Impact of mangrove vegetation on seasonal carbon burial and other sediment characteristics in the Vellar-Coleroon estuary, India
Kandasamy Kathiresan · Venugopal Gomathi · Raj Anburaj Kandasamy Saravanakumar
Results
ORIGINAL PAPER
Impact of mangrove vegetation on seasonal carbon burial and other sediment characteristics in the Vellar-Coleroon estuary, India
Kandasamy Kathiresan · Venugopal Gomathi · Raj Anburaj Kandasamy Saravanakumar
Received: 2012-5-10; Accepted: 2012-12-27
? Northeast Forestry University and Springer-Verlag Berlin Heidelberg 2014
This work quantified the total carbon and 12 other sediment characteristics at 10 soil depths, in planted and or natural mangrove forests in comparison with non-vegetated soil for four seasons of the year 2009-2010 in the Vellar-Coleroon estuarine complex, India. The sediment characteristics varied significantly between mangrove-vegetated and non-vegetated habitats or seasons of analysis, but not between soil depths. The mangrove sediments were rich in total carbon and total organic carbon as compared to non-mangrove sediments (p <0.01). Total carbon was 98.2% higher in mature mangroves and 41.8% in planted mangroves than that in non-mangrove soil. Total organic carbon was as much as 2.5 times greater in mature mangroves and 2 times greater in planted mangroves than that in unvegetated soil. Carbon contents also varied many fold by season. Total carbon content was 8.6 times greater during pre-monsoon, 4.1 times greater during post-monsoon and 2.5 times greater during monsoon than during summer (p <0.01 in all cases). Similarly, total organic carbon was 5.9 times greater during pre-monsoon, 3.1 times greater during post-monsoon and 69% greater during monsoon than during summer. In general, higher levels of sediment carbon were recorded during pre and post-monsoon seasons than during other seasons. Total carbon concentration was correlated negatively to temperature, sand and phosphorus (p <0.01); positively correlated with redox potential, silt, clay, C/N ratio, potassium (p <0.01) and nitrogen (p <0.05); but not correlated with soil depth, pH or salinity. This work revealed that the carbon burial was rapid at the annual rate of 2.8% for total carbon, and 6.7% for total organic carbon in mangrove-planted sediment. Clearing of mangroves can result in significantly and rapidly reduced carbon stores.Our study highlights the importance of natural and plantation mangrove stands for conserving sediment carbon in the tropical coastal domain.
mangrove sediment, carbon burial, total carbon, total organic carbon
Introduction
Covering about 60%?75% of tropical coasts, mangrove forests are among the world’s most productive ecosystems (Kathiresan and Bingham 2001). Their position at the land-sea interface and their role in exchange of material with coastal waters suggests that mangrove forests make important contributions to carbon biogeochemistry in the coastal zone (Bouillon et al. 2008). Mangroves and other coastal vegetation type account for 10% of organic carbon burial in the global coastal domain (Duarte et al. 2005) but they are neglected from accounts of the global oceanic carbon cycle for the main reason that these macrophytes occupy less than 2% of the oceanic surface (Duarte and Cebrain 1996). Mangroves store large amounts of organic carbon to several meters in depth (Alongi 1998; Matsui 1998; Lallier-Verges et al. 1998; Fujimoto et al. 1999; Chumura et al. 2003). Organic carbon in sediment is a crucial indicator of the productivity of the coastal zone (Hasrizal et al. 2009). Besides acting as a sink for carbon, mangroves might also be a source of carbon in that they can supply significant amounts of carbon to adjacent coastal ecosystems and thus play a vital role in coastal fisheries (Ong 1993). Carbon burial in sediments beneath mangroves was, however, ignored in earlier assessments of carbon burial in oceans due to the dearth of information. Seasonal changes in carbon storage in mangrove sediments have been little understood in relation to other soil characteristics and in comparison with mangrove-free soils (Duarte et al. 2005; Kristensen et al. 2008; Bouillon et al. 2009; Xue et al. 2009). This information is needed for management and conservation of mangroves and their stubstrates in the context of global warming and sea level rise, and to respond to anthropogenic damage to mangrove habitats(Gilman et al. 2008). Our objective in the present study was to compare carbon content of sediments beneath natural and/or planted mangroves with the carbon contents of coastal soils lacking mangrove cover, and to describe the influence of other sediment characteristics on soil carbon content by season.
Materials and methods
Study area
Our study area was the Vellar–Coleroon estuary, along the Bay of Bengal on the southeast coast of the state of Tamil Nadu, India (Fig. 1). There were two mangrove forest areas: one a natural formation at Pichavaram on the south side of the estuarine complex and other a planted forest along the Vellar estuary on the north side. Both mangrove forests were dominated by Avicennia marina and Rhizophora mucronata. The mangrove forests of the estuarine complex covered an area of 1300 hectares, of which 50% was forest, 40% was waterways, and the remainder was sand-flats and mud-flats. Local tides were semi-diurnal varied in amplitude from about 15 to 100 cm, reaching the maximum during monsoon and post- monsoon and the minimum in summer. Water depths in the waterways ranged from 0.3 to 3 m. The mean annual temperature of this area was 27°C and annual precipitation was 1465 mm with 52 rainy days each year (Kathiresan 2000).
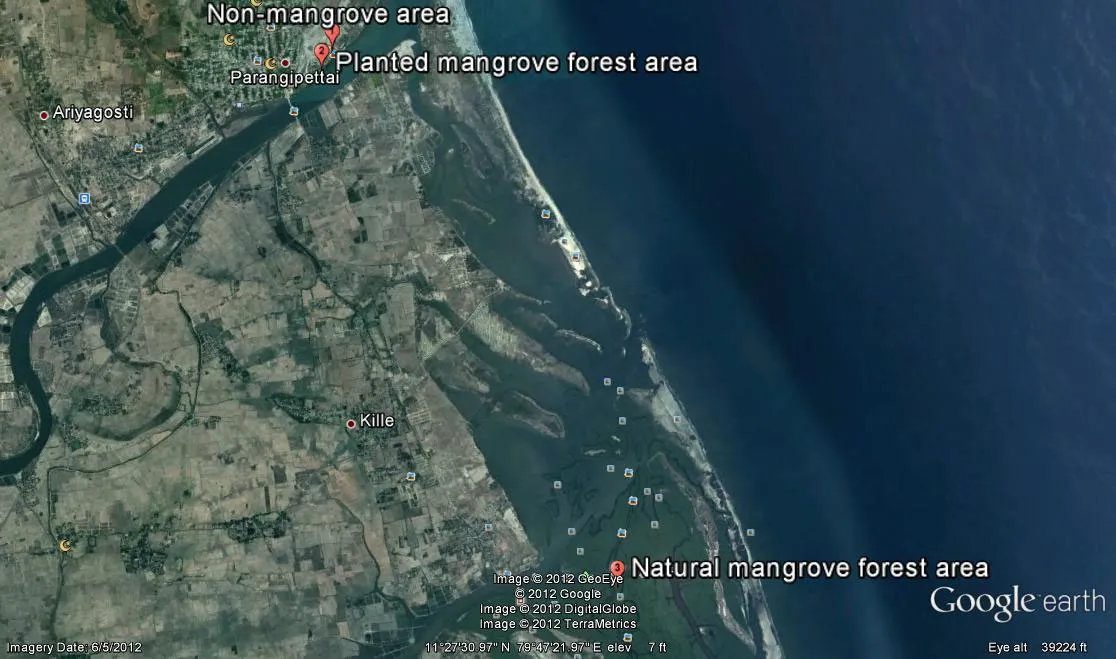
Fig. 1: Location of mangrove sediments at Pichavaram, Vellar estuary and non-mangrove area sampled in four seasons of summer (May 2009), pre-monsoon (July 2009), monsoon (December 2009) and post-monsoon (January 2010)
Sediment collection and analysis
Sediments were sampled at three different sites: (1) non-mangrove barren area (11°29'29.42"N; 79°45'58.13"E) outside the forest along the Vellar estuary, (2) planted mangrove forest aged 15 years along the Vellar (11°29'21.59"N; 79°45'53.12"E), and (3) natural mangrove forest >100 years old at Pichavaram (11°25'46.68"N; 79°47'38.24"E). Sediment samples were drawn using a 1.5 m long stainless steel corer during low tide at 10 depths (10, 20, 30, 40, 50, 60, 70, 80, 90 and 100 cm), for four seasons - Summer (May 2009), pre-monsoon (July 2009), monsoon (December 2009) and post-monsoon (January 2010). Soils were sampled during low tide when the mangrove substrate was fully exposed. Sediment parameters such as temperature, hydrogen ion concentration (pH) and redox potential (Eh), salinity (‰) of pore water were analyzed in situ. Temperature was measured using a thermometer with 0.50C accuracy; pH and redox potential (Eh) were measured using a millivoltmeter with platinum electrode (pH 315i/ SET, Wissenschaftlich Technische Werkstatten, Germany); and pore water salinity was measured using a hand refractometer (Erma INC, Tokyo). In thelaboratory, sediment samples were analyzed for percent composition of silt, clay and sand particles using the pipette method (Buchanan 1984) and for total organic carbon (EI Wakeel and Riley 1956). Sediment samples, dried in an oven at 110°C to a constant weight were ground to fine powder for analysis of total carbon and C/N ratio in a CHN/O analyser (Perkin Elmer-series II 2400), potassium (Guzman and Jimenez 1992), nitrogen and phosphorus using Kjeldahl method (Subbiah and Asija 1956). A suite of statistical analyses was made to quantify the significance of differences in parameters between soil types, seasons, and/or soil depths, and to identify correlations between the parameters.
Results
Levels of total carbon and other sediment characteristics in mangroves and/or barren areas at different soil depths and seasons are shown in Tables 1 and 2. Total carbon content varied significantly between soil types and seasons (p <0.01 in both cases). Differences were insignificant between soil depths. Interaction effects between soil type, season and/or soil depths were also insignificant. Total carbon content in sediment was 1.09% in mature mangroves and 0.78% in planted mangroves, 98.2% higher in the former and 41.8% in the latter than that in unvegetated soil (0.55%). Total carbon varied by season. It was highest in pre-monsoon (1.71%) and lowest in summer (0.2%). Compared to carbon content in summer, pre-monsoon levels were as much as 8.6 times greater, post-monsoon levels 4.1 times greater, and monsoon levels 2.5 times greater.
Total organic carbon (TOC) was 0.193% in mature mangrove sediments and 0.155% in plantation sediments, and the values were 2.5 times greater in the former and 2 times greater in the latter, as compared to levels in non-mangrove soil, which had TOC of 0.076% (Table 1). TOC also varied by season: it was higher during pre-monsoon and post-monsoon than during summer. Compared to TOC levels in summer, pre-monsoon content reached 5.9 times greater, post-monsoon content reached 3.1 times greater, and monsoon levels reached 69% greater.
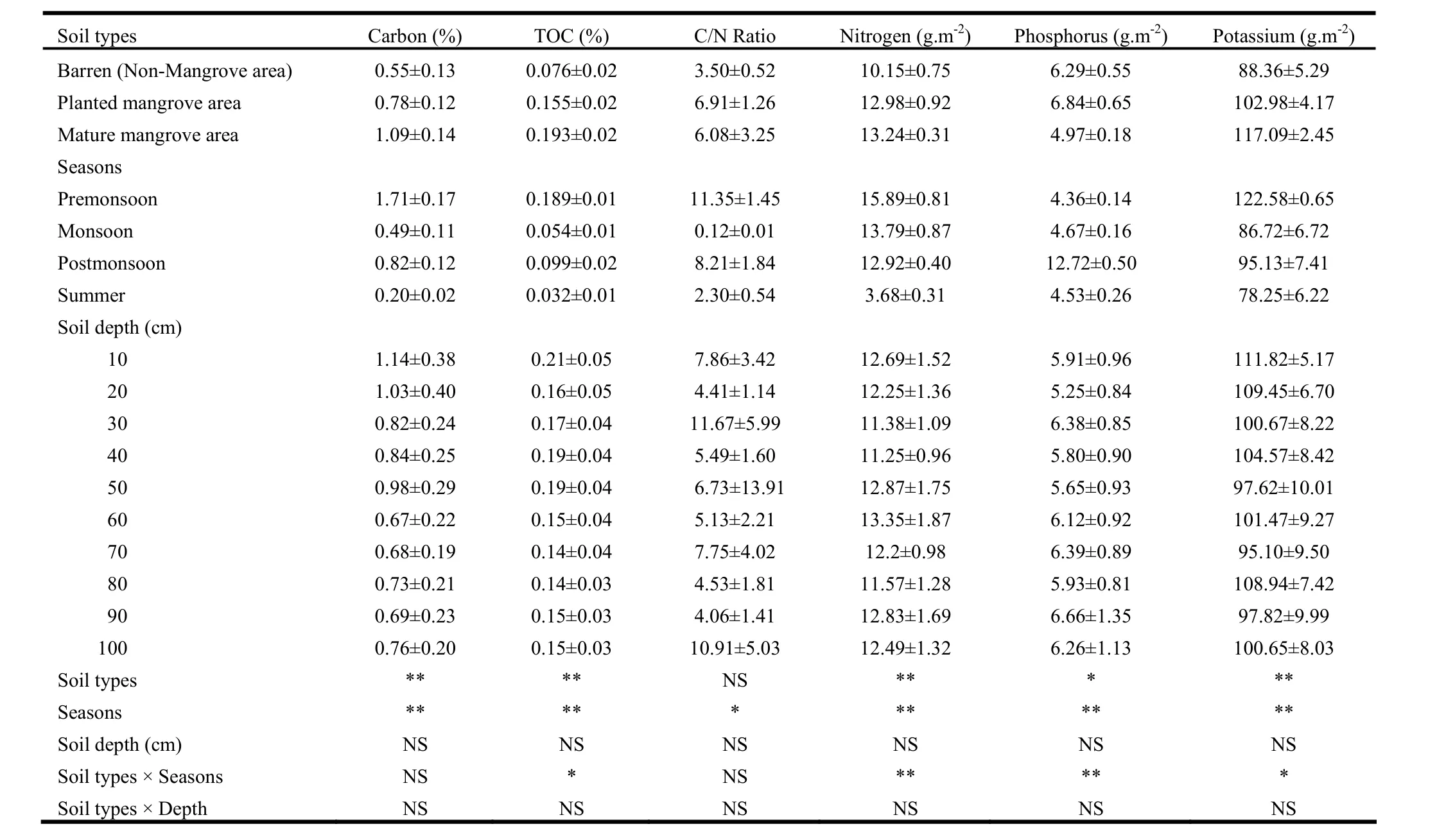
Table 1: Levels of total carbon, total organic carbon (TOC), C/N ratio, nitrogen, phosphorus and potassium in sediments of mangroves and/or non-mangroves at different soil depths and by season.
The C/N ratio varied significantly (p<0.05) only between seasons. The ratio was highest (11.4) in pre-monsoon and lowest (0.12) in monsoon. It was as high as 94.6 fold in pre-monsoon, 68.4 fold in post-monsoon and 19.2 fold in summer, as compared to levels during monsoon (Table 1).
Sediment nutrients (nitrogen, phosphorus and potassium) varied significantly (p<0.01) between soil types and between seasons, and soil type interaction with season. Nutrient levels did not vary by soil depth and interactionwere insignificant between soils depth and other parameters (Table 1). Sediment nitrogen content was greatest in mature mangrove (13.2 g·m-2) followed by planted mangrove (12.0 g·m-2) (Table 1). The content was higher by 30.4% in the former and 27.9% in the latter than that in unvegetated soil. The sediment nitrogen also varied seasonally with maximum content in pre-monsoon (15.9 g·m-2) and minimum in summer (3.68 g·m-2) (Table 1). Compared to levels insummer, N was 4.3, 3.7, and 3.5 times greater in pre-monsoon, monsoon, and post-monsoon, respectively.
Sediment phosphorus content was 8.7% higher in planted mangroves (6.84 g·m-2) and 21% lower in mature mangroves (4.97 g·m-2) than that in barren soil (6.29 g·m-2). Sediment phosphorus content varied seasonally, with the highest value in post-monsoon (12.72 g· m-2) and lowest in pre-monsoon (4.36 g· m-2) (Table 1). Compared to pre-monsoon levels, P content was 2.9 times, 7.1% and 3.9% greater in post-monsoon, monsoon, and summer, respectively. Sediment potassium was higher in mangrove areas than in unvegetated areas. The content was higher by 32.5% in mature mangrove sediment and 15.4% in planted mangrove sediment than in non-mangrove soil. Seasonally, the level of sediment potassium also varied, with the maximum in pre-monsoon (122.6 g·m-2) and minimum in summer (78.3 g·m-2) (Table 1). Potassium levels were 56.7% higher in pre-monsoon, 21.6% in post-monsoon and 10.8% in monsoon than in summer.
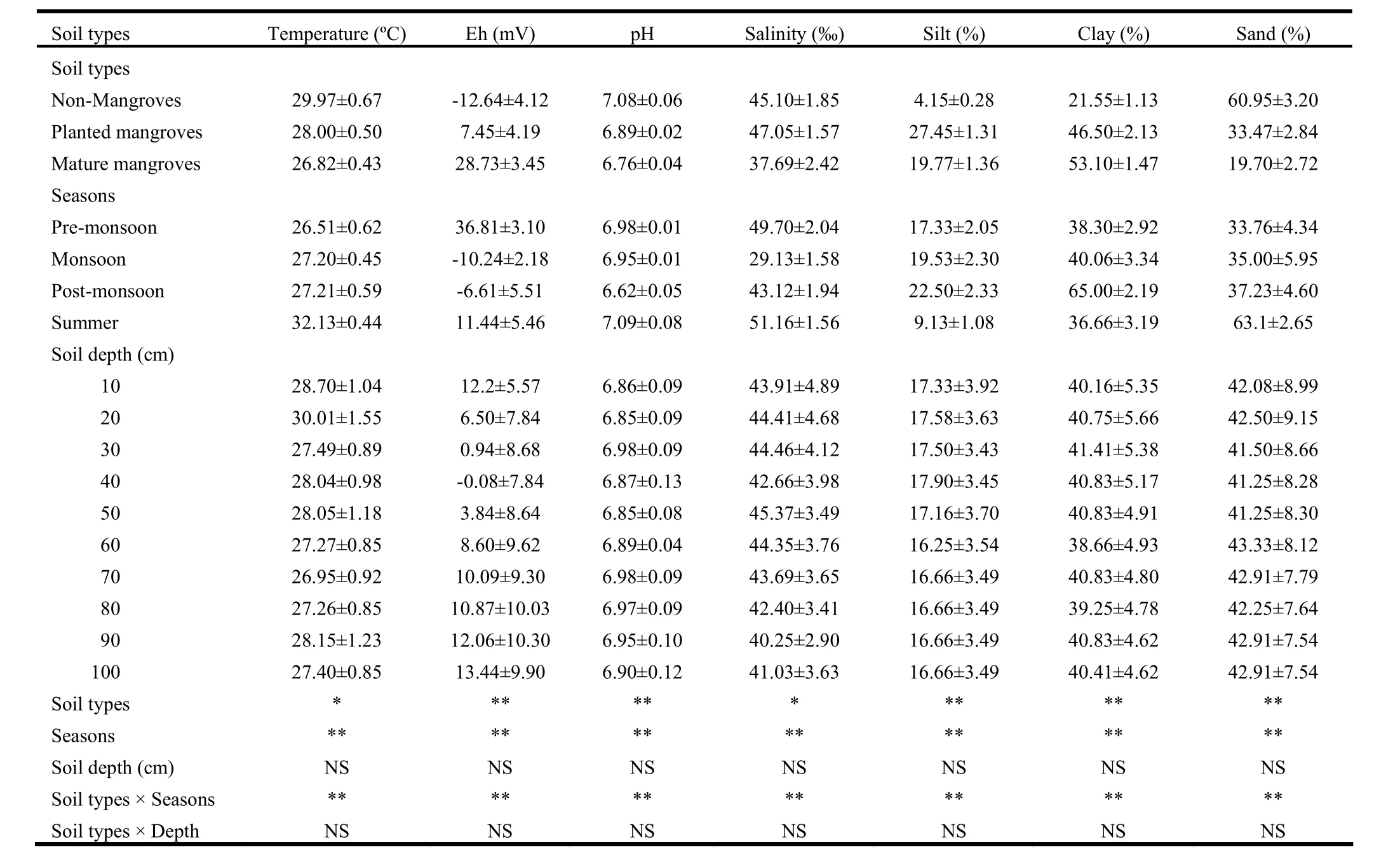
Table 2: Temperature, redox potential, pH, salinity and soil composition in sediments of mangroves and non-mangroves at different soil depths and seasons
Sediment proportions of silt, clay and sand varied significantly (p<0.01) by soil type and season, and interaction between soil type and season was significant. Sediment composition did not vary by soil depth and no interactions with other parameters were significant (Table 2). Silt content was higher in planted (27.5%) and mature (19.8%) mangrove sediments than that in barren area (4.2%). The content was 6.6 fold higher in planted mangrove and 4.8 fold in mature mangroves than in barren area. Silt content also varied significantly by season with the highest value in post-monsoon (2.5%) and lowest in summer (9.13%). Silt content was higher by 2.5 fold during post-monsoon, 2.1 fold during monsoon, and 1.9 fold during pre-monsoon than in summer. Clay content in mature mangrove soil was as high as 2.5 times greater and in planted mangrove 2.2 times greater than in non-mangrove soil. Clay content also varied seasonally with the highest value (65%) in post-monsoon and the lowest (36.7%) in summer (Table 2). The content was higher by 77.3% in post-monsoon, 9.3% in monsoon, 4.5% in pre-monsoon than in summer. Sand content was higher (61%) in non-mangrove soil than in plantation (33.5%) and mature (19.7%) mangrove sediments. Sand content was lower by 67.7% in mature mangroves and 45.1% in planted mangroves than in unvegetated soil. Sand content also varied by season, with the highest value in summer (63.1%) and lowest in pre-monsoon (33.8%) (Table 2). The content was higher by 86.6% in summer, 10.3% in post-monsoon and 3.7% in monsoon than in pre-monsoon.
Salinity, pH, redox potential and temperature varied significantly (p <0.01 in all cases) by soil type and season. Interaction between soil type and season was significant. None of these four parameters varied by soil depth and none interacted significantly with soil depth (Table 2). Salinity was higher (47.1‰) by 4.3% in planted sediment and lower (37.7‰) by 16.4% in mature mangroves than in barren soil. Salinity was 76% greater in summer, 71% in pre-monsoon, 48% in post-monsoon than duringmonsoon. Sediment pH was higher in barren soil than in mangrove sediments and it was lower by 97% in planted and 95% in mature mangrove sediment than in non-mangrove soil. pH was high (7.09) in summer, declining by 1.97% in post-monsoon, 1.55% in monsoon and 6.6% in pre-monsoon. Redox potential was higher in mangrove sediment than that in barren soil, and it was high in pre-monsoon and summer in comparison to post-monsoon and monsoon. Temperature was lower in mangrove sediments than in barren area. It was 1.97°C lower in planted and 3.15°C lower in mature mangroves. Sediment temperatures were highest in summer at 32.13°C, declining by 4.92°C in post-monsoon, 4.93°C in monsoon and by 5.62°C in pre-monsoon.
Discussion
Natural and plantation mangrove sediment stored more total carbon than did barren soil (Table 1). This confirms reports from other mangrove sites. The mangroves store more carbon per unit area by releasing negligible amounts of greenhouse gases. The average soil carbon density of mangrove sediments ((0.055±0.004) g·cm-3) is reported to be 41% higher than the salt marsh average ((0.039±0.003) g·cm-3) at 154 sites from the western and eastern Atlantic and Pacific coasts, as well as the Indian Ocean, Mediterranean Ocean, and Gulf of Mexico (Chmura et al. 2003). The efficiency of carbon burial in sediments increases from 16% to 27% from the youngest to the oldest forest in peninsular Malaysia (Alongi et al. 2004a). This low carbon content in sediments of recently planted mangroves was attributed to the organic matter which was rapidly and efficiently mineralized to a depth of 1 m in Malaysian mangrove forest. Low carbon content was also reflected in the percentage of the ratio of total sediment carbon oxidation to forest net primary productivity, where the ratio declines with increasing age of the forest. Thus the amount of sediment carbon stock is related to the age of mangrove forest (Alongi et al. 2004b). Our results showed that carbon content in mature mangrove forest was higher than in 15-year old planted forest (Table 1).
Mangroves are detritus-based ecosystems and their sediments can store large amounts of organic carbon (Fujimoto et al. 1999; Matsui 1998), and some mangrove ecosystems have organic-rich sediments several meters in depth (Lallier-Verges et al. 1998). Total organic carbon (TOC) values were, however, low at our study area in comparison to other reports. The range of TOC reported is 0.6–1.5% for mangroves of Zhangjiang Estuary, China (Xue et al. 2009) and 1.4–7.0% for western Australian mangroves (Alongi et al. 2000), as compared to the range of 0.032% to 0.193% in the Vellar-Coleroon estuarine complex, India (Table 1). This low value might have been due to the sensitivity of the method used for the analysis. Moreover, our study area experienced high salinity, high wind action, lacking of freshwater, and anthropogenic pressures (Kathiresan 2000; Alongi et al. 2005).
We recorded 2.5 times more total organic carbon (TOC) in mature mangrove sediment and 2 times more TOC in planted sediment than in non-mangrove soil (Table 1). The source of carbon for the mangrove sediments depends on the supply of organic matter derived from mangrove litterfall, bacteria, marine algae, terrestrial plants, protozoa etc., depending on local conditions (Alongi 1998; Volkman et al. 2000; Bouillon et al. 2004; Kristensen et al. 2008). The mangroves may also be a source of carbon in that they may supply significant amounts of carbon to adjacent coastal ecosystems (Ong 1993; Kristensen et al. 2008).
The waters of the present study area are fertile and productive, with high phytoplankton biomass and rates of net phytoplankton production as high as 6.3 g·m-3·d-1. Mangrove ecosystems can efficiently trap suspended organic material from the water column (Alongi et al. 1998; Kathiresan 2000). Further, our mangrove sites were carpeted by thick mats of leaf litter indicating little export by the small tides. Both litter and algae can eventually be transported downwards and mixed into the soil by crabs and other fauna (Alongi et al. 2005). Based on the mean sedimentation rates of 2.3?5.6 mol·m-2·a-1and total carbon decomposition rates of 43?199 mol·m-2·a-1, we estimate carbon burial rates at 3%?5% of total carbon input for our study area. This is apparently little carbon burial, attributed to the vast bulk of the organic carbon being oxidized by soil microbes (Alongi et al. 2005). Organic carbon that escapes microbial degradation is stored in sediments (Kristensen et al. 2008). However, in many mangrove forests, the rate of soil respiration is low possibly because of anaerobic soil conditions, making these forests highly efficient carbon sinks in the tropics (Komiyama et al. 2008).
Carbon content of sediment appears to be determined to a large extent by the degree of linkage of mangroves to adjacent aquatic systems. Mangroves in areas of low tidal amplitude or those high on shorelines have little chance to export organic matter, and also little other material brought in; such systems typically have high carbon contents, and the organic matter accumulating is locally produced (Bouillon et al. 2009). Thus the tidal amplitude and extent of the intertidal zone are important factors determining the carbon content in mangrove sediments. Our study area, however, had low tidal amplitude and limited intertidal area (Kathiresan, 2000).
The total carbon content was <1% in the sediments of our study area (Table 1), which was lower than other mangrove areas (Boto 1992; Alongi et al. 2005). The amount of carbon stored within sediments of individual mangrove ecosystems varies widely from <0.5% to 40% with a global median value of 2.2% (Kristensen et al. 2008). The levels of total carbon and TOC were found significantly higher in premonsoon (Table 1). A similar observation has been made in Terengganu Near shore coastal area, Malaysia, with the highest TOC content of 1.14%±0.29% in premonsoon (Hasrizal et al. 2009).
The ratio between carbon and nitrogen was high in the sediments which were also high in organic carbon (Table 1). A similar observation has been made in the mangroves of Coringa Wildlife Sanctuary in the Godavari Delta, India and Galle and Pambala, south-west Sri Lanka (Bouillon et al. 2003). The C/N ratio was found higher in mangrove sediments (6.91 for mature and 6.08 for planted mangroves) than that in non-mangrove soil (3.5), but the values between the soil types were not significant(Table 1). A similar non-significant difference between forest sediments and bare flat sediments in C/N ratio was recorded in the Zhangjiang Estuary mangrove wetland, China. However, the C/N ratios of all samples are relatively high in the forest sample and low in the bare flat sample in the estuary (Xue et al. 2009), as also observed in the present study. The molar C/N ratios are similarly constant down core sediment in Matang mangrove forest of peninsular Malaysia (Along et al. 2004a). This supports the present study that there was no significant variation of C/N ratio between different soil depths (Table 1).
Compared with other mangrove sediments, C/N ratios were low at our study area. In the previous studies, most mangrove sediments have C/N ratios above 8 (Xue et al. 2009) and this can be attributed to the rich organic carbon (Kennedy et al. 2004; Kristensen et al. 2008). The low C/N ratios of the present study indicated the presence of relatively higher nitrogen content in the mangrove sediment (Table 1).
Regarding the seasonal changes, C/N ratio was high due to high content of carbon in pre-monsoon, and the ratio was low due high input of nitrogen from monsoonal discharges to the mangrove habitats (Kathiresan 2000). The C/N ratio is a measure to identify the source of organic matter. The C/N ratios of sediment are less than 8 for a typical marine origin, but more than 12 for terrestrial sources of organic matter (Bordovskiy 1965; Meyers 1994; Muller and Voss 1999). In our study, the ratio ranged from 0.12 to 11.35 in different seasons (Table 1). These values did not exceed 12 and fell in the average of 5.5, indicating organic matter was derived from marine sources rather than terrestrial sources. A similar observation has been made in the Zhangjiang Estuary mangrove wetland, China which has soil C/N ratios in a range from 6-12 with mean of 8.19 (Xue et al. 2009), as against mean of 5.5 in the present study. This difference in mean value of C/N ratio can be attributed to the analysis which is based on the surface sediments (Xue et al. 2009) and ours was in the sediment up to 1 m depth.
Sediment carbon content was negatively correlated to temperature, sand and soil phosphorus (p <0.01 in all cases); positively correlated with total organic carbon, redox potential, silt, clay , C/N ratio, soil potassium (p <0.01 in all cases) and soil nitrogen) (p <0.05); but was not correlated to soil depth, pH or salinity. The redox potential was negative (-12.6mV) in barren soil, but positive in planted (7.5mV) and mature (28.73 mV) mangrove sediments, indicating that unvegetated soil was less aerated as compared to the vegetated sediment and the latter was well-aerated due to the presence of aerial roots equipped with lenticels. The redox potential also varied by season: redox was positive in pre-monsoon (36.8 mV) and summer (11.4 mV), and negative in post-monsoon (-6.6 mV) and monsoon (-10.2 mV), indicating that sediment was well-aerated during pre-monsoon and summer, and less-aerated in the other two seasons, during which there was frequent submergence of sediment by flooding. Oxmann et al. (2009) reported that the nutritional status of reforested mangrove stands in the Saigon River delta is driven primarily by sediment pH, which in turn is affected by the redox potential (Eh). The latter is influenced by inundation and organic matter decomposition. A lack of organic matter decomposition resulting from destruction of the mangrove vegetation can cause sediment oxidation with an accompanying phosphorus (P) deficiency through sediment acidification. Sediment pH can also produce drastic effects on phosphorus cycling within the relatively narrow pH range 6?7. A predominantly pH-driven P cycling in the mangrove stands deviates from a major Eh influence on P cycling in regions subjected to frequent or permanent submergence. Sediment reduction is generally thought to increase available P levels. Nevertheless, transformation processes during reduction and their effects on P solubility are still a matter of debate (Golterman 2001). In the present study, sediment phosphorus correlated positively with Eh and negatively with pH and this supports the findings of Oxmann et al. (2010) that sediment pH and Eh have impacts on controlling nutrient status, especially phosphorus, in mangrove stands.
In mangrove sediment, total carbon content varied with sediment characteristics, in particular nitrogen, phosphorus, potassium, redox potential, pH and salinity. Nitrogen, phosphorus and potassium are the nutrients that support growth of mangroves, associated microorganisms and fauna. The bioavailability of nutrients is regulated by redox potential, pH and salinity in the sediment. The nutrient dynamics sustain the pristine mangrove ecosystem with significant storage of carbon.
The organic matter concentration is controlled by the particle size of the sediments (Hasarizal et al. 2009). The abundance of greater surface areas of fine particles of silt and clay are responsible for high concentrations of carbon, nitrogen and potassium, whereas the sand particles with large surface areas stock only lesser contents of carbon and nutrients (Ramanathan 1997). Distribution of grain size differed between mangrove vegetated and barren sediment samples. There was a predominance of clay particle in the mangrove sediments of the study area, accounting for 46.5%?53.1%, whereas the sand particle dominated the unvegetated sediment, constituting 61% (Table 2). Silt is predominant in the sediments of the Zhangjiang Estuary mangrove wetland, China, accounting for 61%?72% in the mangrove forest and 59%?76% in the bare flat. Sand content is low with less than 16% in mangrove sediment and 1%?25% in the bare flat, and there was no clear change pattern of grain sizes in the surface sediments of the estuary (Xue et al. 2009). The content of sand particle in the core sediment increases with increasing depth, whereas the content of clay in the core sediment decreases with increasing depth (Xue et al. 2009). However, in the present study, there was no significant difference between the soil depths for clay and sand particles (Table 2).
This work revealed that the mangrove vegetation has an impact on sediment carbon storage. The 15-year old planted mangrove sediment could store the total carbon by 41.8% and total organic carbon by 100% and the annual rate of carbon burial in the sediment would be calculated at 2.8% for total carbon and 6.7% for total organic carbon. Thus the carbon burial rate was rapid, which is in agreement with other reports (Ong 1993; Alongi et al. 2004b). Carbon burial perhaps reduces the problems that go with the 'green house gases’ and global warming. Mangroves are among the most productive ecosystems and their carbon stock per unit area is significant (Twilley et al. 1992).
Clearing of mangroves can rapidly result in significantly reduced carbon stores (Bouillon et al. 2009). Cebrain (2002) estimated that about 35% of the world’s mangroves have been destroyed and this has resulted in a net loss of 3.8-1014g C stored as mangrove biomass. Hence, rehabilitating the degraded mangrove areas would contribute to carbon sequestration to mitigate the global warming threat. Thus, the restoration of lost mangrove forests would be a new countermeasure of global warming.
Acknowledgements
The authors are thankful to the authorities of Annamalai University, India for providing facilities and to the Ministry of Earth Science for financial support.
Alongi DM, 1998. Coastal ecosystem processes. Available at: http://books.google.co.in/books?id=cMQp7bIZOogC.
Alongi DM, 2002. Present state and future of the world’s mangrove forests. Environment Conservation, 29:331?349.
Alongi DM, Ramanathan AL, Kannan L, Tirendi F, Trott LA, BalaKrishna Prasad M. 2005. Influence of human-induced disturbance on benthic microbial metabolism in the Pichavaram mangroves, Vellar-Coleroon estuarine complex, India. Marine Biology, 147: 1033?1044.
Alongi DM, Sasekumar A, Chong VC, Pfitzner J, Trott LA, Tirendi F, Dixon P, Brunskill GJ. 2004b. Sediment accumulation and organic material flux in a managed mangrove ecosystem: estimates of land-ocean-atmosphere exchange in peninsular Malaysia. Marine Geology, 208: 383?402.
Alongi DM, Sasekumar A, Tirendi F, Dixon P. 1998. The influence of stand age on nebthic decomposition and recycling of organic matter in managed mangrove forests of Malaysia. Journal of Experimental Marine Biology and Ecology, 225: 197?218.
Alongi DM, Tirendi F, Trott LA, Xuan XX. 2000. Benthic decomposition rates and pathways in plantation of the mangrove, Rhizophora apiculata, in the Mekong delta, Vietnam. Marine Ecology Progress Series, 194: 87?101.
Alongi DM, Wattayakorn G, Tirendi F, Dixon P. 2004a. Nutrient capital in different aged forests of the mangrove Rhizophora apiculata. Botanica Marina, 47: 116?124.
Bordovskiy OK. 1965. Accumulation and transformation of organic substances in marine sediments. Marine Geology, 3: 3?114.
Boto KG. 1992. Nutrients and mangroves. In: Connell, D.W., Hawker, D.W., (eds.), Pollution in Tropical Aquatic Systems. Boca Raton FL: CRC Press, pp. 129?145.
Bouillon S, Borges AV, Edward Castaneda-Moya, Karen Diele Dittmar T, Duke NC, Erik Krishtensen Lee SY, Cyril Marchand, Middelburg JJ, Rivera-Monroy VH, Smith III TJ, Twilley RR. 2009. Mangrove production and carbon sinks: A revision of global budget estimates. Global Biogeochemical Cycles, 22: 1?12.
Bouillon S, Connolly RM, Lee SY. 2008. Organic matter exchange and cycling in mangrove ecosystems: recent insights from stable isotope studies. Journal of Sea Research, 59: 44?58.
Bouillon S, Dahdouh-Guebas F, Rao AVVS, Koedam N, Dehairs F. 2003. Sources of organic carbon in mangrove sediments: variability and possible ecological implications. Hydrobiologia, 495: 33?39.
Bouillon S, Moens T, Overmeer I, Koedam N, Dehairs F. 2004. Resource utilization patterns of epifauna from mangrove forests with contrasting inputs of local versus imported organic matter. Marine Ecology Progress Series, 278: 77?88.
Buchanan JB. 1984. Sediment analysis. In: Holme, N.A., McIntyre, A.D., (Eds.), Methods for the Study of Marine Benthos. London: Blackwell Scientific Publications, pp. 41?65.
Chmura GL, Anisfeld SC, Cahoon DR, Lynch JC. 2003. Global carbon sequestration in tidal, saline wetland soils. Glob Biogeochem Cycles, 17(4): 1111, doi:10.1029/2002GB001917
Duarte CM, Cebrian J. 1996. The fate of marine autotrophic production. Limnology Oceanography, 41: 1758?1766.
Duarte CM, Middelburg JJ, Caraco N. 2005. Major role of marine vegetation on the oceanic carbon cycle. Biogeosciences, 2: 1?8.
El Wakeel SK, Riley JP. 1956. The determination of organic carbon in marine muds. Journal de conseil permanent Intl. Pourl exploration de la mer, 22: 180?183.
Fujimoto K, Imaya A, Tabuchi R, Kuramoto S, Utsugi H, Murofushi T. 1999. Belowground carbon storage of Micronesian mangrove forests. Ecology Reserves, 14: 409?413.
Gilman EL, Ellison J, Duke NC, Field C. 2008. Threats to mangroves from climate change and adaptation options: a review. Aquatic Botany, 89: 237?250.
Golterman HL. 2001. Phosphate release from anoxic sediments or ‘what did Mortimer really write?’ Hydrobiologia, 450: 99?106.
Guzman HM, Jimenez CE. 1992. Contamination of coral reefs by heavy metals along the Carribeen coast of central America (Coastal Rica and Panama). Marine Pollution Bulletin, 24(11): 554?561.
Hasrizal S, Kamaruzzaman BY, Sakri I, Ong MC, Noor Azhar MS. 2009 Seasonal Distribution of Organic Carbon in the Surface Sediments of the Terengganu Near shore Coastal Area. American Journal of Environmental Sciences, 5(1): 111?115.
Kathiresan K, Bingham BL. 2001. Biology of mangroves and mangrove ecosystems. Advances in Marine Biology 40: 81?251.
Kathiresan K. 2000. A review of studies on Pichavaram mangroves, southeast India. Hydrobiologia, 430: 185?205.
Kennedy H, Gaciab E, Kennedya S, Papdimitrioua DP, Duarte CM. 2004. Organic carbon sources to SE Asian coastal sediments. Estuarine Coastal and Shelf Science, 60: 59?68.
Komiyama A, Ong JE, Poungparn S. 2008. Allometry, biomass and productivity of mangrove forests: A review. Aquatic Botany, 89: 128?137.
Kristensen E, Bouillon S, Dittmar T, Marchand C. 2008. Organic carbon dynamics in mangrove ecosystems: a review. Aquatic Botany, 89: 201?219.
Lallier-Verges E, Perrussel BP, Disnar JR, Baltzer F. 1998. Relationships between environmental conditions and the diagenetic evolution of organic matter derived from higher plants a modern mangrove swamp systems (Guadeloupe, French West Indies). Organic Geochemistry, 29: 1663?1686.
Matsui N. 1998. Estimated stocks of organic carbon in mangrove roots and sediments in Hinchinbrook Channek, Australia. Mangroves and Salt Marshes, 2: 199-204.
Meyers PA. 1994. Preservation of elemental and isotopic source identification of sedimentary organic matter. Chemical Geology, 144: 289?302.
Muller A, Voss M. 1999. The Palaeoenvironments of coastal lagoons in the southern Baltic Sea II. δ13C and δ15N ratios of organic matter-sources andsediments. Palaeoecology, 145: 17?32.
Ong JE. 1993. Mangroves – a carbon source and sink. Chemosphere, 27: 1097?1107.
Oxmann JF, Luitgard Schwendenmann Ruben J Lara. 2009. Interactions among phosphorus, pH and Eh in reforested mangroves, Vietnam: a three-dimensional spatial analysis. Biogeochemistry, 96: 73?85.
Oxmann JF, Pham QH, Schwendenmann L, Stellman JM, Lara RJ. 2010. Mangrove reforestation in Vietnam: the effect of sediment physicochemical properties on nutrient cycling. Plant Soil, 326: 225?241.
Ramanathan AL. 1997. Sediment characteristics of the Pichavaram mangrove environment, south east coast of India. Indian Journal of Marine Science, 26: 319?322.
Subbiah BV, Asija GL. 1956. A rapid procedure for the determination of available nitrogen in soils. Current Science, 31: 196?200.
Volkman JK, Rohjans D, Rullkotter J, Scholz-Bottcher BM, Liebezeit G. 2000. Sources and diagenesis of organic matter in tidal flat sediments from the German Wadden Sea. Continental Shelf Research, 20: 1139?1158.
Xue B. Yan CL, Lu HL, Bai Y. 2009. Mangrove-Derived organic carbon in sediment from Zhangjiang estuary (China) mangrove wetland. Journal of Coastal Research, 25(4): 949?956.
DOI 10.1007/s11676-014-0526-2
The online version is available at http:// www.springerlink.com
Kandasamy Kathiresan (), Venugopal Gomathi, Raj Anburaj,
Kandasamy Saravanakumar ()
Centre of Advanced Study in Marine Biology, Faculty of Marine Sciences, Annamalai University, Parangipettai 608 502, India.
E-mail: kathirsum@rediffmail.com; saravana732@gmail.com
Corresponding editor: Zhu Hong
 Journal of Forestry Research
2014年4期
Journal of Forestry Research
2014年4期
- Journal of Forestry Research的其它文章
- Growth and yield of two grain crops on sites former covered with eucalypt plantations in Koga Watershed, northwestern Ethiopia
- Full length cDNA cloning and expression analysis of annexinA2 gene from deer antler tissue
- Bamboo resources of Sikkim Himalaya: diversity, distribution and utilization
- Implications of crude oil pollution on natural regeneration of plant species in an oil-producing community in the Niger Delta Region of Nigeria
- Enhancement of seed germination in Macaranga peltata for use in tropical forest restoration
- Bio-amelioration of alkali soils through agroforestry systems in central Indo-Gangetic plains of India
