Immobilization of Papain in Biosilica Matrix and Its Catalytic Property*
ZHOU Liya (周麗亞), WANG Cui (王翠), JIANG Yanjun (姜艷軍),2and GAO Jing (高靜),**
1School of Chemical Engineering and Technology, Hebei University of Technology, Tianjin 300130, China
2National Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China
Immobilization of Papain in Biosilica Matrix and Its Catalytic Property*
ZHOU Liya (周麗亞)1, WANG Cui (王翠)1, JIANG Yanjun (姜艷軍)1,2and GAO Jing (高靜)1,**
1School of Chemical Engineering and Technology, Hebei University of Technology, Tianjin 300130, China
2National Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China
A novel method was developed for papain immobilization through a biomimetic silicification process induced by papain. By incubating papain in a silica precursor solution, the papain-silica composite formed rapidly and papain was encapsulated. The encapsulation efficiency and the recovery activity were 82.60% and 83.09%, respectively. Compared with enzymes and biomolecules immobilized in biosilica matrix in the presence of additional silica-precipitating species, this papain encapsulation process, a biomimetic approach, realized high encapsulation efficiency by its autosilification activity under mild conditions (near-neutral pH and ambient temperature). Furthermore, the encapsulated papain exhibits enhanced thermal, pH, recycling and storage stabilities. Kinetic analysis showed that the biomimetic silica matrix did not significantly hinder the mass transport of substrate or the release of product.
papain, silica matrix, encapsulation, biosilicification, biomimetic
1 INTRODUCTION
Encapsulation in a carrier matrix, such as agarose, alginate and silica, is an attractive method for enzyme immobilization, which often provides advantages for enzymes to maintain their activity and improve their stability. However, traditional encapsulation methods, such as sol-gel process, usually inhibit enzyme activity, due to the harsh conditions in the process. In comparison, biomimetic silicification approach is a new way for enzyme encapsulation [1, 2]. This encapsulation process not only prevents the enzyme from contacting with denaturants but also stabilizes the structure of enzyme, so that the activity of the encapsulated enzyme is well preserved.
Many enzymes have been encapsulated in a functional state by the biomimetic approach and have exhibited better activity and longer lifetime than free enzymes [3-6]. For example, Luckarift et al. used R5 peptide as template/catalyst to induce silica formation and realized butyrylcholinesterase immobilization [3]. Subsequently, catalase, horseradish peroxidase, β-galactosidase, glucose isomerase and luciferase have been encapsulated in the biomimetically-induced silica. This encapsulation method shows several inherent advantages for enzyme immobilization such as rapid and mild immobilization process, low diffusion limitations and high volumetric activities, which improve enzyme activity and stability [7-11].
It is worthy of note that in this biomimetic enzyme encapsulation progress, biological templates and/or synthetic analogues are needed to catalyze the formation of silica. The extension of the biomimetic approach to enzyme immobilization is from the observation that silica-precipitating catalysts and enzymes become entrapped during silica formation process. Silaffins [1] and silicateins [12] were first utilized to mineralize silica using tetraethoxysilane (TEOS) as the silicic acid precursor. In place of silica-precipitating proteins obtained difficultly, various silica-precipitating catalysts including 19-amino-acid R5 peptide [1], proteins (such as protamine [13] and anticancer protein number 284 [14]), long-chain polyamines (polyethylenimine (PEI) [15] and polyamidoamine (PAMAM) dendrimers [16]), short-chain amines and even mono-amines [17] have been successfully employed for the precipitation of silica in enzyme immobilization process. In these reports, additional silica-precipitating catalysts are required for enzyme encapsulation, which depends on random entrapment events during silicification and may not ensure efficient and complete enzyme encapsulation [18].
Recent reports indicated that some enzymes had silica-precipitating ability and could be encapsulated in the silica matrix by the silica-precipitating ability of itself. For instance, lysozyme can be encapsulated within silica or titania nanoparticles with appreciable retention of antimicrobial activity by its inorganic speciesprecipitating ability. The resulting lysozyme-silica or lysozyme-titania composites could be used as broadspectrum antifouling materials [19]. Bassindale et al. pointed out that some hydrolase enzymes such as papain and trypsin could co-precipitate with silica under mild conditions [20]. Papain was also demonstrated to be able to induce the formation of titania [21]. Similar report indicated that certain proteolytic enzymes (such as α-chymotrypsin) were capable of inducing the formation of monolithic silica [22]. Buisson et al. reportedthat lipase from Burkholderia cepacia could catalyze the precipitation of silica [23]. However, few systematical studies were on the activity and stability of hydrolase enzymes encapsulated in biomimetic silica by this biomimetic approach.
Thus, a simple, economical, mild method for encapsulating papain in silica nanoparticles without additional silica-precipitating catalyst is reported in this study. Papain induces the formation of silica under ambient conditions and simultaneously encapsulates itself with appreciable retention of its activity. The encapsulation efficiency, activity recovery and stability of papain are investigated. The physical entrapment of papain within biosilica matrix may improve the native catalytic activity of papain.
2 EX PERIMENTAL
2.1 Materials
Papain (EC 3.4.22.2), a protease from Carica papaya, was purchased from Genview (USA) and stored as received at 4 °C. Tetramethyl orthosilicate (TMOS) was obtained from Tianjin Chemical Reagent Research Institute (Tianjin, China). Potassium phosphate buffer (PBS, 0.1 mol·L?1, pH 6.6) was used throughout the immobilization process unless otherwise indicated. All other chemicals were of analytical reagent grade and used without further purification.
2.2 Methods
2.2.1Encapsulation of papain in silica particles
An enzyme solution (10 mg·ml?1) was prepared by dissolving papain into the PBS (0.1 mol·L?1, pH 6.6). Hydrolyzed TMOS used as silicic acid precursor was freshly prepared by hydrolyzing TMOS in 1 mmol·L?1HCl to give a final concentration of 1 mol·L?1[1]. The encapsulation reaction was initiated by mixing the papain solution and hydrolyzed TMOS solution with the volume ratio 10∶1 at room temperature. After 15 min, the mixture was centrifuged for 5 min at 5000 r·min?1. The supernatant was collected to quantitate papain content. Then, the resultant precipitate was washed three times with distilled water to remove the non-encapsulated papain and unreacted silicic acid precursor.
2.2.2Encapsulation efficiency and activity recovery
The encapsulation efficiency is determined as

where csand c0are the papain concentration in the supernatant and in the initial solution, respectively. The concentration of the papain solutions was assayed by UV absorbance at 280 nm, and a standard curve was prepared according to the different absorbance.
The activity recovery is determined as

2.2.3Determination of papain activity
The activity of free and encapsulated papain was determined by using casein as substrate [24]. To appropriate soluble papain or corresponding encapsulated papain, 3 ml PBS (50 mmol·L?1, pH 7.0) and 1.5 ml 10 g·L?1casein solutions were added to start the reaction. The resultant solution was incubated at 40 °C for 30 min with magnetic stirring. Subsequently, 1.5 ml trichloroacetic acid (TCA) of 20% (by mass) was added in order to stop the reaction. Finally, the mixture was statically equilibrated at room temperature for 10 min and centrifuged. The activity of papain was determined spectrophotometrically by directly measuring the tyrosine content in the supernatant at 275 nm. One unit of enzyme activity was defined as 1 μg tyrosine formed per minute at 40 °C and pH 7.0.
2.2.4Papain stability experiments
For determination of the thermal and pH stability, free and encapsulated papain was incubated at 30, 40, 50, 60, 70, 80 and 90 °C for 4 h in PBS (50 mmol·L?1, pH 7.0) and in buffer solutions at pH 3, 4, 5, 6, 7, 8, 9 and 10 at 40 °C for 4 h separately. Then, the activity of papain was measured and the relative activity was calculated as a percentage of this highest activity (the highest values represent 100% of the enzyme activity).
For determination of the storage stability, free and encapsulated papain was stored at room temperature for a certain period of time. The storage stability was compared by storage efficiency defined as
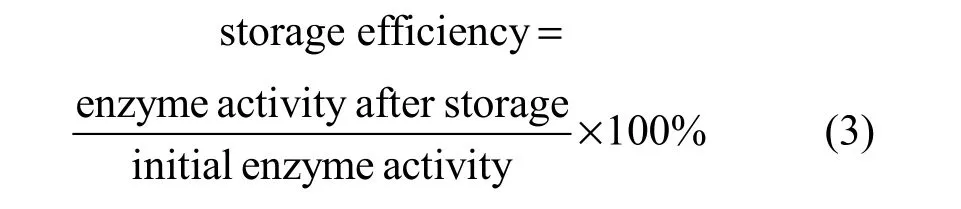
For determination of the recycling stability, the encapsulated papain was collected after each reaction, washed with distilled water, and then utilized in the next cycle. The recycling stability of encapsulated papain was evaluated by measuring the enzyme activity in each successive reaction cycle.

2.2.5Characterization
The morphological characterization of composites was observed by scanning electron microscopy (SEM). The dried samples were coated with gold and investigated using a JSM-6700F (JEOL, Japan) scanning electron microscope operated at 10 kV. Fourier transform infrared (FTIR) spectra of nanocomposites were obtained using a Brukervector 22 (Bruker, Germany).

Figure 1 Scheme for the process of papain encapsulated in silica matrix
3 RESUL TS AND DISCUSSION
3.1 Biomimetic encapsulation of papain
The hydrolyzed TMOS (1 mol·L?1) of 0.5 ml as the silicic acid precursor was added to 10 mg·ml?1papain solution (dissolved in water). No precipitate was observed in this mixture even after being left standing for 12 h at room temperature. However, when the same experiment was conducted in PBS (0.1 mol·L?1, pH 6.6), cloudy solution appeared immediately with the addition of hydrolyzed TMOS and was placed for 15 min to be condensed into white flocculent precipitate. Papain is a cationic polypeptide with a high isoelectric point (pI 8.8-9.6), which is necessary for biosilica formation under mild and simple reaction conditions [20]. Additionally, there are some common properties between papain and the natural silica-precipitating proteins (such as silicateins) [12, 25]. Silicateins are shown to be phosphorylated in native state, and the removal of phosphate would lead to the loss of the silica-precipitating ability. Thus phosphate is required for papain to induce the silica precipitation. The process of papain encapsulation is illustrated in Fig. 1. First, the negatively charged phosphate ions are easily attracted to positively charged papain through electrostatic interaction and aggregates form. Secondly, the silica formed around the papain scaffolds mediates the nucleation and precipitation of the insoluble composite, and papain is entrapped during silica formation. It also indicates that the microscopic phase separation of the silica-precipitating species may be another prerequisite for silica precipitation [26].
The FTIR data of the obtained precipitates is shown in Fig. 2. The typical absorption peaks of silica appeared at 1087 cm?1(Si O Si antisymmetric stretch), 935 cm?1(SiOH stretch), 794 cm?1(SiOSi symmetric stretch) and 466 cm?1(SiOSi bend), as well as the amide I (CO/CN stretch) and amide II (NH bend/CH stretch) vibration bands of polypeptide chain at 1652 and 1540 cm?1are observed. The result indicates that papain is successfully encapsulated within the silica matrix, as concluded in the previous report [13, 20].

Figure 2 FTIR spectrum of the precipitate
Following the rapid formation of precipitates from the reaction mixture, the supernatants were tested for papain content. The result showed that the encapsulation efficiency of papain was about 82.60%. In previous reports, enzymes and biomolecules were often immobilized in the biosilica matrix through biomimetic silicification processes induced by additional silica-precipitating species, relying on random entrapment events during polymerization, which may not ensure efficient and complete enzyme encapsulation. Due to the autosilification activity, the papain encapsulation process overcomes these limitations and realizes high encapsulation efficiency, where papain is capable of initiating silica precursor polycondensation under ambient conditions [18, 27]. The encapsulated papain silica particles were analyzed by SEM to determine the morphology and size distribution. As shown in Fig. 3, the particles were nearly spherical and uniform ranging from approximately 200-500 nm, which is in agreement with previous observations [20, 21].

Figure 3 SEM micrograph of the precipitate
To determine whether papain retained its activity after the immobilization reaction, the activity of papain encapsulated in the silica matrix was compared with the free papain. Activity recovery was about 83.09%, similar to previous reports for encapsulated phosphodiesterase (84%±15%) [18]. Even after 3 times washing of the composite material, papain activity was retained, suggesting that the catalytically active enzyme was embedded within the silica. The high activity recovery can be attributed to the rapid, mild and efficient immobilization procedures.
3.2 Michaelis constant
To further investigate the dynamics about papain-catalyzed reactions, initial reaction rate (V) was determined using various concentrations of casein ([S]) as substrate in order to evaluate its kinetic behavior. The corresponding Lineweaver-Burk plots for free and encapsulated papain were shown in Fig. 4. The Michaelis constant (Km) and maximum velocity (Vmax) were 2.05 mg·ml?1and 0.0207 mg·ml?1·min?1for encapsulated papain, while Kmand Vmaxfor free papain were 1.52 mg·ml?1and 0.0430 mg·ml?1·min?1, respectively.
In comparisons of the kinetic parameters of the free and encapsulated papain, the tendency of a decrease in Vmaxand a increase in apparent Kmafter encapsulation was observed, which is similar with the previous report of encapsulation of fungal protease [16] and phosphodiesterase [18] using the same manner. Additionally, a little increase of Kmsuggests that biomimetic biosilica matrix does not significantly decrease the affinity of papain to the substrate. However, a slight increase in Kmis probably due to the diffusion limitations of substrate through the silica matrix, the steric occlusion of the enzyme or the protein unfolding [28, 29].
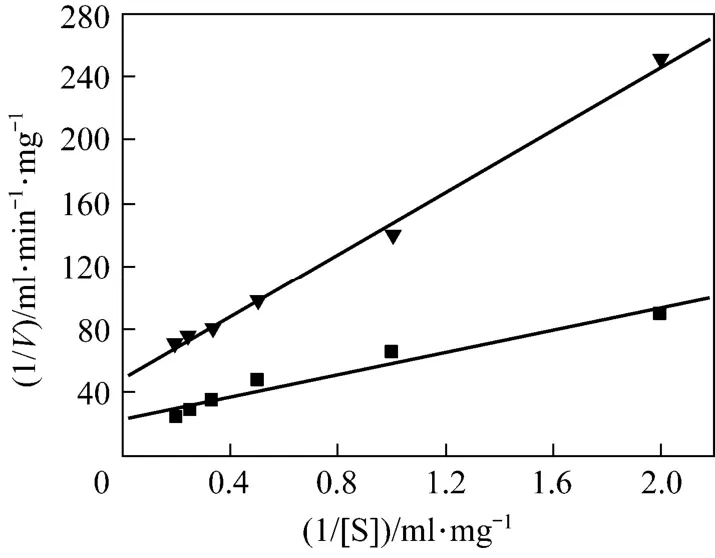
Figure 4 Lineweaver-Burk plots for free papain (■) and encapsulated papain (▼)
3.3 Thermal and pH stability of papain
Temperature and pH are the significant factors that affect the enzyme activity and stability. Thus, the relative activity of free and encapsulated papain was examined after incubation under a variety of pH and temperature conditions. Fig. 5 shows the effect of temperature. Free papain in solution retains high activity at relatively low temperatures (30 and 40 °C). However, free papain denatures drastically and the activity decreases rapidly as the temperature increases from 50 to 90 °C. When incubated at 90 °C for 4 h, free papain only retains 21% of its original activity. In contrast, encapsulated papain retains more than 90% of its original activity under the same condition (90 °C, pH 7.0 for 4 h), which is in agreement with the previousresults for enzymes encapsulation in biomimetic silica matrix. Compared to papain immobilized onto cotton fabric [30] and silica spheres [31], encapsulated papain shows significantly improved heat stability [32]. This can be explained possibly by that the structure of papain is slightly modified upon encapsulation and reverted to the right conformation upon heating.
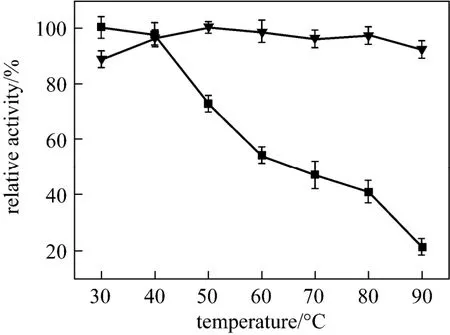
Figure 5 The relative activ ity o f free and encapsulated papain incubated at different temperatures for 4 h■ free papain; ▼ encapsulated papain
The results of pH stability for free and encapsulated papain incubated at different pH are shown in Fig. 6. Activity of the free papain at pH 3 or 9 is approximately 27% or 45% of that at pH 7, indicating the sensitivity of the free papain to acidic or alkaline conditions. In contrast, the encapsulated papain keeps more than 88% of activity in a wide pH range, suggesting that the encapsulated papain is less affected by harsh pH condition. This is probably due to the space limitation by silica matrix where papain molecules are surrounded by rare water molecules, so that even at high or low pH, the OH?or H3O+could not attack the peptide linkage of the protein to inactivate papain [33]. All of the above indicates that the biosilica matrix provides a good microenvironment to protect the encapsulated papain from denaturation under extreme conditions.
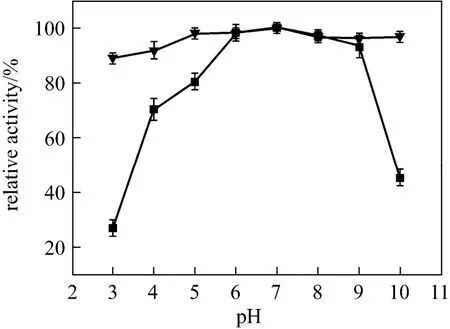
Figure 6 The r elative a ctivity of fr ee and encapsulated papain incubated at different pH■ free papain; ▼ encapsulated papain
3.4 Storage and recycling stability
The storage stability of encapsulated papain at room temperature is critical for its practical application. Therefore, a long-term stability of the encapsulated papain was examined at room temperature, and the results are shown in Fig. 7. Free papain lost most of its activity after storage in aqueous buffer in 30-day period, whereas about 80% of enzyme activity was retained by encapsulation in the silica matrix, which is in agreement with the results in biosilica [3, 28, 32]. Previous studies demonstrated that the loss of the activity was probably due to the microbial degradation [3]. Thus, the high stability of encapsulated papain is attributed to the pore size of silica matrix [13, 32, 34], which is too small to allow the degrading microbial proteins to infiltrate in the composite and degraded papain [28].
The activity of encapsulated papain was assayed in each cycling batch, and the relative activity of enzyme on reuse is shown in Fig. 8. Almost no appreciable loss in activity is observed after 7 cycles. It may be conjectured that the silica matrix effectively prevents enzyme from leaking by encapsulation. Furthermore, the small molecule product (tyrosine) could be timely diffused out through the pores of silica matrix, which is conducive to the reuse of encapsulated papain. The slight loss of papain activity may be due to the breakage of the particles after multiple operations under continuous stirring [32].
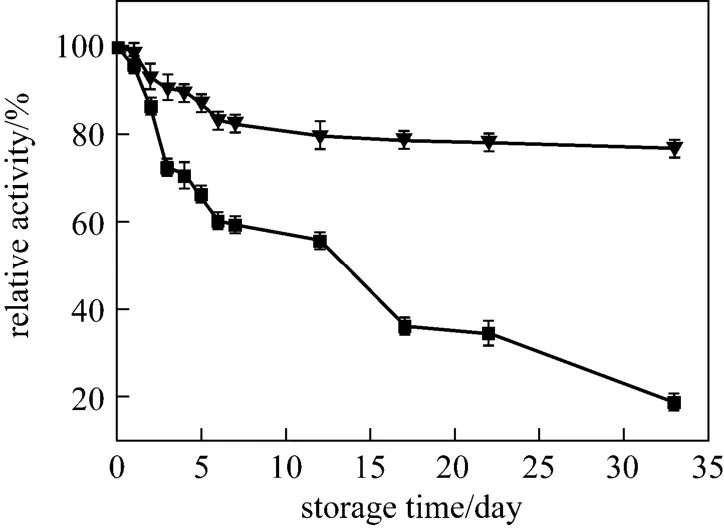
Figure 7 Storage stability of free and encapsulated papain■ free papain; ▼ encapsulated papain
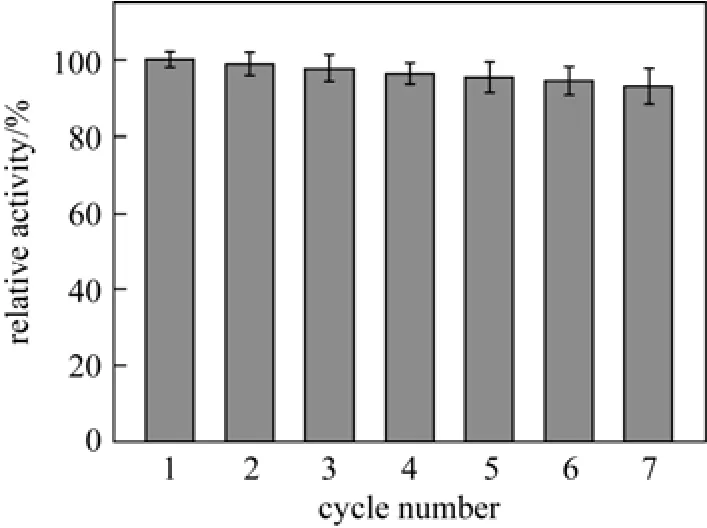
Figure 8 Reusability of the encapsulated papain
4 CONCLU SIONS
In summary, papain can induce the rapid precipitation of silica from silicic acid by a biomimetic mineralization process. Such papain-mediated precipitation is a one-pot procedure that simultaneously results in papain encapsulation within the silica matrix and retention of its appreciable activity. The results showed that 82.60% of papain was able to be encapsulated and 83.09% of its initial activity could be retained. The encapsulated papain exhibits improved thermal, pH, recycling and storage stability. The development of this simple, low-cost processing route to papain/silica nanocomposites and the evaluation of activity of such composites may provide an opportunity to immobilize papain under mild conditions. Additionally, the encapsulated papain may greatly expand the range of its applications, such as the fields of food, medicine and biocatalyst.
REFERENCES
1 Kr?ger, N., Deutzmann, R., Sumper, M., “Polycationic peptides from diatom biosilica that direct silica nanosphere formation”, Science, 286, 1129-1132 (1999).
2 Cha, J.N., Stucky, G.D., Morse, D.E., Deming, T.J., “Biomimetic synthesis of ordered silica structures mediated by block copolypeptides”, Nature, 403, 289-292 (2000).
3 Luckarift, H.R., Spain, J.C., Naik, R.R., Stone, M.O., “Enzyme immobilization in a biomimetic silica support”, Nat. Biotechnol., 22, 211-213 (2004).
4 Betancor, L., Luckarift, H.R., “Bioinspired enzyme encapsulation for biocatalysis”, Trends Biotechnol., 26, 566-572 (2008).
5 Choi, O., Kim, B.C., An, J.H., Min, K., Kim, Y.H., Um, Y., Oh, M.K., Sang, B.I., “A biosensor based on the self-entrapment of glucose oxidase within biomimetic silica nanoparticles induced by a fusion enzyme”, Enzyme Micro. Tech., 49, 441-445 (2011).
6 Das, S., Berke-Schlessel, D., Ji, H.F., McDonough, J., Wei, Y., “Enzymatic hydrolysis of biomass with recyclable use of cellobiase enzyme immobilized in sol-gel routed mesoporous silica”, J. Mol. Catal. B: Enzym., 70, 49-54 (2011).
7 Naik, R.R., Tomczak, M.M., Luckarift, H.R., Spain, J.C., Stone, M.O., “Entrapment of enzymes and nanoparticles using biomimetically synthesized silica”, Chem. Commun., (15), 1684-1685 (2004).
8 Luckarift, H.R., Johnson, G.R., Spain, J.C., “Silica-immobilized enzyme reactors; application to cholinesterase-inhibition studies”, J. Chromatogr. B, 843, 310-316 (2006).
9 Luckarift, H.R., Ku, B.S., Dordick, J.S., Spain, J.C., “Silica-immobilized enzymes for multi-step synthesis in microfluidic devices”, Biotechnol. Bioeng., 98, 701-705 (2007).
10 Swartz, J.D., Miller, S.A., Wright, D., “Rapid production of nitrilase containing silica nanoparticles offers an effective and reusable biocatalyst for synthetic nitrile hydrolysis”, Org. Process Res. Dev., 13, 584-589 (2009).
11 Zamora, P., Narváez, A., Domínguez, E., “Enzyme-modified nanoparticles using biomimetically synthesized silica”, Bioelectrochemistry, 76, 100-106 (2009).
12 Cha, J.N., Shimizu, K., Zhou, Y., Christiansen, S.C., Chmelka, B.F., Stucky, G.D., Morse, D.E., “Silicatein filaments and subunits from a marine sponge direct the polymerization of silica and silicones in vitro”, Proc. Natl. Acad. Sci. USA, 96, 361-365 (1999).
13 Zhang, Y.F., Wu, H., Li, J., Li, L., Jiang, Y.J., Jiang, Z.Y., “Protamine-templated biomimetic hybrid capsules: efficient and stable carrier for enzyme encapsulation”, Chem. Mater., 20, 1041-1048 (2008).
14 Sano, K.I., Minamisawa, T., Shiba, K., “Autonomous silica encapsulation and sustained release of anticancer protein”, Langmuir, 26, 2231-2234 (2010).
15 Berne, C., Betancor, L., Luckarift, H.R., Spain, J.C., “Application of a microfluidic reactor for screening cancer prodrug activation using silica-immobilized nitrobenzene nitroreductase”, Biomacromolecules, 7, 2631-2636 (2006).
16 Madadlou, A., Iacopino, D., Sheehan, D., Emam-Djomeh, Z., Mousavi, M.E., “Enhanced thermal and ultrasonic stability of a fungal protease encapsulated within biomimetically generated silicate nanospheres”, BBA-Gen. Subjects, 1800, 459-465 (2010).
17 Roth, K.M., Zhou, Y., Yang, W., Morse, D.E., “Bifunctional small molecules are biomimetic catalysts for silica synthesis at neutral pH”, J. Am. Chem. Soc., 127, 325-330 (2005).
18 Marner II, W.D., Shaikh, A.S., Muller, S.J., Keasling, J.D., “Enzyme immobilization via silaffin-mediated autoencapsulation in a biosilica support”, Biotechnol. Progr., 25, 417-423 (2009).
19 Luckarift, H.R., Dickerson, M.B., Sandhage, K.H., Spain, J.C.,“Rapid, room-temperature synthesis of antibacterial bionanocomposites of lysozyme with amorphous silica or titania”, Small, 2, 640-643 (2006).
20 Bassindale, A.R., Taylor, P.G., Abbate, V., Brandstadt, K.F., “Simple and mild preparation of silica-enzyme composites from silicic acid solution”, J. Mater. Chem., 19, 7606-7609 (2009).
21 Smith, G.P., Baustian, K.J., Ackerson, C.J., Feldheim, D.L., “Metal oxide formation by serine and cysteine proteases”, J. Mater. Chem., 19, 8299-8306 (2009).
22 Frampton, M., Vawda, A., Fletcher, J., Zelisko, P.M., “Enzyme-mediated sol-gel processing of alkoxysilanes”, Chem. Commun., (43), 5544-5546 (2008).
23 Buisson, P., Rassy, H.E., Maury, S., Pierre, A.C., “Biocatalytic gelation of silica in the presence of a lipase”, J. Sol-Gel Sci. Techn., 27, 373-379 (2003).
24 Lazo-Wasem, E.A., “Standardization of papain activity: Report of a collaborative study”, J. Pharm. Sci., 55, 723-725 (1966).
25 Shimizu, K., Cha, J., Stucky, G.D., Morse, D.E., “Silicatein α: cathepsin L-like protein in sponge biosilica”, Proc. Natl. Acad. Sci. USA, 95, 6234-6238 (1998).
26 Brunner, E., Lutz, K., Sumper, M., “Biomimetic synthesis of silica nanospheres depends on the aggregation and phase separation of polyamines in aqueous solution”, Phys. Chem. Chem. Phys., 6, 854-857 (2004).
27 Ramanathan, M., Luckarift, H.R., Sarsenova, A., Wild, J.R., Ramanculov, E. K., Olsen, E. V., Simonian, A.L., “Lysozyme-mediated formation of protein-silica nano-composites for biosensing applications”, Colloid. Surface. B, 73, 58-64 (2009).
28 Miller, S.A., Hong, E.D., Wright, D., “Rapid and efficient enzyme encapsulation in a dendrimer silica nanocomposite”, Macromol. Biosci., 6, 839-845 (2006).
29 Lai, J.K., Chuang, T.H., Jan, J.S., Wang, S.S., “Efficient and stable enzyme immobilization in a block copolypeptide vesicle-templated biomimetic silica support”, Colloid. Surface. B, 80, 51-58 (2010).
30 Li, F.Y., Xing, Y.J., Ding, X., “Immobilization of papain on cotton fabric by sol-gel method”, Enzyme. Microb. Tech., 40, 1692-1697 (2007).
31 Wang, A., Wang, H., Zhou, C., Du, Z., Zhu, S., Shen, S.,“Ag-induced efficient immobilization of papain on silica spheres”, Chin. J. Chem. Eng., 16, 612-619 (2008).
32 Jiang, Y.J., Sun, Q.Y., Jiang, Z.Y., Zhang, L., Li, J., Li, L., Sun, X.H.,“The improved stability of enzyme encapsulated in biomimetic titania particles”, Mat. Sci. Eng. C, 29, 328-334 (2009).
33 Avnir, D., Coradin, T., Lev, O., Livage, J., “Recent bio-applications of sol-gel materials”, J. Mater. Chem., 16, 1013-1030 (2006).
34 Cardoso, M.B., Luckarift, H.R., Urban, V.S., O'Neill, H., Johnson, G.R., “Protein localization in silica nanospheres derived via biomimetic mineralization”, Adv. Funct. Mater., 20, 3031-3038 (2010).
2012-06-25, accepted 2012-10-20.
* Supported by the National Natural Science Foundation of China (21006020, 21276060, 21276062), the Natural Science Foundation of Hebei Province (B2010000035, B2011202095), the Science and Technology Research Key Project of Higher School in Hebei Province (ZD2010118), the Application Basic Research Plan Key Basic Research Project of Hebei Province (11965150D) and Open Funding Project of the National Key Laboratory of Biochemical Engineering (China).
** To whom correspondence should be addressed. E-mail: jgao@hebut.edu.cn
 Chinese Journal of Chemical Engineering2013年6期
Chinese Journal of Chemical Engineering2013年6期
- Chinese Journal of Chemical Engineering的其它文章
- Adsorption and Desorption Behavior of Tannic Acid in Aqueous Solution on Polyaniline Adsorbent*
- Synthesis of 2-Methyl-4-methoxyaniline from o-Nitrotoluene Using Pt/C and Acidic Ionic Liquid as Catalyst System*
- Supercritical Fluid Extraction of a Novel Template from Mesoporous Zirconia and the Effect on Porous Structure*
- Preparation of Mesoporous Carbons from Acrylonitrile-methyl Methacrylate Copolymer/Silica Nanocomposites Synthesized by in-situ Emulsion Polymerization*
- Effect of Hydrogen Reduction of Silver Ions on the Performance and Structure of New Solid Polymer Electrolyte PEI/Pebax2533/AgBF4Composite Membranes*
- Comparison on Thermal Conductivity and Permeability of Granular and Consolidated Activated Carbon for Refrigeration*
