中國肝癌肝移植臨床實踐指南
中華醫學會器官移植學分會
中華醫學會外科學分會移植學組
中國醫師協會器官移植醫師分會
1 前 言
據統計中國每年超過30 萬人死于肝細胞肝癌(以下簡稱肝癌),占全球肝癌死亡人數的一半左右。而肝移植是被全世界認可的治療終末期肝病最有效的手段之一。我國自20 世紀90 年代掀起第二次肝移植熱潮以來,肝移植事業發展迅猛,呈專業化和規模化發展態勢,在移植數量和質量方面已接近或達到西方發達國家水平。截止2014 年4 月,中國肝移植注冊網站登記肝移植26 751 例。目前,肝移植在全國范圍內已得到廣泛開展,亟待相關實踐指南來指導全國肝移植工作更規范、有效、安全地開展。中華醫學會器官移植學分會、中華醫學會外科學分會移植學組及中國醫師協會器官移植醫師分會組織專家制定肝癌肝移植臨床實踐指南,重點闡述肝移植受者選擇標準、術前降期治療、抗病毒治療、免疫抑制劑應用、術后復發防治五部分內容。本指南采用的循證醫學證據分級主要參考2001 牛津證據分級(詳見表1),推薦意見強度主要參考GRADE系統推薦分級等[1-2]。
2 肝癌肝移植受者選擇標準(表2)
供肝短缺是世界性難題,故應將寶貴的供肝資源優先分配給肝移植的最大獲益者。心臟死亡器官捐獻是中國現今拓展供肝來源的主要方向,而活體肝移植在有豐富移植經驗的醫療單位已成為一項成熟技術[3]。1996 年Mazzaferro 等提出米蘭標準后,符合米蘭標準的肝癌肝移植受者獲得了長期存活[4-7]。但米蘭標準對肝癌大小和數目的限制過于嚴格,更重要的是忽略了腫瘤的生物學特性。如果根據米蘭標準,中國大多數肝癌患者將失去肝移植機會。近年來國際上涌現出一些新的肝癌肝移植受者選擇標準,如加州大學洛杉磯分校(University of California,San Francisco,UCSF)標準、Up-to-Seven標準等,這些新標準提出的共同目的是擴大受者人群并取得與米蘭標準相似的移植生存率[8-9]。2008 年,中國提出的杭州標準是國際上首個引入腫瘤生物學特性和病理學特征的移植標準,這是對以往標準局限于腫瘤形態學的巨大突破。研究證實,無論是尸體肝移植還是活體肝移植,符合杭州標準的肝癌受者均獲得滿意的術后生存率[10-15]。近年來,對于肝癌切除術后復發者,如符合肝移植準入標準,多數專家主張行搶救性肝移植[16-17];對于肝癌肝移植術后移植物失功者,再次肝移植應審慎考慮[17-18]。
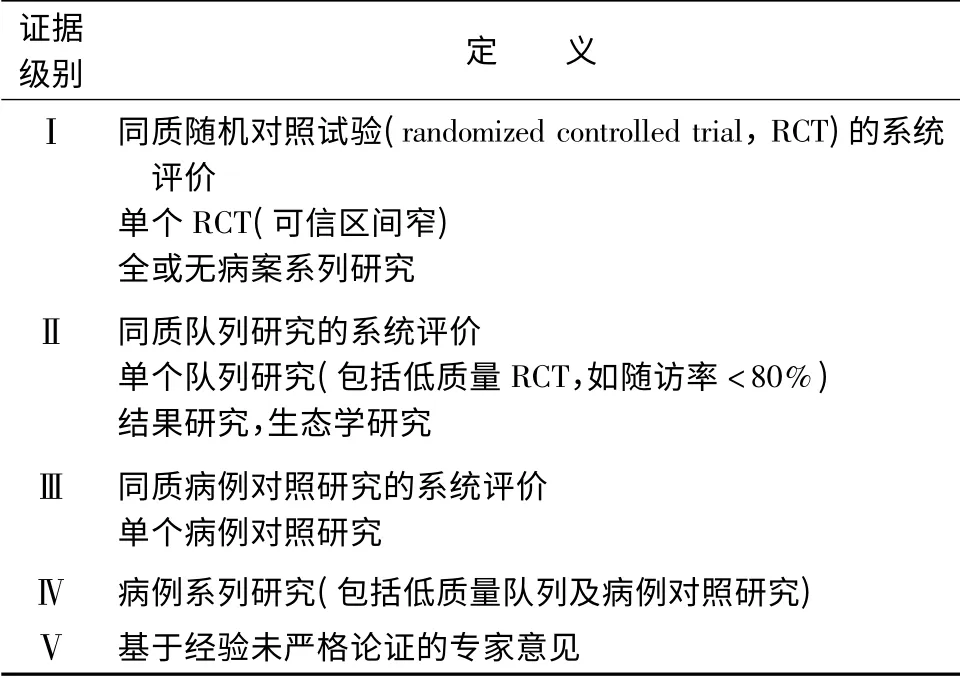
表1 循證醫學證據分級

表2 肝癌肝移植受者選擇標準
3 肝癌肝移植術前降期治療(表3)
肝癌肝移植術前腫瘤降期治療是通過一系列治療手段,減輕腫瘤負荷,降低分期,使超出肝癌肝移植受者選擇標準的患者能夠被納入移植標準,獲得肝移植機會。降期治療主要適用于不符合現有肝癌肝移植標準,且無門靜脈主干或下腔靜脈等大血管侵犯、無遠處轉移的肝癌患者[17,19-21]。降期治療的方法主要有局部消融治療和肝動脈栓塞化療(transcatheter hepatic arterial chemoembolization,TACE)等[17,19,22]。局部消融治療包括射頻消融、微波消融、冷凍消融和經皮無水乙醇注射等方法。降期治療的療效一般采用對比增強CT 和MRI,并結合甲胎蛋白(alpha fetal protein,AFP)水平變化進行評估,評價指標包括腫瘤大小、數目和AFP 水平等[22-28]。目前研究認為多種治療方法的聯合應用可達到更好的降期療效[29]。

表3 肝癌肝移植術前降期治療
4 肝癌肝移植受者抗病毒治療(表4)
中國肝癌肝移植受者90%以上與乙型肝炎病毒(hepatitis B virus,HBV)感染相關。肝移植前HBV 載量高以及肝移植后乙型肝炎(以下簡稱乙肝)復發的受者,肝癌復發的風險增加,因此對乙肝肝移植受者盡早行抗病毒治療,盡快降低HBV 水平,有助于降低移植術后乙肝復發率,提高受者長期生存率[30-32]。HBV 載量高的等待肝移植患者應采用恩替卡韋等強效、高耐藥屏障核苷類似物(nucleostide analogues,NAs)。移植術中無肝期應給予乙型肝炎免疫球蛋白(hepatitis B immunoglobulin,HBIG)。移植后的主要抗病毒治療方案為NAs 聯合低劑量HBIG,其中恩替卡韋或替諾福韋的聯合方案能更好地預防移植術后乙肝復發[33-38]。近年來,研究表明應用無激素免疫抑制方案可降低移植術后乙肝復發率[39]。此外也有移植術后接種乙肝疫苗預防乙肝復發的報道,其臨床應用尚有爭議[40-42]。中國丙型肝炎病毒(hepatitis C virus,HCV)感染患者呈增多趨勢,HCV RNA 陽性患者如肝功能Child-Pugh 評分≤7,術前宜進行抗病毒治療,移植術后須經病理確認丙型肝炎復發后方可給予抗HCV 治療[43]。
5 肝癌肝移植受者免疫抑制劑應用(表5)
鈣調磷酸酶抑制劑(calcineurin inhibitor,CNI)的應用是肝移植后肝癌復發的獨立危險因素[44]。對于肝癌肝移植受者,腫瘤的復發風險與其侵襲性及機體的免疫功能有關,受者處于強免疫抑制狀態時其免疫監視系統受到破壞,促進腫瘤復發、轉移,而免疫抑制劑量不足則容易誘發排斥反應。如何維持這一平衡,目前尚無定論[45-47]。肝癌肝移植受者目前尚不建議免疫抑制劑的全線撤除,但主張個體化的低劑量免疫抑制方案[45]。近年來臨床上有糖皮質激素早期撤除、無糖皮質激素及使用具有腫瘤抑制作用的mTOR 抑制劑(西羅莫司為代表)的成功應用方案[48-51]。目前臨床上主要的免疫抑制方案為:①他克莫司或環孢素+嗎替麥考酚酯+糖皮質激素;②白介素-2 受體阻滯劑+西羅莫司+嗎替麥考酚酯+糖皮質激素;③白介素-2 受體阻滯劑+嗎替麥考酚酯+他克莫司/西羅莫司[52-55]。
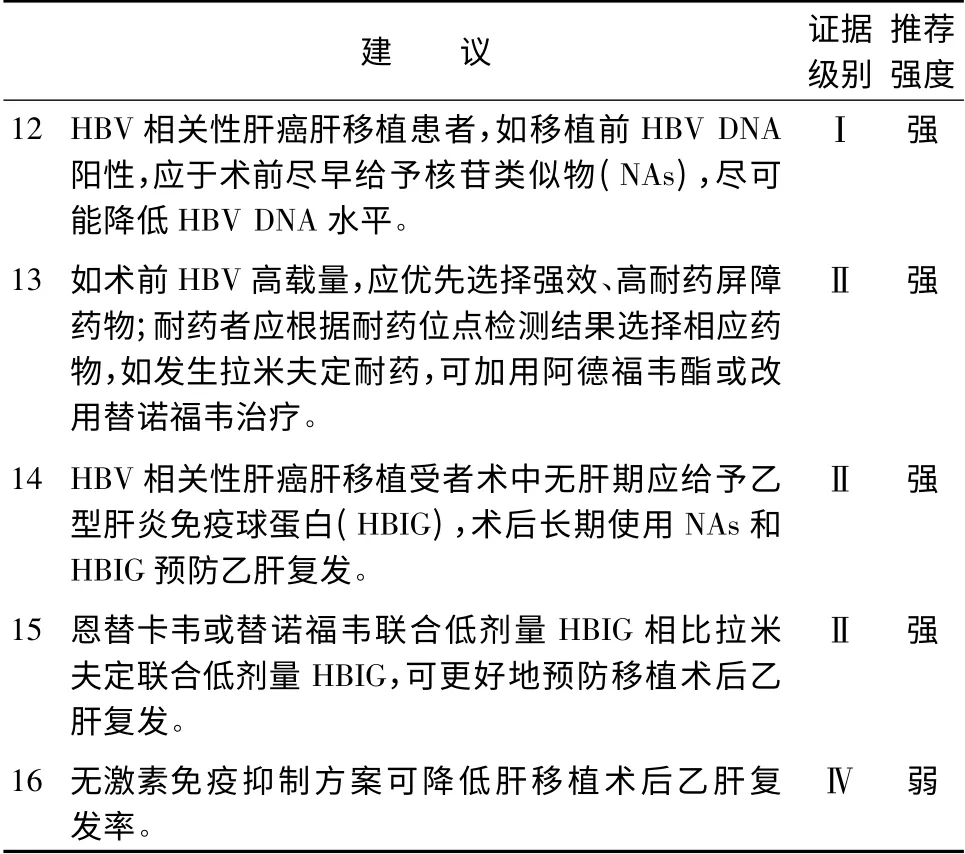
表4 肝癌肝移植受者抗病毒治療

表5 肝癌肝移植受者免疫抑制劑應用
6 肝癌肝移植術后復發的防治(表6)
文獻報道,肝癌肝移植術后5 年肝癌復發率可達20% ~57. 8%,故復發轉移的防治十分重要[56-57]。肝癌的形態學特征(大小、數目等)、分期、組織學分級以及生物學特性等應作為術后用藥的重要參考,制定個體化治療方案。
肝癌肝移植術后可能存在針對腫瘤的免疫逃逸,故應給予受者一定療程的術后治療,以期盡可能地減少微小轉移灶,降低術后復發率。選用碘131美妥昔單抗放射免疫治療、索拉非尼治療以及系統性化療(如奧沙利鉑或阿霉素分別與氟尿嘧啶聯合使用),均可為部分受者提供一定的生存獲益[58-61]。
對于肝移植術后肝癌復發轉移者,應用索拉非尼治療,可延長受者生存期[17,62-64]。肺轉移灶如可切除,首選手術切除[65]。移植肝內復發病灶的局部治療包括手術切除、TACE,局部消融等[66-68]。此外,有專家提出放療、再次肝移植等可作為治療的選擇。對于晚期患者,可考慮減少或停止免疫抑制劑的使用。

表6 肝癌肝移植術后復發的防治
編審專家組組長: 鄭樹森
編審專家組成員( 按姓氏拼音排序) : 陳規劃、陳實、陳孝平、陳燕凌、陳知水、陳忠華、丁義濤、董家鴻、竇劍、竇科峰、杜國盛、段偉東、傅志仁、高杰、高良輝、何曉順、賀強、景鴻恩、李波、李立、李寧、李玉民、劉景豐、劉軍、盧實春、呂國悅、明英姿、彭承宏、彭貴主、彭志海、錢建民、沈巖、沈中陽、石承先、時軍、孫玉嶺、王偉林、溫浩、吳健、吳忠鈞、夏強、徐驍、嚴律南、楊廣順、楊家印、楊揚、楊占宇、葉啟發、臧運金、張峰、張珉、張水軍、鄭樹森、周琳、朱繼業、朱志軍
執筆: 徐驍、李建輝、高峰、陳峻、舒哲悅、方維佳、衛強
1 Oxford Centre for Evidence-based Medicine—Levels of Evidence(March 2009)[DB/OL]. Oxford Centre for Evidence-based Medicine,2009[2014-04-15]. http://www. cebm. net/oxfordcentre-evidence-based-medicine-levels-evidence-march-2009/.
2 Schünemann HJ,Oxman AD,Brozek J,et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies[J]. BMJ,2008,336(7653):1106-1110.
3 Liang WH,Wu LW,Ling XT,et al. Living donor liver transplantation versus deceased donor liver transplantation for hepatocellular carcinoma:a meta-analysis[J]. Liver Transpl,2012,18(10):1226-1236.
4 Mazzaferro V,Regalia E,Doci R,et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis[J]. N Engl J Med,1996,334(11):693-699.
5 European Liver Transplant Registry Results[DB/OL]. European Liver Transplant Registry,2011[2014-04-15]. http://www.eltr.org.
6 OPTN/SRTR Annual Report[DB/OL]. Organ Procurement and Transplantation Network,2011[2014-04-15]. http://www.ustransplant.org.
7 Mazzaferro V,Bhoori S,Sposito C,et al. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience[J]. Liver Transpl,2011,17(Suppl 2):S44-S57.
8 Yao FY,Ferrell L,Bass NM,et al. Liver transplantation for hepatocellular carcinoma:expansion of the tumor size limits does not adversely impact survival[J].Hepatology,2001,33(6):1394-1403.
9 Mazzaferro V,Llovet JM,Miceli R,et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria:a retrospective,exploratory analysis[J]. Lancet Oncol,2009,10(1):35-43.
10 Zheng SS,Xu X,Wu J,et al. Liver transplantation for hepatocellular carcinoma:Hangzhou experiences[J]. Transplantation,2008,85(12):1726-1732.
11 Lei JY, Wang WT, Yan LN. Hangzhou criteria for liver transplantation in hepatocellular carcinoma: a single-center experience[J]. Eur J Gastroenterol Hepatol,2014,26(2):200-204.
12 Audet M,Panaro F,Piardi T,et al. Are the Hangzhou criteria adaptable to hepatocellular carcinoma patients for liver transplantation in Western countries?[J]. Liver Transpl,2009,15(7):822-823.
13 Chen J,Xu X,Wu J,et al. The stratifying value of hangzhou criteria in liver transplantation for hepatocellular carcinoma[J]. PLoS One,2014,9(3):e93128.
14 徐驍,楊家印,鐘林,等. 肝癌肝移植“杭州標準”多中心應用研究——1163 例報道[J]. 中華器官移植雜志,2013,34(9):524-527.
15 鄭樹森,汪愷,徐驍,等. 肝移植治療肝癌的受者選擇杭州標準在親屬活體供肝移植中的應用價值[J]. 中華器官移植雜志,2011,32(6):330-333.
16 Hu ZH,Wang W,Li ZW,et al. Recipient outcomes of salvage liver transplantation versus primary liver transplantation:a systematic review and meta-analysis[J]. Liver Transpl,2012,18(11):1316-1323.
17 Clavien PA,Lesurtel M,Bossuyt PM,et al. Recommendations for liver transplantation for hepatocellular carcinoma:an international consensus conference report[J]. Lancet Oncol,2012,13(1):e11-e22.
18 Olthoff KM,Merion RM,Ghobrial RM,et al. Outcomes of 385 adult-to-adult living donor liver transplant recipients:a report from the A2ALL Consortium[J]. Ann Surg,2005,242(3):314-323.
19 Galuppo R,McCall A,Gedaly R. The role of bridging therapy in hepatocellular carcinoma[J]. Int J Hepatol,2013:419302.
20 European Association for the Study of the Liver. EASL-EORTC clinical practice guidelines:management of hepatocellular carcinoma[J]. J Hepatol,2012,56(4):908-943.
21 Washburn K,Edwards E,Harper A,et al. Hepatocellular carcinoma patients are advantaged in the current liver transplant allocation system[J]. Am J Transplant,2010,10(7):1643-1648.
22 Lewandowski RJ,Kulik LM,Riaz A,et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma:chemoembolization versus radioembolization[J]. Am J Transplant,2009,9(8):1920-1928.
23 Merani S,Majno P,Kneteman NM,et al. The impact of waiting list α-fetoprotein changes on the outcome of liver transplant for hepatocellular carcinoma[J]. J Hepatol,2011,55(4):814-819.
24 Mailey B,Artinyan A,Khalili J,et al. Evaluation of absolute serum α-fetoprotein levels in liver transplant for hepatocellular cancer[J].Arch Surg,2011,146(1):26-33.
25 Ravaioli M,Grazi GL,Piscaglia F,et al. Liver transplantation for hepatocellular carcinoma:results of down-staging in patients initially outside the Milan selection criteria[J]. Am J Transplant,2008,8(12):2547-2557.
26 Yao FY,Kerlan RK Jr,Hirose R,et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation:an intention-to-treat analysis[J]. Hepatology,2008,48(3):819-827.
27 Duvoux C, Roudot-Thoraval F, Decaens T, et al. Liver transplantation for hepatocellular carcinoma:a model including αfetoprotein improves the performance of Milan criteria [J].Gastroenterology,2012,143(4):986-994.
28 Lai Q,Avolio AW,Graziadei I,et al. Alpha-fetoprotein and modified response evaluation criteria in solid tumors progression after locoregional therapy as predictors of hepatocellular cancer recurrence and death after transplantation[J]. Liver Transpl,2013,19(10):1108-1118.
29 Ashoori N,Bamberg F,Paprottka P,et al. Multimodality treatment for early-stage hepatocellular carcinoma:a bridging therapy for liver transplantation[J]. Digestion,2012,86(4):338-348.
30 Koda M,Nagahara T,Matono T,et al. Nucleotide analogs for patients with HBV-related hepatocellular carcinoma increase the survival rate through improved liver function[J]. Inter Med,2009,48(1):11-17.
31 Wong JS,Wong GL,Tsoi KK,et al. Meta-analysis:the efficacy of anti-viral therapy in prevention of recurrence after curative treatment of chronic hepatitis B-related hepatocellular carcinoma[J]. Aliment Pharmacol Ther,2011,33(10):1104-1112.
32 Wu JC,Huang YH,Chau GY,et al. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma[J]. J Hepatol,2009,51(5):890-897.
33 European Association for the Study of the Liver. EASL clinical practice guidelines:Management of chronic hepatitis B virus infection[J]. J Hepatol,2012,57(1):167-185.
34 Chen CJ,Yang HI,Iloeje UH;REVEAL-HBV Study Group.Hepatitis B virus DNA levels and outcomes in chronic hepatitis B[J].Hepatology,2009,49(5 Suppl):S72-S84.
35 Lai CL, Yuen MF. Prevention of hepatitis B virus-related hepatocellular carcinoma with antiviral therapy[J]. Hepatology,2013,57(1):399-408.
36 Yin JH,Li N,Han YF,et al. Effect of antiviral treatment with nucleotide/nucleoside analogs on postoperative prognosis of hepatitis B virus-related hepatocellular carcinoma:a two-stage longitudinal clinical study[J]. J Clin Oncol,2013,31(29):3647-3655.
37 Huang G,Lai EC, Lau WY, et al. Posthepatectomy HBV reactivation in hepatitis B-related hepatocellular carcinoma influences postoperative survival in patients with preoperative low HBV-DNA levels[J]. Ann Surg,2013,257(3):490-505.
38 Hu TH,Chen CL,Lin CC,et al. Section 14. Combination of entecavir plus low-dose on-demand hepatitis B immunoglobulin is effective with very low hepatitis B recurrence after liver transplantation[J]. Transplantation,2014,97(Suppl 8):S53-S59.
39 Kim JM,Joh JW,Kim SJ,et al. Steroid withdrawal in adult liver transplantation:occurrence at a single center[J]. Transplant Proc,2010,42(10):4132-4136.
40 Sánchez-Fueyo A,Rimola A,Grande L,et al. Hepatitis B immunoglobulin discontinuation followed by hepatitis B virus vaccination:A new strategy in the prophylaxis of hepatitis B virus recurrence after liver transplantation[J]. Hepatology,2000,31(2):496-501.
41 Chung Mau Lo,Chi Leung Liu,See Ching Chan,et al. Failure of hepatitis B vaccination in patients receiving lamivudine prophylaxis after liver transplantation for chronic hepatitis B[J]. J Hepatol,2005,43(2):283-287.
42 Lo CM,Lau GK,Chan SC,et al. Efficacy of a pre-S containing vaccine in patients receiving lamivudine prophylaxis after liver transplantation for chronic hepatitis B[J]. Am J Transplant,2007,7(2):434-439.
43 Watt K,Veldt B,Charlton M. A practical guide to the management of HCV infection following liver transplantation [J]. Am J Transplant,2009,9(8):1707-1713.
44 Vivarelli M,Cucchetti A,La Barba G,et al. Liver transplantation for hepatocellular carcinoma under calcineurin inhibitors:reassessment of risk factors for tumor recurrence[J]. Ann Surg,2008,248(5):857-862.
45 Chen K,Man K,Metselaar HJ,et al. Rationale of personalized immunosuppressive medication for hepatocellular carcinoma patients after liver transplantation[J]. Liver Transpl,2014,20(3):261-269.
46 Miyagi S,Kawagishi N,Sekiguchi S,et al. The relationship between recurrences and immunosuppression on living donor liver transplantation for hepatocellular carcinoma[J]. Transplant Proc,2012,44(3):797-801.
47 Saigal S,Shah S. Liver transplantation-economics in the less developed world[J]. Indian J Gastroenterol,2012,31(1):13-14.
48 Foroncewicz B,Mucha K,Ryszkowska E,et al. Safety and efficacy of steroid-free immunosuppression with tacrolimus and daclizumab in liver transplant recipients:6-year follow-up in a single center[J].Transplant Proc,2009,41(8):3103-3106.
49 Vivarelli M,Cucchetti A,La Barba G,et al. Liver transplantation for hepatocellular carcinoma under calcineurin inhibitors:reassessment of risk factors for tumor recurrence[J]. Ann Surg,2008,248(5):857-862.
50 Nair S,Eason J,Loss G. Sirolimus monotherapy in nephrotoxicity due to calcineurin inhibitors in liver transplant recipients[J]. Liver Transpl,2003,9(2):126-129.
51 Menon KV,Hakeem AR,Heaton ND. Meta-analysis:recurrence and survival following the use of sirolimus in liver transplantation for hepatocellular carcinoma[J]. Aliment Pharmacol Ther,2013,37(4):411-419.
52 Liang W,Wang D,Ling X,et al. Sirolimus-based immunosuppression in liver transplantation for hepatocellular carcinoma:a meta-analysis[J]. Liver Transpl,2012,18(1):62-69.
53 Soliman T,Hetz H,Burghuber C,et al. Short-term induction therapy with anti-thymocyte globulin and delayed use of calcineurin inhibitors in orthotopic liver transplantation[J]. Liver Transplant,2007,13(7):1039-1044.
54 Martin-Mateos RM,Graus J,Albillos A,et al. Initial immunosuppression with or without basiliximab:a comparative study[J].Transplant Proc,2012,44(9):2570-2572.
55 Pillai AA,Levitsky J. Overview of immunosuppression in liver transplantation[J]. World J Gastroenterol,2009,15(34):4225-4233.
56 Mazzaferro V,Llovet JM,Miceli R,et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria:a retrospective,exploratory analysis[J]. Lancet Oncol,2009,10(1):35-43.
57 Zimmerman MA,Ghobrial RM,Tong MJ,et al. Recurrence of hepatocellular carcinoma following liver transplantation:a review of preoperative and postoperative prognostic indicators[J]. Arch Surg,2008,143(2):182-188.
58 Xu J,Shen ZY,Chen XG,et al. A randomized controlled trial of Licartin for preventing hepatoma recurrence after liver transplantation[J]. Hematology,2007,5(2):269-276.
59 Huang L,Li GM,Zhu JY,et al. Efficacy of sorafenib after liver transplantation in patients with primary hepatic carcinoma exceeding the Milan criteria:a preliminary study[J]. Onco Targets Ther,2012,5:457-462.
60 Zhang Q,Chen H,Li Q,et al. Combination adjuvant chemotherapy with oxaliplatin, 5-fluorouracil and leucovorin after liver transplantation for hepatocellular carcinoma:a preliminary open-label study[J]. Invest New Drugs,2011,29(6):1360-1369.
61 張照輝,馬力文,宋世兵,等. 肝癌肝移植術后輔助化療的臨床分析[J]. 中華腫瘤雜志,2005,27(1):45-47.
62 Waghray A,Balci B,El-Gazzaz G,et al. Safety and efficacy of sorafenib for the treatment of recurrent hepatocellular carcinoma after liver transplantation[J]. Clin transplant,2013,27(4):555-561.
63 Pfeiffenberger J,Koschny R,Hoffmann K,et al. Sorafenib treatment is save and may affect survival of recurrent hepatocellular carcinoma after liver transplantation[J]. Langenbecks Arch Surg,2013,398(8):1123-1128.
64 Sposito C,Mariani L,Germini A,et al. Comparative efficacy of sorafenib versus best supportive care in recurrent hepatocellular carcinoma after liver transplantation:a case-control study[J]. J Hepatol,2013,59(1):59-66.
65 Hwang S,Kim YH,Kim DK,et al. Resection of pulmonary metastases from hepatocellular carcinoma following liver transplantation[J]. World J Surg,2012,36(7):1592-1602.
66 Taketomi A,Fukuhara T,Morita K,et al. Improved results of a surgical resection for the recurrence of hepatocellular carcinoma after living donor liver transplantation[J]. Ann Surg Oncol,2010,17(9):2283-2289.
67 Ko HK,Ko GY,Yoon HK,et al. Tumor response to transcatheter arterial chemoembolization in recurrent hepatocellular carcinoma after living donor liver transplantation[J]. Korean J Radiol,2007,8(4):320-327.
68 Ho CK,Chapman WC,Brown DB. Radiofrequency ablation of recurrent hepatocellular carcinoma in a patient after liver transplantation:two-year follow-up[J]. J Vasc Interv Radiol,2007,18(11):1451-1453.

