Drug sensitivity of thirty-four reference slowly growing mycobacteria to first and second line antituberculous agents
(1. , , , 410078, ;2. , , 046000, ;3. , , , 102206, ;4. s, 310000, )
Drug sensitivity of thirty-four reference slowly growing mycobacteria to first and second line antituberculous agents
(1.DepartmentofImmunology,XiangyaSchoolofMedicine,CentralSouthUniversity,Changsha410078,China;2.DepartmentofImmunology,ChangzhiMedicalCollege,Changzhi046000,China;3.StateKeyLaboratoryforInfectiousDiseasePreventionandControl,NationalInstituteforCommunicableDiseaseControlandPrevention,ChineseCenterforDiseaseControlandPrevention,Beijing102206,China;4.CollaborativeInnovationCenterforDiagnosisandTreatmentofInfectiousDiseases,Hangzhou310000,China)
Slowly growing mycobacteria (SGM) are distributed in the environment, for example in soil and dirty water. SGM can cause human infections, especially lung diseases. In this article, first and second line antituberculous agents were examined in order to identify the optimum drugs for the treatment of SGM disorders. The fewest SGM in our study (4/34) were susceptible to isoniazid. Rifampicin (13/34) and ethambutol (14/34) were effective against similar numbers of strains. Ofloxacin (23/34), kanamycin (26/34), tobramycin (26/34) and streptomycin (27/34) were active against most of the tested strains. Ciprofloxacin (31/34), levofloxacin (31/34), amikacin (33/34) and capreomycin (33/34) showed an excellent range of activity. Moxifloxacin (34/34) showed the widest range of activity against the SGM species. Among the tested SGM species,M.simiaeandM.africanumwere resistant to the highest number of drugs.M.szulgaiandM.duvaliiwere susceptible to all the first and second line antituberculous agents tested. Overall, the second-line antituberculous agents were good candidates for the treatment of infection by SGM species and can be widely used in the therapy of SGM diseases.
drug sensitivity; reference slowly growing mycobacteria; antituberculous agents; minimum inhibitory concentration (MIC)
Nontuberculous mycobacteria (NTM) are opportunistic pathogens that are widely distributed in the environment[1], for example in tap water, shower heads, soil, dust, and food products, in addition to domestic and wild animals[2]. Slowly growing mycobacteria (SGM) species belong to the NTM. SGM form visible colonies in more than 7 days on subculture media[3]and includeM.avium,M.intracellulare,M.kansasii,M.haemophilum,M.marinum,M.ulceransand other species[3]. The distribution of species varies from region to region[4]. Among SGM strains,M.avium,M.intracellulare,M.silvaticum,M.hominissiusandM.paratuberculosiscomprise theMycobacteriumaviumcomplex (MAC);M.aviumandM.intracellulare, are the most common species in pulmonary and disseminated SGM diseases[2,5-6]. After MAC,M.kansasiiis the second most common cause of pulmonary infections caused by NTM in the United States of America (USA) and England. SGM strains can form biofilms that contribute to resistance to disinfectants and antibiotic therapy[2]. The most serious drawback for SGM therapeutic regimens in clinical practice is problematic[7]. In the present study, drug sensitivity tests were performed on 34 reference SGM organisms using Clinical Laboratory Standards Institute (CLSI) (USA)[8]and World Health Organization (WHO)[9]guidelines in order to indicate treatment criteria for physicians.
Materials and methods
Experimental strains
Thirty-four international reference RGM strains were purchased from Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ) and the American Type Culture Collection (ATCC). They were incubated at room temperature.
Antituberculous agents
Twelve drugs were purchased from Sigma-Aldrich Company:rifampicin (RFP), isoniazid (INH), ethambutol (EMB), streptomycin (SM), amikacin (AM), kanamycin (KN), capreomycin (CPM), tobramycin (TOB), ofloxacin (OF), ciprofloxacin (CIP), levofloxacin (LEV) and moxifloxacin (MOX). All the antibacterial liquids were freshly prepared.
Drug sensitivity test
Bacteria were cultured in 7H10 agar with 5% oleic-acid-albumin-dextrose-catalase (OADC)[8]and drug susceptibility tests were performed by cation-adjusted Mueller-Hinton (CAMH) broth microdilution adding 5% OADC supplement, per the standard operating procedure of the CLSI[8]. All the tests were performed in 96-well microplates in duplicate. The mean value obtained from the two tests was considered the minimum inhibitory concentration (MIC). The bacteria were modulated by saline to a density of 0.5 McFarland standard, then diluted to 1:200 of 0.5 McFarland standard using CAMH broth with 5% OADC. The drugs were continuously double attenuated from wells 1 to 11 in every row. The levels of rifampicin, isoniazid, ethambutol, streptomycin and tobramycin were 0.25-256 μg/mL. The concentrations of amikacin, kanamycin, capreomycin, ofloxacin, ciprofloxacin, levofloxacin and moxifloxacin were 0.03-32 μg/mL. Two negative controls were used. One was no drug (CAMH + OADC + microorganism) and the other was no bacteria (merely CAMH and OADC)[5]. The MIC breakpoints of the antibiotics displaying sensitivity, moderate sensitivity, and resistance were as per the guidelines of the CLSI[8]and the WHO[9]and are shown in Table 1.
Results
The results of tests of 12 antituberculous agents against 34 SGM species are shown in Table 1 and Figure 1. The fewest bacteria (4/34), includingM.szulgai,M.celatum,M.duvaliiandM.elephantiswere susceptible to isoniazid. Note that these values include the total number of strains that were sensitive and moderately sensitive to the named drug. Rifampicin (13/34) and ethambutol (14/34) were effective against similar numbers of strains. Streptomycin (27/34), ofloxacin (23/34), kanamycin (26/34) and tobramycin (26/34) were active against most of the tested strains, but ciprofloxacin (31/34), levofloxacin (31/34), amikacin (33/34) and capreomycin (33/34) showed even wider activity. Moxifloxacin (34/34) was the best drug, active (or moderately active) against all the SGM species tested in this report.
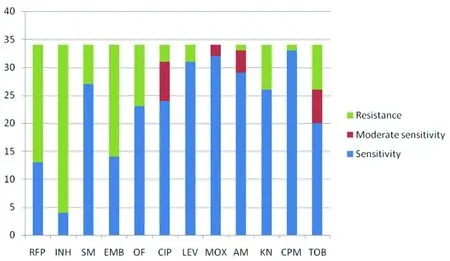
INH:isoniazid; RFP:rifampicin; SM:streptomycin; EMB:ethambutol; OF:ofloxacin; CIP:ciprofloxacin; LEV:levofloxacin; MOX:moxifloxacin; AM:amikacin; KN:kanamycin; CPM:capreomycin; TOB:tobramycin.
Fig.1 Sensitivity distribution of 34 reference slowly growing mycobacteria to 12 antituberculous agents
Considering the tested SGM species,M.simiae(9/12) andM.africanum(8/12) were resistant to the most drugs.M.szulgaiandM.duvaliiwere susceptible to all to the first and second line antituberculous agents.
Discussion
In this study, 12 antituberculous agents were tested against 34 SGM organisms by microdilution. Our findings show that the second-line antituberculous agents, especially moxifloxacin, amikacin and capreomycin, were more effective than the first-line agents against SGM.
Of the tested SGM species,M.aviumandM.intracellulareare the most common SGM organisms and, alongsideM.chimaera, form part of the MAC[10-11]. In previous studies, some MAC strains were resistant to first-line antituberculous agents[12].M.aviumwas largely resistant to rifampicin and ethambutol[13-15]. Sometimes rifampicin and ethambutol were combined with macrolide antibiotics in order to amplify the latter drugs’ activity[10]. Our data indicated that isoniazid had the lowest activity against the tested strains. This is consistent with the literature[3,16-17].

Tab.1 The MIC values (μg/mL) of antituberculous drugs tested against 34 international reference slowly growing mycobacteria
Note 1. INH:isoniazid, RFP:rifampicin, SM:streptomycin, EMB:ethambutol, OF:ofloxacin, CIP:ciprofloxacin, LEV:levofloxacin, MOX:moxifloxacin, AM:amikacin, KN:kanamycin, CPM:capreomycin, TOB:tobramycin.
Note 2. Numbers in bold indicate resistance; numbers in bold and italics indicate moderate sensitivity.
Among the fluoroquinolone and aminoglycoside antibiotics, moxifloxacin and amikacin showed excellent activity against SGM organisms in previous studies[3,17], which was confirmed by our findings. In the current report, ofloxacin and tobramycin were less effective than other second-line antituberculous agents. Capreomycin was a good candidate for the therapy of SGM diseases. Of the tested SGM strains,M.africanum,M.simiae,M.chimaeraandM.immunogenumwere resistant to most of the antibacterial agents, but were susceptible to moxifloxacin and amikacin.
In general, SGM strains were resistant to the conventional first-line antituberculous drugs. However, the second-line antituberculous agents can be widely used in the therapy of the SGM infections. Combination of two or more drugs against SGM strains will be investigated in the future.
Acknowledgments
We thank the staffs of the National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention.
Disclosure of conflict of interest
The authors have declared that no competing interests exist.
Ethical approval
Not required.
[1]Velayati AA, Farnia P, Mozafari M, et al. Nontuberculous mycobacteria isolation from clinical and environmental samples in Iran:twenty years of surveillance[J]. Biomed Res Int, 2015, 2015:254285.
[2]Al-Anazi KA, Al-Jasser AM, Al-Anazi WK. Infections caused by non-tuberculous mycobacteria in recipients of hematopoietic stem celltransplantation[J]. Front Oncol, 2014, 4:311. DOI:10.3389/fonc.2014.00311
[3]Philley JV, Griffith DE. Treatment of slowly growing mycobacteria[J]. Clin Chest Med, 2015, 36:79-90.
[4]Ferro BE, van Ingen J, Wattenberg M, et al. Time-kill kinetics of slowly growing mycobacteria common in pulmonary disease[J]. J Antimicrob Chemother, 2015, pii:dkv180.
[5]Foongladda S, Pholwat S, Eampokalap B, et al. Multi-probe real-time PCR identification of common Mycobacterium species in blood culture broth[J]. J Mol Diagn. 2009, 11(1):42-48. DOI:10.2353/jmoldx.2009.080081
[6]Falkinham JO, Hilborn ED, Arduino MJ, et al. Epidemiology and ecology of opportunistic premise plumbing pathogens:Legionellapneumophila,Mycobacteriumavium, andPseudomonasaeruginosa[J]. Environ Health Perspect, 2015, 123(8):749-758.
[7]Hombach M, Somoskovi A, Homke R, et al. Drug susceptibility distributions in slowly growing non-tuberculous mycobacteria using MGIT 960 TB eXiST[J]. Int J Med Microbiol, 2013, 303 (5):270-276.
[8]Clinical and Laboratory Standards Institute. Susceptibility Testing of Mycobacteria, Nocardiae, and Other aerobic actinomycetes; Approved Standard--Second Edition[S]. CLSI document 2011, M24-A2.
[9]World Health Organization. Policy guidance on drug-susceptibility testing (DST) of second-line antituberculosis drugs. WHO/HTM/TB/2008.392[M]. Geneva:World Health Organization, 2008.
[10]Renvoise A, Bernard C, Veziris N, et al. Significant difference in drug susceptibility distribution betweenMycobacteriumaviumandMycobacteriumintracellulare[J]. J Clin Microbiol, 2014, 52 (12):4439-4440. DOI:10.1128/JCM.02127-14
[11]Liu G, Chen ST, Yu X, et al. Bacteriological and virulence study of aMycobacteriumchimaeraisolate from a patient in China[J]. Antonie Van Leeuwenhoek, 2015, 107 (4):901-909.
[12]Venugopal D, Kumar S, Isa M, et al. Drug resistance profile of humanMycobacteriumaviumcomplex strains from India[J]. Indian J Med Microbiol, 2007 , 25 (2):115-120.
[13]Saggese MD, Tizard I, Gray P, et al. Evaluation of multidrug therapy with azithromycin, rifampin, and ethambutol for the treatment ofMycobacteriumaviumsubsp avium in ring-neck doves (Streptopeliarisoria):an uncontrolled clinical study[J]. J Avian Med Surg, 2014, 28 (4):280-289. DOI:10.1647/2012-067R1
[14]Jeong BH, Jeon K, Park HY, et al. Intermittent antibiotic therapy for nodular bronchiectaticMycobacteriumaviumcomplex lung disease[J]. Am J Respir Crit Care Med, 2015, 191 (1):96-103. DOI:10.1164/rccm.201408-1545OC
[15]van Ingen J, Kuijper EJ. Drug susceptibility testing of nontuberculous mycobacteria[J]. Future Microbiol, 2014, 9 (9):1095-1110. DOI:10.2217/fmb.14.60
[16]Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement:diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases[J]. Am J Respir Crit Care Med, 2007, 175 (4):367-416.
[17] Li G, Lian LL, Wan L, et al. Antimicrobial susceptibility of standard strains of nontuberculous mycobacteria by microplate Alamar Blue assay[J]. PLoS One, 2013, 8 (12):e84065. DOI:10.1371/journal.pone.0084065
Received:2015-06-11;Revision accepted:2015-08-30
PANG Hui1,2,3,LI Gui-lian3,4,WAN Kang-lin3,4,YU Ping1
10.3969/j.issn.1002-2694.2015.10.004
s:Yu Ping, Email:yuping1953@sina.com; Wan Kang-lin, Email:wankanglin@icdc.cn

Financially supported by the Project of the National Key Program of Mega Infectious Diseases (No. 2013ZX10004-101), the Key Project of the State Key Laboratory for Infectious Disease Prevention and Control(No. 2014SKLID104), and a Science and Technology Innovation Team Support Project (No. CX201412) from Changzhi Medical College

