Effects of Experimental Conditions on The Morphology and Photocurrent Density of TiO2 Nanorods
SUN Xian-miao,SUN Qiong,XIE Cui-cui,DONG Li-feng,2*
(1.College of Materials Science and Engineering,Qingdao University of Science and Technology,Qingdao 266042;2.Department of Physics,Astronomy,and Materials Science,Missouri State University,Springfield,Missouri,65897,USA)
*Corresponding Author,E-mail:DongLifeng@qust.edu.cn
1 Introduction
Oriented single-crystalline TiO2nanorod without surface trap sites and grain boundary is a potential material to improve the photoelectric conversion efficiency of DSSCs.However,the synthesis of TiO2kept a challenge until a facial hydrothermalmethod was reported to fabricate single-crystalline rutile TiO2nanorod arrays directly on fluorine-doped tin oxide(FTO)glass substrates by Liu et al[8].So far,hydrothermalmethod has been largely explored to synthesize single-crystalline TiO2nanorods,but there is no detailed report on the correlations between experimental parameters and the morphology and photoelectronic properties of TiO2nanorods.
In this study,TiO2nanorods were fabricated through a facial hydrothermalmethod,and the effects of experimental parameters including acidity,reactant concentration,growth time and reaction temperature on the morphologies and photocurrent densities of TiO2nanorodswere systematically investigated.
2 Experiments
2.1 Synthesis of TiO2 Nanorods
TiO2nanorod arrays were synthesized by a facial hydrothermal method[8].In brief,60 mL of a mixed solution containing 30 mL of deionized(DI)water and 30mL of hydrochloric acid(HCl,36.5%)was stirred for 5 min under ambient conditions,and then 1.2 mL of titanium isopropoxide[Ti(iPro)4]was added to the mixture and stirred for another 5 min.The ultimate mixture was transferred into a Teflon-lined stainless steel autoclave.A piece of FTO substrate was placed at an angle against the wall of the Teflon-liner with the conducting side facing down.Hydrothermal synthesiswas carried out at 120~180℃for 3~16 h in an oven.After the synthesis,the autoclave was naturally cooled to room temperature.The FTO substrate was taken out,rinsed extensively with DIwater,and allowed to dry in air.Meanwhile,a series of experiments with different concentration of HCl and Ti(iPro)4were conducted to investigate the effects of acidity and reactant concentration.
2.2 Physical Characterization and Photovoltaic Measurement
Surfacemorphologies of the sampleswere examined by field emission scanning electron microscopy(FESEM,JEOL JSM-6700F).Photocurrent response vs.time of TiO2nanorod films wasmeasured on a CHI660D electrochemical station withoutexternal bias electrical potential using a three-electrode cell configuration with a saturated calomel electrode(SCE)as a reference electrode,a platinum wire as a counter electrode,and the round TiO2/FTO film with an exposed area of0.502 4 cm2as aworking electrode.The illumination was provided by a xenon lamp(120 W,Newport 96000)and the status of the lamp was changed between on and off every 10 s.0.1 mol/L of NaSO4aqueous solution was used as electrolyte throughout this study.
3 Results and Discussion
3.1 M orphology of TiO2 Nanorod Arrays
Fig.1 shows the top surface(a)and cross-section(b)of TiO2nanorods fabricated using Ti(iPro)4as the titanium precursor at150℃for 4 h.It is obviously that the FTO substrate is uniformly covered with a film of TiO2nanorods with a diameter of circa 160 nm and a length of 1.5 m.These nanorods are highly ordered and perpendicular to the substrate.
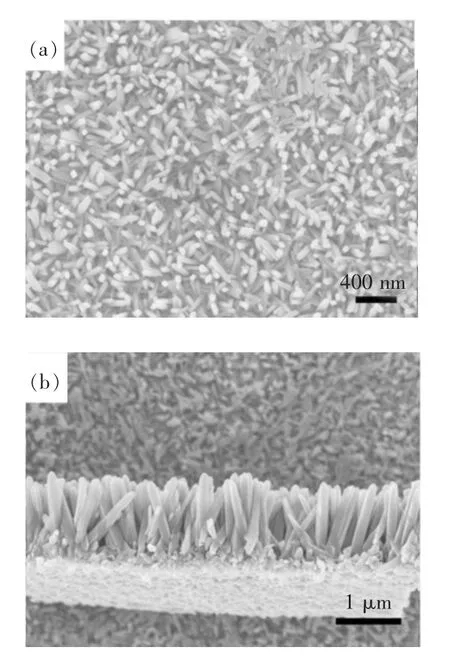
Fig.1 SEM images of TiO2 nanorod arrays synthesized on FTO substrates from Ti(iPro)4 at150°C for 4 h
3.2 Effects of Acidity and Reactant Concentration
With the volume of total reaction solutions kept constant,the volume of HCl changed from 20 mL to 40 mL with V(DIwater)∶V(HCl)=2∶1,1∶1,2∶3,1∶2(samples1 ~4).As shown in Fig.2(a),there are no obvious products from the ratios of 2∶3 and 1∶2;and the product from the ratio of 1∶1 ismore abundant than that of 2∶1.Photoelectricalmeasurements also demonstrated a higher photocurrent density of the ratio of1∶1 than thatof2∶1(Fig.2(b)).On the other hand,Fig.2(c)displays the photographs of the samples 5~9 obtained with different Ti(iPro)4concentrations by adding 0.6,1.2,1.8,2.4,3.0mL of Ti(iPro)4,respectively.Among the five samples,sample 6 seems to have the thickest film on the substrate surface.Electrical measurements show that sample 5 and 8 are conductive,indicating that the film is too thin or non-uniform on the conductive FTO substrate.Fig.2(d)shows the photocurrent density vs.time(I-t)curves of samples 6,7,and 9.Among the three samples,sample 6 exhibits a higher photocurrent density than sample 9 and a better reproducibility than sample 7.Based on our experimental observations,amixture solution of 30 mL of DIwater,30 mL of HCl and 1.2 mL of Ti(iPro)4was chosen to further investigate effects of growth time and reaction temperature.
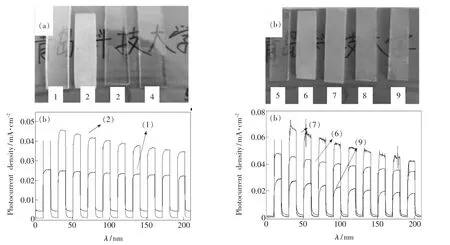
Fig.2 Photos and I-t curves of TiO2 nanorods synthesized with different acidity(a,b)and reactant concentrations(c,d).Samples 1~4 were synthesized at150 °C for 4 h in the solutions of 1.2 mL of Ti(iPro)4 with various V(DIwater)∶V(HCl):(1)40mL to20mL(2∶1);(2)30mL to30mL(1∶1);(3)24mL to 36mL(2∶3),and(4)20mL to 40 m L(1∶2).Samples5 ~9 were obtained using 30mL of DIwater and 30mL of HClwith the Ti(iPro)4 addition of0.6,1.2,1.8,2.4 and 3.0 mL,respectively.
3.3 Effects of Grow th Time
As shown in Fig.3(a),photocurrent density fluctuates with the change of growth time from 3 h to 16 h,and no clear correlations can be concluded between photocurrent density and the growth time.Similar case exists in the relationship between growth time and the nanorod diameter(Fig.3(b)).However,the length of TiO2nanorods can be controlled by changing the growth time.For instance,when the growth time changed from 3 h to 10 h,the nanorod length increased from circa 200 nm to 660 nm.It was reported that the length of TiO2nanorods has an important influence on their photoelectric properties and the optimum length is about2.0 μm[9].However,in our experiments,the longest nanorod is twice longer than that of the shortest one,and the diameter of the largest nanorod is nearly two times that of the smallest one.This indicates that the photocurrent density of TiO2nanorods can be affected by both their length and diameter,as illustrated by the fluctuation of photocurrent density with the change of growth time(Fig.3(a)).
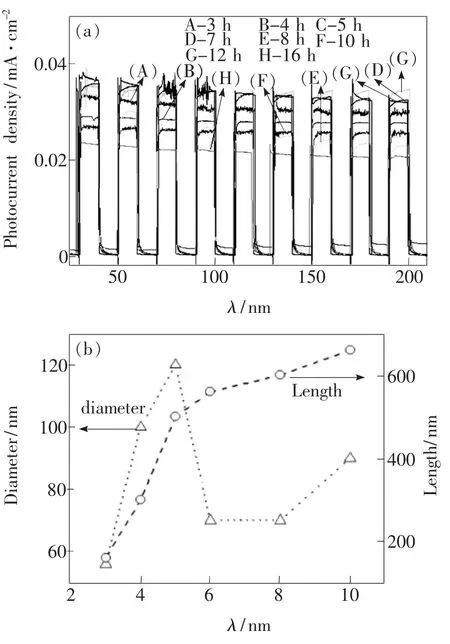
Fig.3 Effects of growth time(3~16 h)on I-t characteristics(a)and morphology(b)of TiO2 nanorods
3.4 Effect of Reaction Tem perature
As demonstrated in Fig.4,different from the effects of growth time,reaction temperature greatly affects both photocurrent density and themorphology of TiO2nanorods.Fig.4(a)and(b)exhibit that,with the increase of reaction temperature,the photocurrent density was firstly enhanced and then declined over 155℃.Fig.4(c)show that both the diameter and length of TiO2nanorodswere enhanced with the increase of reaction temperature.When the temperature increased from 120℃ to 180℃,the diameter and length increased from 80 nm and 0.4 μm to 200 nm and 2.8μm,respectively.It is well known that a high temperature promotes the nucleation and aggravates the aggregation of TiO2nanorods,and thereby increases their diameter and length.The changes in the length aremore obvious than those in the diameter,indicating that the photocurrent density ismainly affected by the length of TiO2nanorods.The optimum length in our research is about 1.5 Mm,which is slightly different from the literature[9],which can be due to the effects of the diameter.
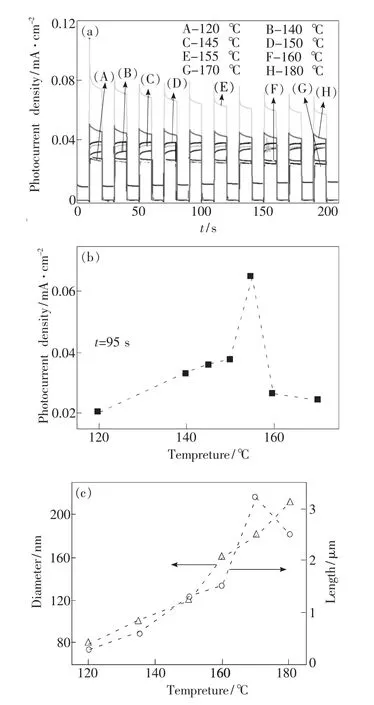
Fig.4 Effects of reaction temperature(120~180℃)on I-t characteristics(a);instantaneous current density at 95 s(b);and the diameters and lengths of TiO2 nanorods(c).
4 Conclusion
In summary,TiO2nanorod arrays were synthesized through a facial hydrothermalmethod,and the effects of acidity,reactant concentration,growth time and temperature were systematically studied.Experiment results indicate that the optimum reaction solution is themixture of30mL of DIwater,30 mL of HCl and 1.2 mL of titanium precursor.The length of TiO2nanorods increasing with the growth time,while there are no clear correlations between the growth time and the diameter/photocurrent density.However,compared with growth time,reaction temperature greatly affects the length and diameter of TiO2nanorods and their photocurrent density.
[1]O'regan B,Gr?tzel M.A low-cost,high-efficiency solar cell based on dye-sensitized colloidal TiO2films[J].Nature,1991,353(6436):737-740.
[2]Liu Z C,Zheng JT,Zhao D F,et al.Effects of forbidden bandwidth and optical absorption coeffcient on photocatalytic ability of TiO2[J].Chin.J.Lumin.(發光學報),2012,33(12):1329-1334(in Chinese).
[3]Yang X,Qu Y,Fan Y,et al.Y-branched TiO2nanotubes prepared by electrochemical anodization[J].Chin.J.Lumin.(發光學報),2012,33(3):269-274(in Chinese).
[4]Xin X,Scheiner M,Ye M,et al.Surface-treated TiO2nanoparticles for dye-sensitized solar cells with remarkably enhanced performance[J].Langmuir,2011,27(23):14594-14598.
[5]GuoW,Chen W,Wang X X,et al.Rectangular bunched rutile TiO2nanorod arrays grown on carbon fiber for dye-sensitized solar cells[J].J.Am.Chem.Soc.,2012,134(9):4437-4441.
[6]Zhu K,Neale N R,Miedaner A,et al.Enhanced charge-collection efficiencies and light scattering in dye-sensitized solar cells using oriented TiO2nanotubes arrays[J].Nano Lett.,2007,7(1):69-74.
[7]Dloczik L,Ileperuma O,Lauermann I,et al.Dynamic response of dye-sensitized nanocrystalline solar cells:Characterization by intensity-modulated photocurrent spectroscopy[J].J.Phys.Chem.B,1997,101(49):10281-10289.
[8]Liu B,Aydil E S.Growth of oriented single-crystalline rutile TiO2nanorods on transparent conducting substrates for dyesensitized solar cells[J].J.Am.Chem.Soc.,2009,131(11):3985-3990.
[9]Zhou Z,Fan J,Wang X,etal.Effectof highly ordered single-crystalline TiO2nanowire length on the photovoltaic performance of dye-sensitized solar cells[J].ACSAppl.Mater.Interfaces,2011,3(11):4349-4353.

