Effect of Temperature on Segregation Coefficients of Impurities in Phosphorus
LIU Xuefeng (劉雪峰), LI Jun (李 軍),, LUO Jianhong (羅建洪), ZHOU Kun (周 堃)and YANG Zhaopeng (楊兆鵬)
1College of Chemical Engineering, Sichuan University, Chengdu 610065, China
2College of Materials and Chemistry & Chemical Engineering, Chengdu University of Technology, Chengdu 610059, China
Effect of Temperature on Segregation Coefficients of Impurities in Phosphorus
LIU Xuefeng (劉雪峰)1, LI Jun (李 軍)1,*, LUO Jianhong (羅建洪)1, ZHOU Kun (周 堃)2and YANG Zhaopeng (楊兆鵬)1
1College of Chemical Engineering, Sichuan University, Chengdu 610065, China
2College of Materials and Chemistry & Chemical Engineering, Chengdu University of Technology, Chengdu 610059, China
The present work is focused on the relationship between effective segregation coefficient keffand temperature of melting zone for purification of phosphorus by zone melting method. Values of keffat four temperatures of melting zone are obtained for zone pass n=1 at travel velocity of molten zone v=5×10?3m·h?1and initial impurity concentration C0≤10 μg·g?1. lnkeffis a linear function of 1/T. The keffvalues of Al, Ca, Cr, Fe, Cd and Sb increase with temperatures while that of Mg is almost constant. The purification is acceptable at lower temperature of melting zone such as 323 K. The variations of enthalpy and entropy between impurities and phosphorus in the liquid and solid phases are also presented.
phosphorus, purity, temperature, effective segregation coefficient, zone melting
1 INTRODUCTION
High purity phosphorus is used in the preparation of high quality phoschemical and electronic products. The performance of these products depends on the purity of phosphorus used as raw material. GaP [1] and InP [2] are widely used in light emitting diode (LED) and semiconductor is prepared by ultrapure (6N) phosphorus. Several methods of purification have been studied, such as oxidation [3], distillation [4], active carbon adsorption [5], electromagnetic purification [6] and zone melting. Zone melting is prevailing in preparation of ultrapure substances.
The method of purification by zone melting has found widespread applications since the first description by Pfann in the early 1950s [7, 8]. Ultrapurification is achieved by a directional movement of a series of zones through a solid ingot [9, 10]. With the difference of concentrations in the liquid and solid phases, measured by effective segregation coefficient keff[11], impurities are enriched in the front or the end of the ingot. The experimental conditions, such as temperature of molten zone [12], travel velocity of molten zone [13], number of passes n [14], initial impurity content, clean environment, zone length l and ingot length L [15, 16], affect the zone melting and should be optimized in the purification process to obtain optimal value of keff.
In the stagnant film model, keffis defined as [17]
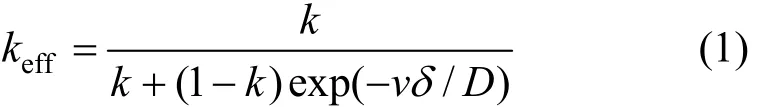
where k is the equilibrium segregation coefficient, D is the impurity diffusion coefficient, δ is the diffusion boundary layer thickness, and v the travel velocity of molten zone. D is affected by temperature, pressure and component and k is affected by properties of material and temperature. Some methods have been reported to determine k [18, 19], but no efficient method is available to measure the diffusion boundary layer thickness δ. Because the melting point of phosphorus is 318 K, the experiments are conducted under the normal condition. Generally it is difficult to obtain uniformly moving solidification surface since the velocity of zones is so slow. Besides the vibration caused by the running motor, a small change of temperature may result in fluctuation at solid-liquid interface. The temperature of melting zone is an important parameter, which affects the solute distribution and thermodynamic properties of phosphorus, so the relation between effective segregation coefficient keffand molten zone temperature is investigated in this study.
2 MODEL DEVELOPMENT
2.1 Assumptions and calculation function
Diffusion process in the melting zone is not easy to be detected, but the result of diffusion can be obtained after the zone melting process. Following assumptions are made in order to analyze the solute distribution in the melting zone [20-22].
(1) The initial concentration of impurities, C0, is uniform in the sample throughout. The effective segregation coefficient keffis a constant.
(2) The temperature and the zone travel velocity are well controlled.
(3) The volume of phosphorus does not change in the process of melting and solidification.
(4) The content of impurities changes along the axial direction of ingot only.
(5) Diffusion of impurities in the solid is negligible and the distribution of impurities in the liquid zone isuniform.
In the calculation, following dimensionless variables will be used:and, where y is the location of sampling points.
Simulations of impurity redistribution during zone melting are generally based on keffvalue, which is calculated from the solute equilibrium distribution in liquid and solid phases at particular temperatures. The phase diagram at such a low content of impurities is not reported, but the axial distribution of impurities along the ingot after one molten zone pass can be obtained. Pfann has given the distribution [7, 8]:
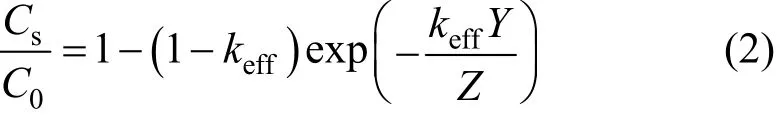
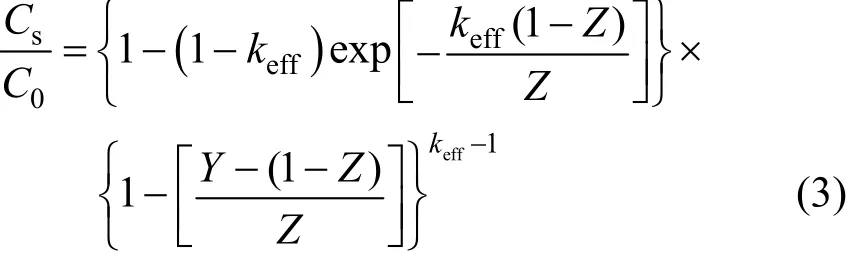
where Csis the impurity concentration after one pass.
2.2 Thermodynamic analysis
The temperature is not the only variable in the process, and the content of impurities along the ingot is not uniform after a molten zone pass. Because the phosphorus used in the experiment contains extremely small quantities of impurities, the liquidus and solidus in the binary phase diagram are almost straight. The equilibrium segregation coefficient k is a constant, which is given by the slopes of liquidus and solidus [23, 24]. The influence of impurity content could be ignored.
With the interactions among metal impurities neglected, the Gibbs free energy (G) of the system composed of impurity i and phosphorus (P) is
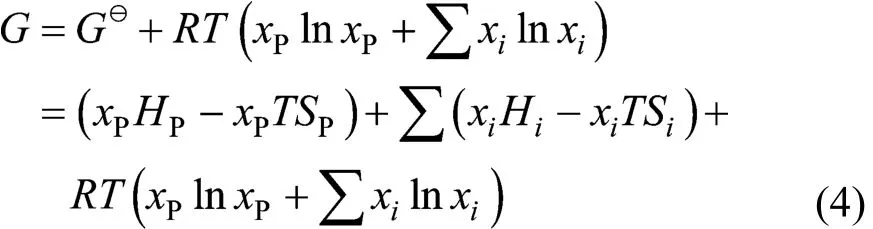
where χiand χPrepresent the concentrations of impurity and phosphorus and R=8.314 J?K·mol?1. Impurity diffusion equilibrium is reached at the solid-liquid interface, and the power that the Gibbs free energy towards the minimum acts as the driving force for redistribution of impurities. In the diffusion boundary layer of liquid (l) and solid (s):, then
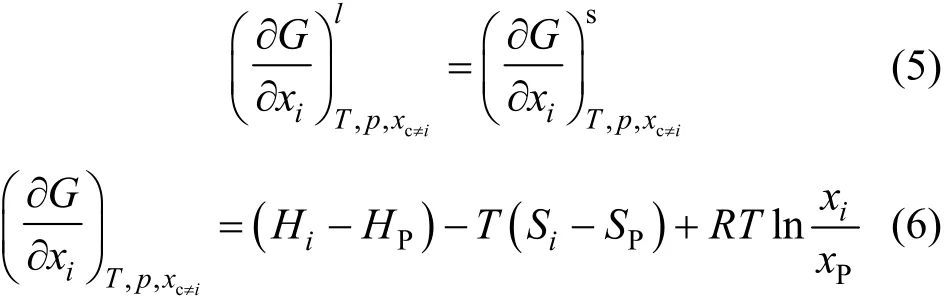
The linear relation between the logarithm of effective segregation coefficient keffand 1/T has been reported [25, 26] for this system, and so that
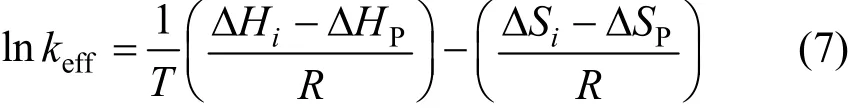
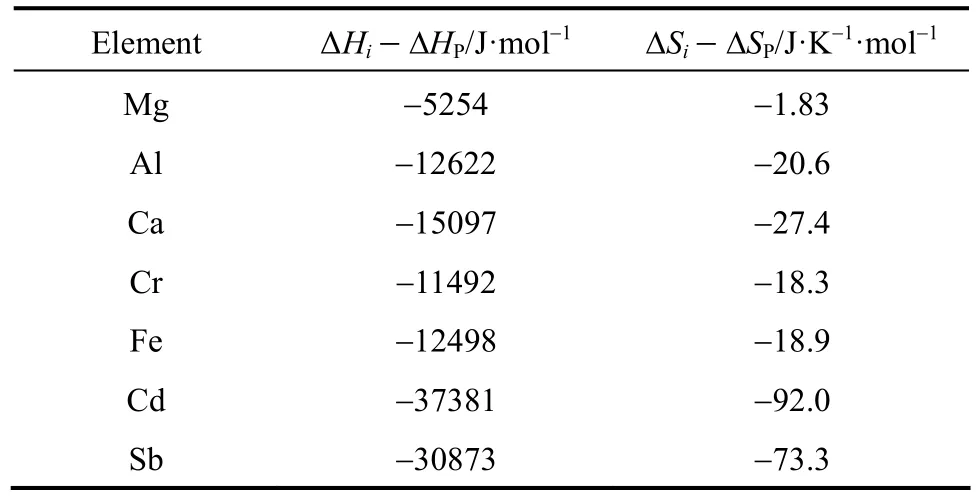
where ?Hi, ?HP, ?Siand ?SPare the variations of enthalpy and entropy between the liquid and solid phases in the course of solidification.
2.3 Experimental method
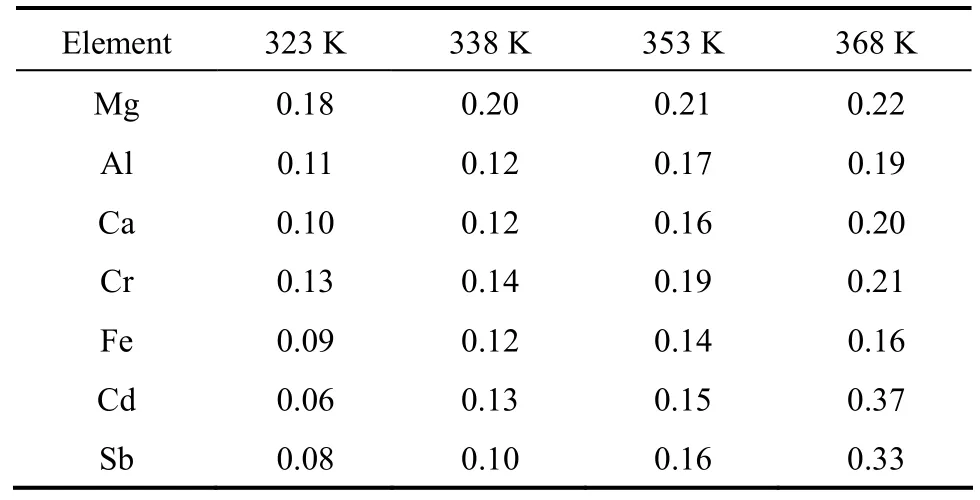
In order to obtain the data for production of high purity phosphorus, the content for each impurity was below 10 μg·g?1in the experimental material. The length L of phosphorus ingots is 0.1 m, while the length l of molten zone is 1×10?2m. After a single pass, a few samples were obtained at the points y=2×10?2, 4×10?2, 6×10?2, 8×10?2m. The travel velocity of the zone was constant, v=5×10?3m·h?1, to make the value of keffclose to k and the effect of travel speed on the experiment is negligible.
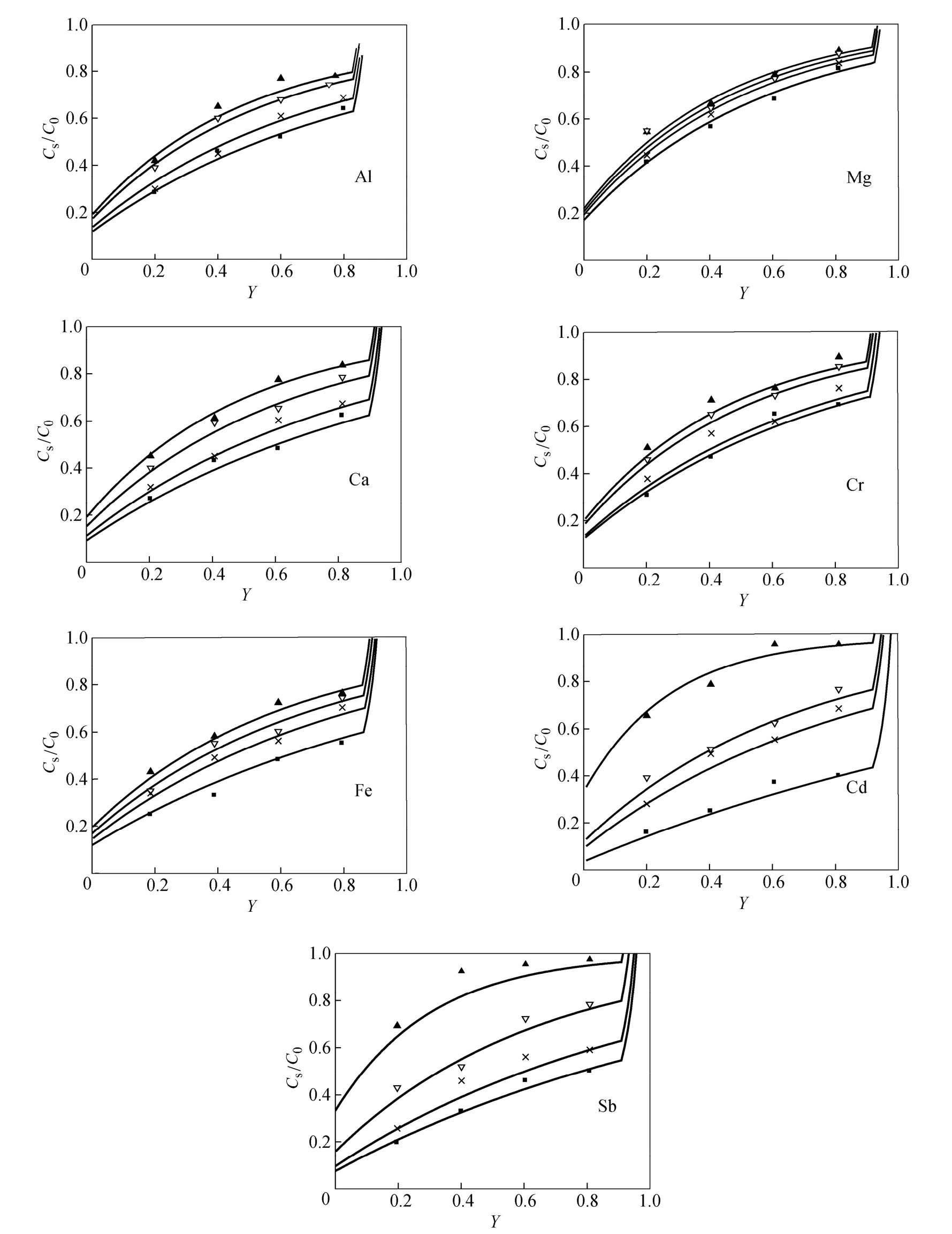
The samples react with trace-metal grade nitric acid from Fisherscience, Canada, phosphorus is oxidized to phosphate, while the metal impurities are oxidized to metal ions. The reaction product is analyzed by inductively coupled plasma mass spectrometry (Agilent 7500a ICP-MS) to examine the changes in the distribution of impurities. The segregation coefficient keffcould be calculated using the experimental data with Pfann’s model, Eq. (2), with all the experimental data in the range of 0 Changes of organic content are neglected as the metal impurities in phosphorus are the main objective of this study and the distribution of each substance is considered to be independent. The axial distribution of impurities along the ingot after one zone melting pass can be detected. Eq. (2) can be written as Table 1 Values of keffat different temperatures in molten phosphorus The slope keff/lZ and keffare calculated and shown in Table 1. The correlation coefficients are larger than 0.9 for all impurities. Figure 1 shows the curves of concentration Cs/C0versus distance Y with the values of keffat different temperatures for 7 impurities. The experimental data are in accordance with Pfann’s model, Eqs. (2) and (3). The values of keffare lower than 1 and increase with temperature. The effect of temperature on Mg is less,while that on Cd and Sb is more significant. Figure 1 Experimental points of Cs/C0fitted with computed distribution curves for 7 impurities in molten phosphorus (v=5 mm·h?1; n=1 pass) From Eq. (7), the effect of temperature on segregation coefficient is determined by the differences of thermodynamic properties between impurities and phosphorus, which is given in Table 2. The correlation coefficients are larger than 0.9 for all impurities, so lnkeffis linear with 1/T. Table 2 Change of enthalpy and entropy between impurities and phosphorus For all impurities, ?Hi??HP<0, so that the transfer of these impurities into liquid phosphorus needs less energy than phosphorus melts. It needs a lower melting zone temperature to achieve better separation, and the absolute value of ?Hi??HPmeans the significance of the influence of temperature on the separation. Entropy is a thermodynamic property to measure the disorder of material, so ?Si??SPrepresents the confusion degree of impurities from solid phosphorus to liquid phosphorus contrasting pure phosphorus. The result shows that ?Si??SP<0 for all impurities. The values of ?Siand ?SPare positive in melting, so the increase of impurities confusion is weaker than phosphorus. The segregation coefficient keffand the change of enthalpy and entropy for seven impurities, Mg, Al, Ca, Cr, Fe, Cd and Sb, in phosphorus are determined in this work. The distribution of impurities at different temperatures indicates that a lower molten zone temperature T is required to achieve the optimal segregation coefficient for the seven impurities and lnkeffis linear with 1/T. Considering the melting point of phosphorus, the temperature of melting zone should be 323 K to make the melting zone stable in the process. The seven impurities in phosphorus can be purified by zone melting, because their keffare smaller than 1. The values of kefffor 7 impurities increase with temperature, but the effect of temperature on Mg is much less. NOMENCLATURE C0initial content of impurity, μg·g?1 Cscontent of impurity at sampling points, μg·g?1 D diffusion coefficient of impurity, m2·s?1 G Gibbs free energy, J·mol?1 G standard Gibbs free energy, J?mol?1 H enthalpy, J·mol?1 k equilibrium distribution coefficient keffeffective distribution coefficient L ingot length, m l zone length, m n number of zone passes R gas constant, J·K?1·mol?1 S entropy, J·K?1·mol?1 T temperature of molten zone, K v travel velocity of molten zone, m·h?1 χ content of impurity Y dimensionless location of sampling point y location of sampling point, m Z dimensionless length of zone δ diffusion boundary layer thickness, m μ chemical potential Subscripts l liquid P phosphorus s solid REFERENCES 1 Umeno, K., Furukawa, Y., Wakahara, A., Noma, R., Okada, H., Yonezu, H., Takagi, Y., Kan, H., “MBE growth of GaAsN/GaP(N) quantum wells with abrupt hetero-interfaces for photonics applications on Si substrates”, J. Cryst. Growth., 311, 1748-1753 (2009). 2 Depreter, B., Moerman, I., Baets, R., Van Daele, P., Demeester, P.,“InP 1.3 μm microcavity LEDs with high quantum efficiency”, J. Cryst. Growth., 221, 674-678 (2000). 3 Ren, Y.S., Li, J., Duan, X.X., “Application of the central composite design and response surface methodology to remove arsenic from industrial phosphorus by oxidation”, Can. J. Chem. Eng., 89, 491-498 (2011). 4 Dore, J.C., Rosenhouse, H., “Process for phosphorus purification”, US Pat., 4483746A (1984). 5 Hartlapp, G., Hermulheim, H., Haas, H., Kuapsack, H., Stendenbach, K.H., “Purification of yellow phosphorus”, US Pat., 3836675 (1974). 6 Liu, Z.H., Shen, Q.H., Chen, W., “Yellow phosphorus refining process by electromagnetic purification method”, US Pat., 1456496 A (2003). 7 Pfann, W.G., “Techniques of zone melting and crystal growth”, Solid State Physics, 4, 423-521 (1957). 8 Pfann, W.G., Zone Melting, 2nd Ed., Wiley, New York (1966). 9 Munirathnam, N.R., Prasad, D.S., Sudheer, C., Prakash, T.L., “Purification of tellurium to 6N+ by quadruple zone refining”, J. Crys. Growth., 254, 262-266 (2003). 10 Zanio, K., “Purification of CdTe from tellurium-rich solutions”, J. Electron. Mater., 3 (2), 327-351 (1974). 11 Cheung, N., Bertazzoli, R., Garcia, A., “Experimental impurity segregation and numerical analysis based on variable solute distribution coefficients during multi-pass zone refining of aluminum”, J. Cryst. Growth, 310, 1274-1280 (2008). 12 Nishimura, B.T., Vasylkiv, O., Sakka, Y., Loboda, P., “Microstructure and high-temperature strength of B4C-TiB2composite prepared by a crucibleless zone melting method”, J. Alloys and Compd., 485, 677-681 (2009). 13 Mei, P.R., Moreira, S.P., Cardoso, E., C?rtes, A.D.S., Marques, F.C.,“Purification of metallurgical silicon by horizontal zone melting”,Sol. Energy Mater. Sol. Cells, 98, 233-239 (2012). 14 Roumié, M., Zahraman, K., Zaiour, A., Mohanna, Y., Hage-Ali, M.,“Study of segregation process of impurities in molten tellurium after one pass of three conjoint zones in zone refining”, J. Cryst. Growth., 289, 260-268 (2006). 15 Prasad, D.S., Munirathnam, N.R., Rao, J.V., Prakash, T.L., “Effect of multi-pass, zone length and translation rate on impurity segregation during zone refining of tellurium”, Mater. Lett., 60, 1875-1879 (2006). 16 Ho, C.D., Yeh, H.M., Yeh, T.L., “Numerical analysis on optimal zone lengths for each pass in multipass zone-refining processes”, Can. J. Chem. Eng., 76 (1), 113-118 (1998). 17 Kurz, W., Fisher, D., Fundamentals of Solidification, 2nd Ed., Trans. Tech. Publications Co., Switzerland (1989). 18 Bloem, J., Giling, L.J., “Determination of segregation coefficients from zone melting experiments under extrinsic conditions”, Mat. Res. Bull., 9, 265-272 (1974). 19 Zaiour, A., Hage-Ali, M., Koebel, J.M., Bentz, A., Siffert, P., “Equilibrium segregation coefficient and liquid diffusion coefficient of some impurities in tellurium”, Appl. Phys. A, 43 (3), 219-222 (1987). 20 Cahoon, J.R., “Zone melting calculations: an ideal spreadsheet application”, Int. J. Comp. Appl. Technol., 25 (1), 1-8 (2006). 21 Cheung, N., Bertazzoli, R., Garcia, A., “Numerical and experimental analysis of an approach based on variable solute distribution coefficients during purification by zone refining”, Sep. Purif. Technol., 52, 504-511 (2007). 22 Spim, J.J.A., Bernadou, M.J.S., Garcia, A., “Numerical modeling and optimization of zone refining”, J. Alloys Compd., 298, 299-305 (2000). 23 Jannke, P.J., Friedenberg, R., “Achieving ultrapurity in pharmaceuticals by zone melting”, J. Chem. Educ., 42 (3), 157-159 (1965). 24 Ren, Y.S., Li, J., Duan, X.X., “Determination of equilibrium distribution coefficients of impurities in phosphorus by zone melting”, Chin. J. Chem. Eng., 19 (2), 223-226 (2011). 25 Zaiour, A, Hamdoun, B., “Effect of temperature on segregation coefficients of impurities in tellurium”, Phys. Scr., 70, 193-196 (2004). 26 Baralis, G., “Distribution coefficients during the solidification of an ideal binary system in the presence of heat flow”, J. Cryst. Growth. 3 (4), 627-632 (1968). SEPARATION SCIENCE AND ENGINEERING Chinese Journal of Chemical Engineering, 22(3) 294—298 (2014) 10.1016/S1004-9541(14)60044-6 2012-08-28, accepted 2013-04-05. *To whom correspondence should be addressed. E-mail: lijun@scu.edu.cn3 RESULTS AND DISCUSSION
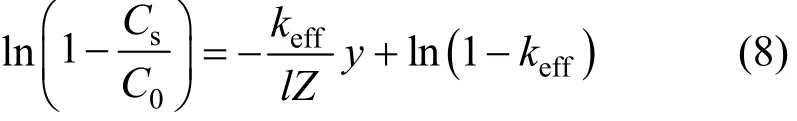
4 CONCLUSIONS
 Chinese Journal of Chemical Engineering2014年3期
Chinese Journal of Chemical Engineering2014年3期
- Chinese Journal of Chemical Engineering的其它文章
- Determination of Transport Properties of Dilute Binary Mixtures Containing Carbon Dioxide through Isotropic Pair Potential Energies
- Preparation and Characterization of Sodium Sulfate/Silica Composite as a Shape-stabilized Phase Change Material by Sol-gel Method*
- Superparamagnetic Supported Catalyst H3PW12O40/γ-Fe2O3for Alkylation of Thiophene with Olefine*
- A Bi-component Cu Catalyst for the Direct Synthesis of Methylchlorosilane from Silicon and Methyl Chloride
- A Contraction-expansion Helical Mixer in the Laminar Regime*
- Effects of Shape and Quantity of Helical Baffle on the Shell-side Heat Transfer and Flow Performance of Heat Exchangers*
