D-和L-丙氨酸晶體的突現順磁性:準一維N+H…O-氫鍵的自旋-軌道分離
王文清 沈新春 張玉鳳 龔
(1北京大學化學與分子工程學院應用化學系,北京分子科學國家實驗室,北京100871;2山東大學化學與分子工程學院,濟南250061;3北京服裝學院材料科學與工程學院,北京100029)
D-and L-alanine crystals are recognized as diamagnetic materials1which exist as main chains of hydrogen bonded zwitterions(+NH3―C(CH3)H―CO2-)and form handed arrangement of N―H…O=C peptide bond which plays a critical role in the secondary structural elements of protein molecules resulting in right-handed α-helix by L-amino acids.Emergent paramagnetism of DNA molecules has been discovered by Juyeon.2Paramagnetism originated from the N+H…O?hydrogen bond containing magnetic dipole moment associated with the intrinsic spin and the orbital motion of the electron.We have found the electron spin-flip transition of N+H…O-hydrogen bond around 270 K under magnetic induction of B=+1 T in D-alanine crystal lattice and B=-1 T in L-alanine crystal lattice.Because the energy is lower when the dipole moment in D-alanine is parallel to the field than when it is antiparallel in L-alanine,the parallel alignment is preferred.The energy difference is around 10-5eV·molecule-1.3This article focuses on the field-dependent magnetic properties of the chiral alanine crystals,especially associated with the electronic orbital motions.We design to apply a strong external field of 5 T parallel to the preferred axis of spin magnetic dipole moment and expect that D-alanine will be manifested emergent paramagnetism and L-alanine will be found a different process from spin-flop state to paramagnetic state transition.4,5
2 Experimental

Fig.1 dc-Magnetic susceptibility of D-and L-alanine from 2 to 320 K
The polycrystalline powders of D-and L-alanine(Sigma Chemical Co.)are twice recrystallized from sterilization aqueous solution at 277 K by slow evaporation for 2 to 3 weeks,producing well formed crystal elongated along the c axis and with principal faces{110}6,7washed with dropping absolute alcohol,evacuated and kept in a desiccator.Both chiral alanine crystals are determined by X-ray diffraction(XRD)crystallography on a Rigaku RAXIS-RAPID imaging plate diffractometerwith graphitemonochromated Mo-Karadiation (λ=0.071069 nm)at 300,270,250 K8and neutron diffraction data from crystals at 60,240,250,260,295,300 K,9-11respectively.The crystal purity was identified by the positive-ion electrospray mass spectra(EI-MS)with a detection limit of 2 fmol of organic impurity.12The enantiomeric purity of D-and L-alanine single crystals were identified by gas chromatography mass spectrometry(GC-MS)of their N-trifluoroacetyl(TFA)isopropyl esters on Chirasil-Val capillary columns.13Fourier transform infrared(FTIR)spectrometer(Nicolet iN 10 MX,USA)with a detection limit of 10 mg was precluded the possible H2O content in the measuring alanine crystals.
3 Results and discussion
3.1 dc-Magnetic susceptibility of D-and L-alanine under B=±1 T
The mass magnetic susceptibility of D-and L-alanine single crystals was measured with a Quantum MPMS XL-5 SQUID system from 2 to 330 K by Direct Current(dc)Option.Two crystals were selected(mass of 390.89 mg for D-alanine and 338.50 mg for L-alanine)and rectangularly mounted in the long plastic tube.The crystals were placed that the c axis was exactly parallel to the direction of the applied field.Based on the calculation of the orientational potential energy,an external magnetic induction B=±1 T was applied and then the dc-magnetic susceptibility was measured by scanning three times.We have found an electron spin-flip transition of N+H…O-hydrogen bond under magnetic induction+1 T in D-alanine and-1 T in L-alanine along c(z)axis(Fig.1).

Fig.2 N+H4···O-bonding of L-and D-alanine viewed down the a axis9
Based on the magnetic susceptibility measurements(Fig.1)and neutron diffraction data(Fig.2),we consider the phase transition mechanism:first,the magnetic dipole moment of N+H…O-hydrogen bond might be associated with motion of charges in the nucleus.The magnitude of such a magnetic dipole moment would be of the order ofe?/2M,where M is the mass of a proton, ?=h/2π (h is Planck constant).But the magnetic dipole moment measured experimentally from the size of splitting is of the order of μb=e?/2m,where m is the mass of an electron,which is about 2000 times larger.Therefore,the nucleus cannot be responsible for the observed magnetic dipole moment.Its source must be the electron.
Second,the electrons of hydrogen atom have an intrinsic angular momentum called spin,and an associated spin magnetic dipole moment.Because the electron has a negative charge,its magnetic dipole moment μlis antiparallel to its orbital angular momentum L.14When it is placed in an external magnetic field of+1 T,the magnetic dipole will tend to align with the field.The orientational potential energy when the dipole is parallel to the field is-μlB,and it is+μlB when the dipole is antiparallel to the field.So the energy that must be supplied to turn the dipole is

Conversely,the dipole N+H…O-of L-alanine is originally aligned antiparallel to the field,it cannot turn to align itself parallel to the field unless it can get rid of the same amount of energy.
This experiment proved that the electron of hydrogen in N+H…O-bond is spin-up(↑)parallel to the c(z)axis in D-alanine but spin-down(↓)antiparallel to the c(z)axis in L-alanine under external magnetic field of+1 T,and vice versa under-1 T.We find the true chirality and parity-time(PT)asymmetry of N+H…O-hydrogen bond in zwitterionic model and discover that the quintessential truly chiral influence is the helicity of the lattice structure of peptide bond.This is the easy way to solve the puzzle of“false chirality”of translating and rotating ammonia model of Compton.15
Additionally,the measurements of the specific heat16evidence for the electron spin-flip transition of N+H…O-hydrogen bond of D-/L-alanine crystal at 272.02 K/271.85 K with the heat of transition of 1.87 J·mol-1/1.46 J·mol-1,respectively.The energy difference between D-and L-alanine crystals is(1.87-1.46)J·mol-1=4.2×10-6eV·molecule-1,which is approximatively coincident with dc-magnetic susceptibility measurement in external magnetic field of+1 T in D-alanine and-1 T in L-alanine.Since spin is axial vector,all the above results are not seen from the polycrystalline powder measurements.
3.2 RSO-magnetic susceptibility of D-and L-alanine under B=5 T
We use the same crystals(390.89 mg for D-alanine and 338.50 mg for L-alanine)to study the magnetic properties under strong magnetic induction B=5 T with a quantum design model MPMS-7 SQUAD-based magnetometer from 5 to 330 K by Reciprocating Sample Option(RSO).In the measurements,D-alanine manifests emergent paramagnetism at 297.6 K(Figs.3(a),4(a)).However in L-alanine,when the field reaches a critical value there is a discontinuity in the magnetization,the moments flop firstly perpendicular to the field,as the field reaches a value high enough,then an electron spin-flop state to paramagnetic state transition is observed around 303.9 K(Figs.3(b),4(b)).We have found emergent paramagnetism in D-and L-alanine crystals with different process.
The magnetic interaction energy under magnetic induction B=5 T is
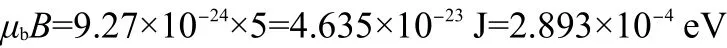
At room temperature T=300 K,the thermal energy
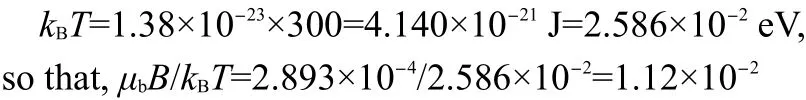
Hence,μbB being about 1.12%of kBT.Assuming that μbB<kBT is valid at room temperature under magnetic induction of 5 T,electron spin-flop to paramagnetic state transition is occurred.
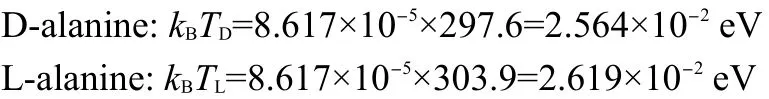
The thermal energy difference can be estimated:
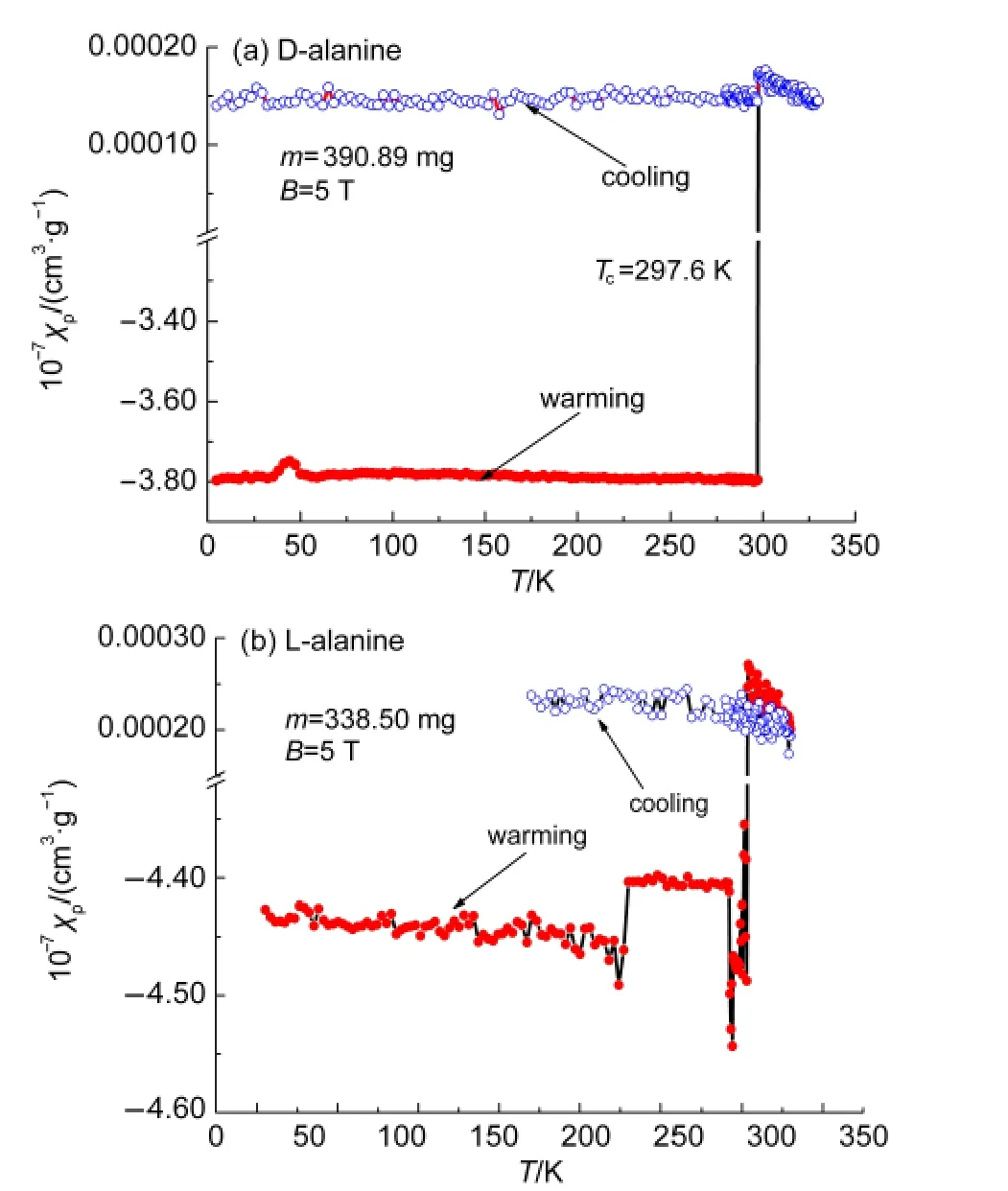
Fig.3 Magnetic susceptibility versus temperature from 5 to 330 K

Fig.4 Magnetic susceptibility versus temperature from 280 to 325 K
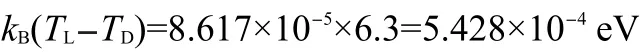
According to the paramagnetic susceptibility data,
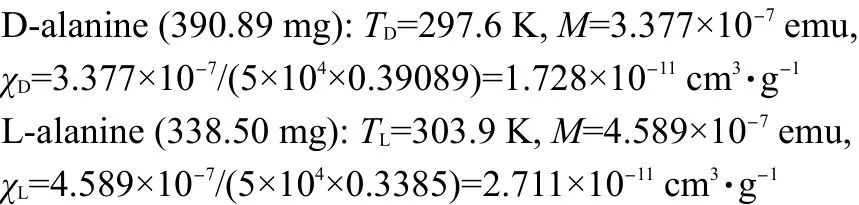
The difference of mass paramagnetic susceptibility can be estimated:

As the field reaches a value high enough of 5 T,the paramagnetism is conserved at a fixed temperature range warming from 297.6 to 310 K and cooling from 309 to 5 K.This is the saturated condition,in which all the spins with their individual magnetic dipole moment are aligned.When the applied field is removed,the thermal motion randomizes the dipole directions so that the magnetization is zero(Fig.5).

Fig.5 Magnetic susceptibility versus temperature(5-50 K)of D-and L-alanine
4 Remark and prospects
The field-dependent magnetic properties of chiral alanine crystals associated with electronic motions are investigated.The key finding is the observation of spin-orbital separation in the quasi-one-dimensional N+H…O-hydrogen bond of L-and D-alanine crystals.The requirement for paramagnetism in chiral alanine crystals is that the dipole moments of N+H…O-hydrogen bond have some degree of isolation.The hallmark of one-dimensional physics is a breaking up of the elementary electron into its separate degree of freedom.Even if electrons in crystals form bound state and delocalize from the nuclei,they retain their fundamental quantum numbers:spin,charge,and orbital.The hallmark of one-dimensional physics is a breaking up of the elementary electron into its separate degree of freedom.The separation of the electron into independent quasi-particles that carry either spin(spinons),charge(holons)or orbital degree of freedom(orbiton).17This article clearly shows the quasi-one-dimensional of N+H…O-hydrogen bond in chiral alanine crystalline lattice manifested a paramagnetic response to external magnetic field of 5 T around room temperature.The applied field splits a degenerate energy level in paramagnetic system of chiral-alanine.Degeneracy also can be driven into ground state by the field.This can lead to the phenomenon of field-induced magnetic ordering.18,19
(1) Kwok,R.S.;Maxton,P.;Migliori,A.Solid State Communication 1990,74(11),1193.
(2) Juyeon,Y.Physical Review B 2006,74,212406-1-4.doi:10.1103/PhysRevB.74.212406
(3)Wang,W.Q.;Shen,X.C.;Gong,Y.Acta Phys.-Chim.Sin.2008,24(5),743.[王文清,沈新春,龔.物理化學學報,2008,24(5),743.]doi:10.1016/S1872-1508(08)60031-5
(4) Carlin,R.L.;van Duyneveldt,A.J.Accounts Chem.Res.1980,13,231.doi:10.1021/ar50151a007
(5) Carlin,R.L.Magnetochemistry;Springer-Verlag:Berlin,Heidelberg,1986.
(6) Simpson,H.J.;Marsh,R.E.Acta Cryst.1966,20,550.doi:10.1107/S0365110X66001221
(7) Destro,R.;Marsh,R.E.J.Phys.Chem.1988,92,966.doi:10.1021/j100315a022
(8)Wang,W.Q.;Min,W.;Bai,F.;Sun,L.;Yi,F.;Wang,Z.M.;Yan,C.H.;Ni,Y.M.;Zhao,Z.X.Tetrahedron-Asymmetry 2002,13,2427.doi:10.1016/S0957-4166(02)00658-4
(9)Wilson,C.C.;Myles,D.;Ghosh,M.;Johnson,L.N.;Wang,W.Q.New J.Chem.2005,29,1318.doi:10.1039/b419295h
(10)Wang,W.Q.;Liu,Y.N.;Gong,Y.Acta Phys.-Chim.Sin.2004,20,1345.[王文清,劉軼男,龔.物理化學學報,2004,20,1345.]doi:10.3866/PKU.WHXB20041112
(11)Wang,W.Q.;Gong,Y.;Yao,N.Acta Phys.-Chim.Sin.2005,21,774.[王文清,龔,姚 楠.物理化學學報,2005,21,774.]doi:10.3866/PKU.WHXB20041112
(12) Gledhill,M.Analyst 2001,126,1359.
(13) Cronin,J.R.;Pizzarello,S.Science 1997,275,951.doi:10.1126/science.275.5302.951
(14) Robert,E.;Robert,R.Quantum Physics of Atoms 1980,4(3),194.
(15) Compton,R.;Pagni,R.M.Advances in Atomic,Molecular and Optical Physics 2002,48,231.
(16)Wang,W.Q.;Shen,X.C.;Wu,J.L.;Gong,Y.;Shen,G.H.;Zhao,H.K.Acta Phys.-Chim.Sin.2012,28,773.[王文清,沈新春,吳季蘭,龔,申國華,趙洪凱.物理化學學報,2012,28,773.]doi:10.3866/PKU.WHXB201202132
(17) Schlappa,J.;Wohlfeld,K.;Zhou,K.J.;Mourigal,M.;Haverkort,M.W.;Strocov,V.N.;Hozoi,L.;Monney,C.Nature 2012,485,82.doi:10.1038/nature10974
(18) Tsunehisa,K.;Fumiko,K.;Masashi,Y.Langmuir 2006,22,3464.doi:10.1021/la053479n
(19) Abdrzej,R.;Jirawat,W.S.;Suchada,R.Science 2001,294,1503.doi:10.1126/science.1065477

