column and finally purified by sephadax LH-20 column eluted with CHCl3-MeOH(1∶1)to afforded 7(30 mg)and 1(29 mg).15(16 mg),4(6 mg),3(30 mg)and 2(63 mg)were isolated from fraction 4(20 g)by chromatography on MCI gel column(85%MeOH-H2O to 100%MeOH),and repeated purified over silica gel CC,RP-18 and Sephadex LH-20 columns.
Result and Discussion
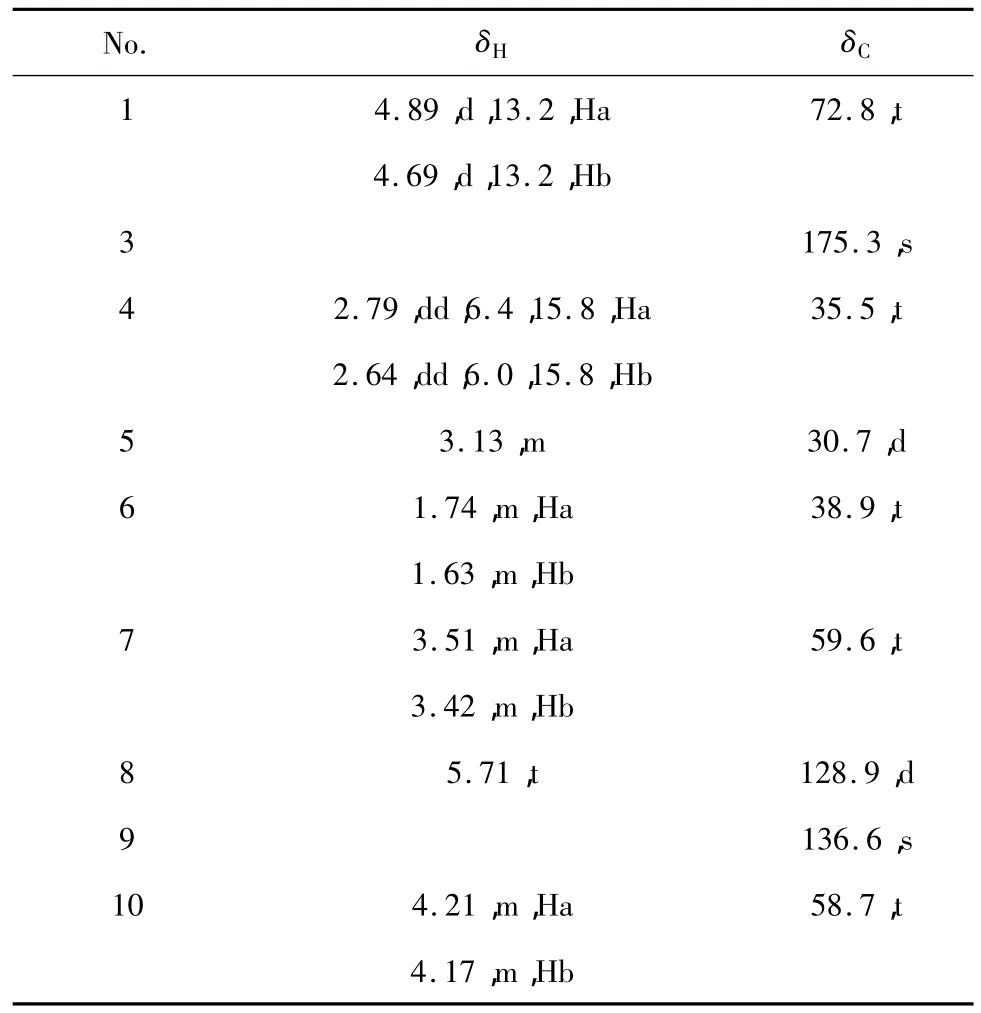
Table 1 NMR data of compound 1 in CD3OD(δ in ppm,J in Hz)
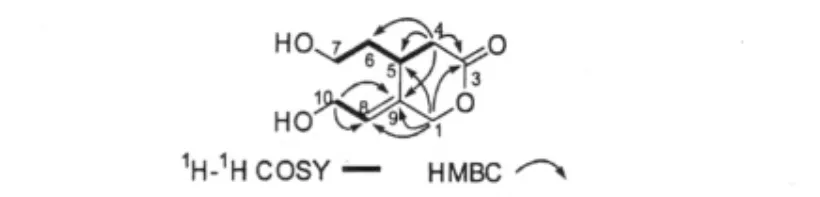
Fig.2 Key1H-1H COSY and HMBC correlations
Compound 1 was obtained as colorless oil.Its molecular formula C9H14O4was established by HR-ESI-MS(m/z 209.0789[M+Na]+,calcd 209.0789),indicating three degrees of unsaturation.Its IR spectrum exhibited absorption bands for hydroxy(3395 cm-1),ester carbonyl groups(1727 cm-1),and double band(1646 cm-1).An analysis of the1H NMR spectrum indicated the typical signals of one olefinic protons at δ 5.71 (1H,m)and six oxygenated methylene protons[δH4. 89(1H,d,J=13.2 Hz,H-1a),4.69(1H,d,J=13. 2 Hz,H-1b),3.51(1H,m,H-7a),3.42(1H,m,H-7b),4.21(1H,m,H-10a),4.17(1H,m,H-10b)]. The13C NMR(DEPT)spectra of 1 showed two methylenes(δC35.5,C-4;38.9,C-6),one methane(δC30.7,C-5),three oxygenated methylenes(δC72.8,C-1;59.6,C-7;58.7,C-10),one trisubstituted double bond(δC128.9,C-8;136.6,C-9)and an ester carbonyl(δC175.3,C-3).The above evidence implied that compound 1 was a C9seco-iridoid.Its 1D NMR data was similar to those of floribundane B[16].The obvious differcnce was that the methyl group in floribundane B was substituted by a hydroxymethyl group in 1,which was supported by the 1D NMR data(Table 1). In addition,the ester carbonyl was substituted at C-3 in 1 rather than C-1 in floribundane B,as deduced from the1H-1H COSY correlations of H2-4/H-5/H2-6/H2-7,and confirmed by the HMBC correlations from H2-1,H-4a(δH2.79,dd,J=6.4,15.8 Hz),and H-4b(2. 64,dd,J=6.0,15.8 Hz)to the ester carbonyl.The ROESY correlation of H-8 with H-1a suggested the E-orientation of the double bond in compound 1.On the basis of the above evidence,compound 1 was determined to be a new C97,8-secoiridoid derivative and named as sladenfolin A.
Sladeniafolin A(1):colorless oil,C9H14O4;[α]-2.18(c 0.67,CH3OH).UV(CH3OH):λmax(log ε):201(3.47).IR(KBr):3395,2935,2883,1727,1384,1318,1296,1268,1087,1086,1055,1023 cm-1.1H and13C NMR:Tables 1,ESI-MS(positive): 209([M+Na]+).HR-ESI-MS(positive):209. 0787([M+Na]+,C9H14O4Na;calc.209.0789).
1 Kunming Institute of Botany,Chinese academy of sciences. Flora of Yunnan.Science Press,1997,8:384-385.
2 He R,Qi RP,Yang W,et al.Studies on the chemical constit-uents of Sladenial celastrifolia Lurz.J Yunnan Univ National (Nat Sci Ed),2010,19:102-105.
3 Kuang HX,Yang BY,Xia YG,et al.Chemical constituents from the flower of Datura metel L.Arch Pharm Res,2008,31: 1094-1097.
4 Susumu K,Katsuyoshi M,Hideaki O,et al.Crotonionosides A-G:megastigmane glycosides from leaves of Croton cascarilloides R?schel.Phytochemistry,2011,72:147-153.
5 Zhu WM,Zhao Q,Li SL,et al.Sesquiterpenoids from Hedychium yunnanense and Porana discifera,and the structural revision of two sesquiterpenoids from Laggera pterodonta.J Asian Nat Prod Res,2007,9,277-283.
6 Brigida DA,Marina DG,Antonio F,et al.Structure elucidation and phytotoxicity of C13nor-isoprenoids from Cestrum parqui.Phytochemistry,2004,65:497-505.
7 Shi DY,Han LJ,Sun J,et al.A new halogenated biindole and a new apo-carotenone from green alga Chaetomorpha basiretorsa Setchell.Chin Chem Lett,2005,16:777-780.
8 LI XH,Shen DD,Li N,et al.Bioactive triterpenoids from Symplocos chinensis.J Asian Nat Prod Res,2003,5:49-56.
9 Cheng JJ,Zhang LJ,Cheng HL,et al.Cytotoxic hexacyclic triterpene acids from Euscaphis japonica.J Nat Prod,2010,73:1655-1658.
10 Yin K,Gao HY,Li XN,et al.Chemical constituents of Chaenomeles speciosa(Sweet.)Nakai.J Shenyang Pharm Univ,2006,23:760-763.
11 Raj KH,Maringanti B.Terpenoids from the resin of Shorea robust.Phytochemistry,1994,35:1073-1074.
12 Bina SS,Aneela W,Sabira B.Two new pentacyclic terpenoids from the aerial parts of Lantana camara Linn.Heterocycles,2000,53:681-687.
13 Susana IP,Carmen SR,Carlos PN,et al.Chemical composition of the epicuticular wax from the fruits of Eucalyptus globules.Phytochem Anal,2005,16:364-369.
14 Sabira B,Qayyum A,Bina SS,et al.Constituents of the leaves of Thevetia neriifolia.J Nat Prod,1993,56:613-617.
15 Han SY,Jong CP,Jae SC.Triterpenoid glycosides from Rosa rugosa.Arch Pharm Res,1987,10:219-222.
16 Cristina MPB,Carlos D,Dina IMDM.Iridoids from Hymenodictyon floribundum.J Braz Chem Soc,2010,21:1121-1125.
- 天然產物研究與開發的其它文章
- 盤龍參中的兩個新二氫菲類