Ultraviolet-inactivated Sendai virus strain Tianjin induced apoptosis in human glioma cell line LN229
SHI Li-ying,LI Mei,ZHANG Lei,GENG Peng,LI Yong-mei
(Department of Microbiology, College of Basic Medicine, Tianjin Medical University, Tianjin 300070, China)
Ultraviolet-inactivated Sendai virus strain Tianjin induced apoptosis in human glioma cell line LN229
SHI Li-ying,LI Mei,ZHANG Lei,GENG Peng,LI Yong-mei
(DepartmentofMicrobiology,CollegeofBasicMedicine,TianjinMedicalUniversity,Tianjin300070,China)
Sendai virus strain Tianjin was a novel genotype of Sendai virus. This study was designed to investigate the effect of ultraviolet-inactivated, replication-defective Sendai virus strain Tianjin (UV-Tianjin) on apoptosis of human glioma cell line LN229 and the possible mechanism. MTT assay showed that treatments with UV-Tianjin dose-dependently inhibited the proliferation of LN229 cells. Flow cytometric analysis after Annexin V/PI staining showed that UV-Tianjin induced apoptosis of LN229 cells in a dose-dependent manner. Colorimetric detection of caspase activity suggested that the activities of caspase-3, -8 and -9 increased with the increasing of viral titer. Moreover, Western blotting analysis showed that treatments with UV-Tianjin increased the expression levels of Bax, cytc, Fas, FasL and decreased the expression levels of Bcl-2, pro-caspase-8, pro-caspase-9 and pro-caspase-3 in LN229 cells in a dose-dependent manner. Our study suggests that UV-Tianjin exhibits the anticancer activity in human glioma cell line LN229 through inducing apoptosis, which may involve in both the endogenous mitochondrial pathway and the exogenous death receptor pathway.
Sendai virus; human glioma cell line LN229; apoptosis; mitochondrial pathway; death receptor pathway
Funded by the National Natural Science Foundation of China (No. 81172168)
In recent decades, oncolytic virotherapy has received widespread concern and related research has made great progress. A variety of viruses, such as adenovirus, herpes simplex virus-1, reovirus, vesicular stomatitis virus, Newcastle disease virus, measles virus and vaccinia virus, have been widely used in the treatment of tumor[1-5]. Oncolytic virus refers to the virus that kills tumor cells selectively, without harming the normal surrounding tissue. It mainly depends on its capability of self-replicating within the infected cells to directly induce tumor cell death or activate the body immune system to infiltrate and mediated the destruction of tumor mass[6-7]. Recent studies have shown that ultraviolet-inactivated, replication-defective Sendai virus (SeV) also showed strong antitumor effect[8-11]. However, the exact mechanism remains to be further explored.
Sendai virus strain Tianjin was isolated from the lungs of marmoset with respiratory infection in 1999. Whole genome sequencing and phylogenetic analysis has confirmed that the virus belonged to the genusRespiroviruswithin the familyParamyxoviridaeand was a novel genotype of Sendai virus[12]. In our previous study, in order to explore its antitumor effect and also ensure the safety of the application, we prepared ultraviolet-inactivated Tianjin strain (UV-Tianjin) and found that UV-Tianjin suppressed the growth of colon carcinomas in mice by inducing an antitumor immune response and tumor-cell apoptosis[13].
Brain glioma, originated in glial cells in brain, is the most common intracranial tumor, accounting for about 40%~50% of all intracranial tumors. It is also the second most frequent malignant tumor among children[14]. This study was designed to investigate the effect of UV-Tianjin on the proliferation and apoptosis of the human glioma cell line LN229invitro, and the possible mechanism.
Materials and methods
Cell culture and virus
Human glioma cell line LN229 was obtained from Type Culture Collection, Chinese Academy of Sciences (Shanghai, China) and was propagated in Dulbecco's modifed Eagle's medium (DMEM) (Hyclone Laboratories, Inc., Tianjin, China) supplemented with 10% fetal bovine serum (Hyclone Laboratories, Inc., Tianjin, China) at 37 ℃ in an incubator containing 5% CO2. Sendai virus strain Tianjin (GenBank: EF679198) was propagated in the chorioallantoic fluid from 9 to 11 day-old chicken eggs, after which it was purified by centrifugation and inactivated by UV irradiation (100 mJ/cm2), as previously described[15]. Inactivated virus could not replicate, but its capacity for viral fusion remains intact.
Reagents and antibodies
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) was purchased from Sigma-Aldrich (Shanghai, China). The Annexin V-FITC / Propidium iodide (PI) apoptosis detection kit was purchased from Biovision (Wuhan, China). Caspase-3, -8, -9 colorimetric assay kits were purchased from KeyGEN BioTECH (Nanjing, China). Anti-caspase-3 (9665), anti-caspase-8 (9746) and anti-caspase-9 (9502) antibodies were obtained from Cell Signaling Technology (Shanghai, China). Anti-Bcl-2 (sc-509), anti-Bax (sc-20067), anti-cytc(sc-7159), anti-Fas (sc-715), anti-FasL (sc-6237) and anti-β-actin (sc-47778) antibodies were purchased from Santa Cruz Biotechnology (Beijing, China).
Cell viability assay
Cell viability was assessed using MTT assay. LN229 cells were seeded at a density of 2 × 104cells/well into 96-well plates. After 24 h incubation, UV-Tianjin [multiplicity of infection (MOI): 10-320, the amount of viruses was defined as the equivalent of MOI before deactivation] was added to the monolayer and the culture continued for another 24 h. Then 10 μL of 5 mg/mL MTT was added to each well and re-incubated for an additional 4 h at 37 ℃. After removal of the MTT-containing medium, 100 μL of DMSO was added to dissolve the water insoluble formazan. Then the optical density (OD) values of each well were measured using a microplate reader (Bio-Rad, USA) at 570 nm. The following formula was used to calculate cell viability. Viability (%) = (OD570, sample-OD570,blank) / (OD570,control-OD570,blank)×100 %
Flow cytometric analysis of apoptosis
Apoptotic cells were examined using an Annexin V-FITC / PI apoptosis detection kit according to the manufacturer's instruction. In brief, 5×105cells of each group were harvested by trypsinization after treatment with UV-Tianjin (MOI 20, 40, 80) for 24 h. Then the cells were washed twice with PBS and resuspended in 500 μL of binding buffer. Cell suspensions were then incubated with 5 μL of annexin V-FITC and 5 μL of PI for 10 min at room temperature in the dark. The cells were evaluated immediately by flow cytometry (BD FACSVerse, USA) and the data were analyzed using FlowJo 7.6 software.
Caspase activity assay
Caspase activity was determined by using the caspase-3, -8, -9 colorimetric assay kits according to the manufacturer's instructions. Briefly, LN229 cells were cultured in 6-well plates overnight and then treated with UV-Tianjin (MOI 20, 40, 80) for 24 h. The cells were collected, washed in PBS, and lysed in 100 μl of lysis buffer on ice for 30 min. After centrifugation, the supernatant containing 150 μg protein were incubated with caspase-3 (Ac-DEVD-pNA), caspase-8 (Ac-IETD-pNA) and caspase-9 (Ac-LEHD-pNA) substrates in reaction buffer at 37 ℃ for 4 h in the dark. The levels of releasedpNA were measured at 405 nm wave length with a microplate reader. Fold increases in caspase-3, -8, or -9 activities were determined by comparison with the untreated control, which was set to 1.
Western blot analysis
LN229 cells were harvested by using cell scraper after treatment with UV-Tianjin for 24 h. The cell pellets were resuspended in 100 μL of lysis buffer containing protease inhibitor cocktail and lysed for 30 min on ice. Following the BCA assay, forty micrograms of protein from each cell lysate was loaded onto a gel and separated by SDS-PAGE. The proteins were then transferred to a PVDF membrane. Membranes were blocked with 5% nonfat milk at 4 ℃ overnight and then incubated with primary antibodies at room temperature for 1 h, after which membranes were washed in Tris-buffered saline Tween-20 (TBST) for three times and incubated with HRP-linked secondary antibodies (anti-rabbit or anti-mouse IgG) for 1 h at room temperature. After washing, the protein bands were visualized on X-ray film by TMB staining.
Statistical analysis
All of the data are represented as the mean±standard deviation (SD) from at least three independent experiments. Statistical comparisons were performed by one-way analysis of variance, and further post-hoc testing was conducted using the statistical software GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA, USA). AP<0.05 was considered statistically significant.
Results
UV-Tianjin inhibited the growth of human glioma cell
When we treated human glioma cell line LN229 with various amounts of UV-Tianjin for 24 h, as shown in Figure 1A, the cell viability was suppressed by UV-Tianjin in a dose-dependent manner. Microscopic examination showed that UV-Tianjin induced extensive cell fusion in LN229 cells (Figure 1B).
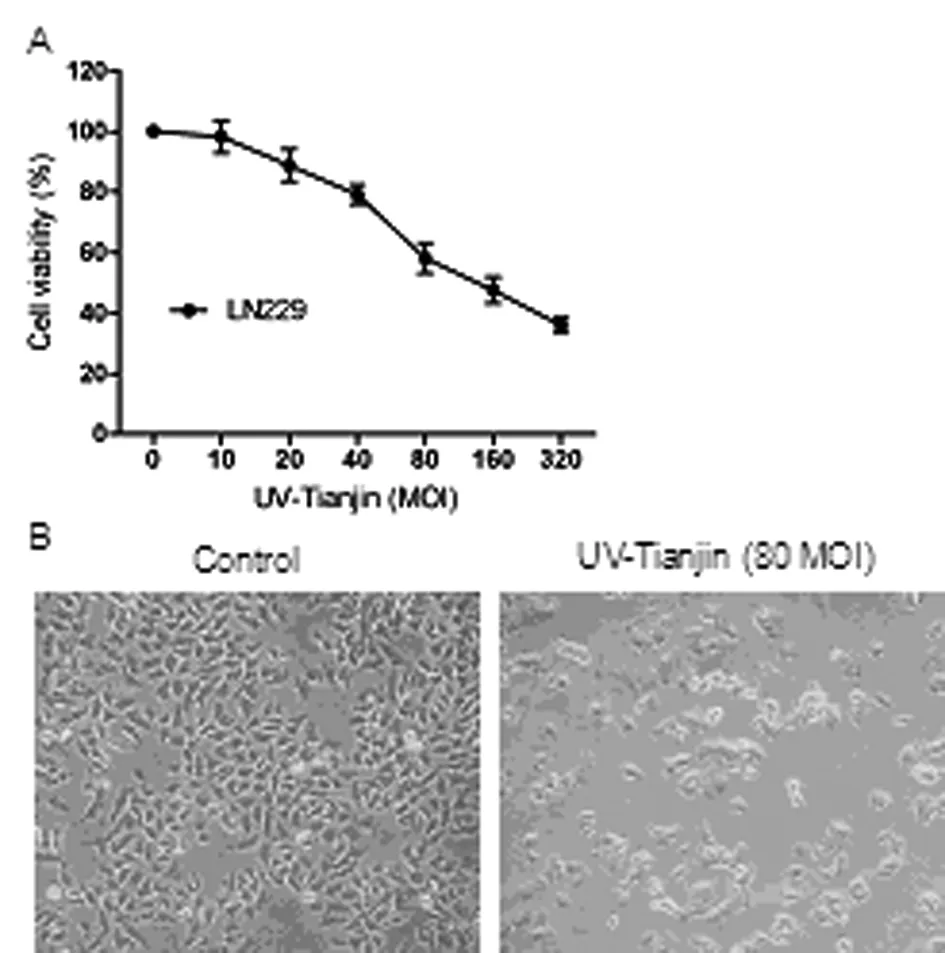
(A) The cell viability of LN229 cells were determined by MTT assay after UV-Tianjin treatment for 24 h. The results were expressed as mean±SD of three experiments in quadruplicate.
(B) Representative photographs of LN229 cells under microscope after UV-Tianjin treatment (MOI 80) for 24 h (×100).
Fig.1 Effects of UV-Tianjin on viability of LN229 cells
UV-Tianjin induced apoptosis of LN229 cells
To determine whether UV-Tianjin-mediated growth inhibition resulted from apoptosis, we subsequently estimated the number of apoptotic cells by flow cytometry. The results demonstrated that the apoptotic cells (early apoptosis and late apoptosis) accounted for 23.4%±2.2%, 33.2 %±2.8 % and 51.7%±3.2% of the cells with UV-Tianjin treatment for 24 h at MOI 20, 40 and 80, respectively (Figure 2). Thus, our results showed that UV-Tianjin could effectively initiate apoptosis in LN229 cells.
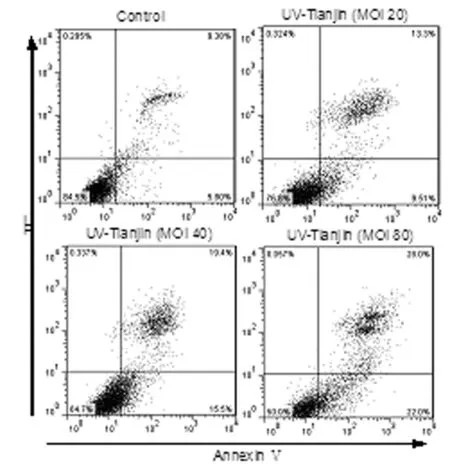
Annexin V-FITC / PI double staining was performed to determine the apoptotic rate following treatment with different doses of UV-Tianjin for 24 h. The lower right quadrant (annexin V+/PI-) represents early apoptosis, and the upper right quadrant (annexin V+/PI+) represents late apoptosis and necrosis.
Fig.2 UV-Tianjin-induced apoptosis in LN229 cells
UV-Tianjin-induced apoptosis in LN229 cells is caspase dependent
To investigate the possible mechanism underlying UV-Tianjin-induced apoptosis in LN229 cells, caspase-3, -8 and -9 activities were assayed using a colorimetric method. The results showed that the caspase-3, -8 and -9 activities increased in a dose-dependent manner following UV-Tianjin treatment for 24 h (Figure 3,*P<0.05). Caspase-8 and caspase-9 were essential proteases of extrinsic and intrinsic apoptotic pathways, caspase-3 acts as downstream effectors of both these pathways. Taken together, our data suggested that UV-Tianjin-induced apoptosis was caspase dependent and might involve in both the endogenous mitochondrial pathway and the exogenous death receptor pathway.
Both mitochondrial pathway and death receptor pathway play a role in UV-Tianjin-induced apoptosis in LN229 cells To investigate the involvement of mitochondrial pathway in UV-Tianjin-induced apoptosis, we examined the expression levels of Bax, Bcl-2, cytc, pro-caspase-9 and -3 inside the cytoplasm by western blotting. It was found that when the doses of
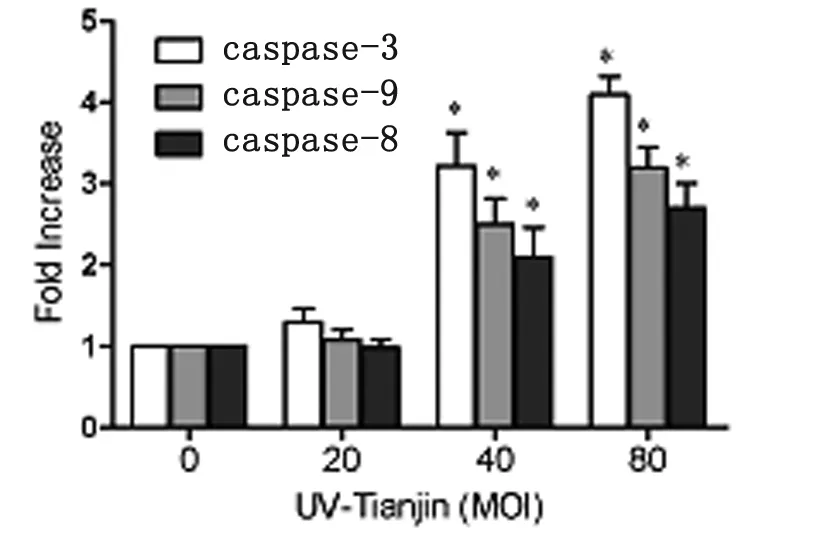
The data are representative of three independent experiments.*P<0.05 compared with control.
Fig.3 Effects of UV-Tianjin on caspase activities in LN229 cells after UV-Tianjin treatment using a colorimetric method
UV-Tianjin were increased, the level of Bax and cytcwas upregulated, while the expression of Bcl-2, pro-caspase-9 and -3 proteins was downregulated (Fig.4).
Following this, to confirm the involvement of the death receptor pathway in UV-Tianjin-induced apoptosis, we examined the effect of UV-Tianjin on the expression levels of Fas, FasL and downstream pro-caspase-8. Results demonstrated that a visible increase in Fas, FasL, and decrease in pro-caspase-8 was observed after UV-Tianjin treatment, as compared with control cells (Figure 4).
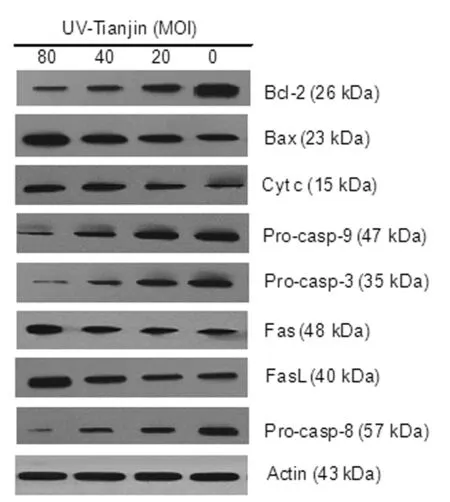
Fig.4 Effects of UV-Tianjin on the expression levels of apoptosis-associated proteins
Discussion
Sendai virus strain Tianjin was a novel genotype of Sendai virus. In the present study, we found that Tianjin strain significantly inhibited the proliferation of human glioma cell line LN229 in a dose-dependent manner although it has been inactivated by ultraviolet. And flow cytometric analysis confirmed that UV-Tianjin-mediated anti-proliferation effect resulted from apoptosis. We speculate that although the virus can't normally replicate within infected cells due to the damage of nucleic acid by ultraviolet irradiation, viral antigens on the envelop still remains integrity and may mediate virus to enter the cells and then exert the role of inducing apoptosis within the cells.
Apoptosis occurs through two main pathways: the mitochondrial-mediated intrinsic and the death receptor-mediated extrinsic pathway. Both of them are dependent on caspase activation. The mitochondrial pathway is regulated by the anti-apoptotic proteins (Bcl-2, etc.) and the proapoptotic proteins (Bax, etc.). Upregulation of Bax results in its mitochondrial translocation, subsequent depolarization of mitochondrial membrane and formation of outer membrane channels, leading to cytcrelease. The release of cytccauses the activation of downstream protein of caspase family, such as caspase-9 and caspase-3, which then cleaves the corresponding substrates and ultimately leads to cell death[16-18]. Our results indicated that UV-Tianjin-induced apoptosis might be through the intrinsic pathway.
The death-receptor pathway is another important pathway of apoptosis. Death receptors belong to the tumor necrosis factor receptor (TNFR) gene superfamily. Eight members of the death receptor family have been characterized so far. The Fas/ FasL system represents one of the main apoptotic pathways. Activation of the Fas receptor by Fasl triggers a complex cascade of intracellular events that requires Fas-associated death domain (FADD) adapter protein and the formation of death-inducing signaling complex (DISC), leading to caspase-8 activation and apoptosis[19-21]. Our results showed that Fas/FasL system is involved in UV-Tianjin-induced apoptosis.
In summary, our study has demonstrated that UV-Tianjin induced apoptosis of human glioma LN229 cells, which may involve in both the endogenous mitochondrial pathway and the exogenous death receptor pathway. However, more research needs to be performed to confirm whether the two pathways play a role in UV-Tianjin-induced apoptosis. Our study may provide new theoretical basis for the development of antitumor biological agents.
[1]Felt SA, Moerdyk-Schauwecker MJ, Grdzelishvili VZ. Induction of apoptosis in pancreatic cancer cells by vesicular stomatitis virus[J]. Virology, 2015, 474: 163-173. DOI: 10.1016/j.virol.2014.10.026
[2]Ma B, Wang Y, Zhou X, et al. Synergistic suppression effect on tumor growth of hepatocellular carcinoma by combining oncolytic adenovirus carrying XAF1 with cisplatin[J]. J Cancer Res Clin Oncol, 2015, 141(3): 419-429. DOI: 10.1007/s00432-014-1835-8
[3]Koks CA, Garg AD, Ehrhardt M, et al. Newcastle disease virotherapy induces long-term survival and tumor-specific immune memory in orthotopic glioma through the induction of immunogenic cell death[J]. Int J Cancer, 2015, 136(5): E313-325. DOI: 10.1002/ijc.29202
[4]Miest TS, Cattaneo R. New viruses for cancer therapy: meeting clinical needs[J]. Nat Rev Microbiol, 2014, 12(1): 23-34. DOI: 10.1038/nrmicro3140
[5]Ilkow CS, Swift SL, Bell JC, et al. From scourge to cure: tumour-selective viral pathogenesis as a new strategy against cancer[J]. PLoS Pathog, 2014, 10(1): e1003836. DOI: 10.1371/journal.ppat.1003836
[6]Kim M. Replicating poxviruses for human cancer therapy[J]. J Microbiol, 2015, 53(4): 209-218. DOI: 10.1007/s12275-015-5041-4
[7]Jefferson A, Cadet VE, Hielscher A. The mechanisms of genetically modified vaccinia viruses for the treatment of cancer[J]. Crit Rev Oncol Hematol, 2015, pii: S1040-8428(15)00067-0. DOI: 10.1016/j.critrevonc.2015.04.001
[8]Fujihara A, Kurooka M, Miki T, et al. Intratumoral injection of inactivated Sendai virus particles elicits strong antitumor activity by enhancing local CXCL10 expression and systemic NK cell activation[J]. Cancer Immunol Immunother, 2008, 57(1): 73-84.
[9]Matsushima-Miyagi T, Hatano K, Nomura M, et al. TRAIL and Noxa are selectively upregulated in prostate cancer cells downstream of the RIG-I/MAVS signaling pathway by nonreplicating Sendai virus particles[J]. Clin Cancer Res, 2012, 18(22): 6271-6283. DOI: 10.1158/1078-0432.CCR-12-1595
[10]Zhang Q, Xu X, Yuan Y, et al. IPS-1 plays a dual function to directly induce apoptosis in murine melanoma cells by inactivated Sendai virus[J]. Int J Cancer, 2014, 134(1): 224-234. DOI: 10.1002/ijc.28340
[11]Nomura M, Shimbo T, Miyamoto Y, et al. 13-Cis retinoic acid can enhance the antitumor activity of non-replicating Sendai virus particle against neuroblastoma[J]. Cancer Sci, 2013, 104(2): 238-244. DOI: 10.1111/cas.12063
[12]Shi LY, Li M, Yuan LJ, et al. A new paramyxovirus, Tianjin strain, isolated from common cotton-eared marmoset: genome characterization and structural protein sequences analysis[J]. Arch Virol, 2008, 153(9): 1715-1723. DOI: 10.1007/s00705-008-0184-9
[13]Shi LY, Chen J, Zhong QP, et al. Inactivated Sendai virus strain Tianjin, a novel genotype of Sendai virus, inhibits growth of murine colon carcinoma through inducing immune responses and apoptosis[J]. J Transl Med, 2013, 11: 205.DOI: 10.1186/1479-5876-11-205
[14]McKean-Cowdin R, Razavi P, Barrington-Trimis J, et al. Trends in childhood brain tumor incidence, 1973-2009[J]. J Neurooncol, 2013, 115(2): 153-160. DOI: 10.1007/s11060-013-1212-5
[15]Kaneda Y, Nakajima T, Nishikawa T, et al. Hemagglutinating virus of Japan (HVJ) envelope vector as a versatile gene delivery system[J]. Mol Ther, 2002, 6(2): 219-226.
[16]Bi MC, Rosen R, Zha RY, et al. Zeaxanthin induces apoptosis in human uveal melanoma cells through Bcl-2 family proteins and intrinsic apoptosis pathway[J]. Evid Based Complement Alternat Med, 2013, 2013: 205082. DOI: 10.1155/2013/205082
[17]Strasser A, Cory S, Adams JM. Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases[J]. EMBO J, 2011, 30(18): 3667-3683. DOI: 10.1038/emboj.2011.307
[18]Li P, Nijhawan D, Budihardjo I, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade[J]. Cell, 1997, 91(4): 479-489.
[19]Gu TW, Bae WY, Park HT, et al. Expression profile of fas-fas ligand in spiral ganglion cells during apoptosis[J]. Clin Exp Otorhinolaryngol, 2014, 7(1): 1-6. DOI: 10.3342/ceo.2014.7.1.1
[20]Nagata S, Golstein P. The Fas death factor[J]. Science, 1995, 267(5203): 1449-1456.
[21]Kruidering M, Evan GI. Caspase-8 in?apoptosis: the beginning of “the end”?[J]. IUBMB Life, 2000, 50(2): 85-90.
Received:2014-08-19;Revision accepted:2015-05-09
紫外線滅活仙臺病毒誘導人膠質瘤細胞系LN229凋亡作用
石立瑩,李 梅,張 磊,耿 鵬,李詠梅
目的 探討紫外線滅活仙臺病毒致人膠質瘤LN229細胞凋亡作用及其機制。方法 用不同劑量滅活病毒感染LN229細胞,24 h后,MTT法檢測滅活病毒對細胞增殖的影響;流式細胞儀檢測細胞凋亡情況;分光光度法檢測caspase活性;Western blotting檢測凋亡相關蛋白的表達水平。結果 MTT檢測顯示滅活仙臺病毒能夠劑量依賴性抑制LN229細胞增殖;流式細胞儀檢測顯示滅活病毒組細胞凋亡率呈劑量依賴性升高;caspase活性測定顯示滅活病毒組細胞中caspase-3, -8和-9蛋白活性呈劑量依賴性增加;Western blotting結果顯示,隨著滅活病毒滴度增加,細胞中Bax、細胞色素c、Fas、FasL表達水平升高、而Bcl-2、caspase-8前體、caspase-9前體和caspase-3前體蛋白表達下降。結論 滅活仙臺病毒能夠誘導LN229細胞劑量依賴性凋亡,其機制與線粒體途徑和死亡受體途徑相關。
仙臺病毒;人膠質瘤細胞系LN229;凋亡;線粒體途徑;死亡受體途徑
R373.1
A
1002-2694(2015)07-0597-05
天津醫科大學基礎醫學院微生物學教研室,天津 300070; Email: sly.good@126.com
10.3969/cjz.j.issn.1002-2694.2015.07.001
國家自然科學基金面上項目(No.81172168)


