吡喃花色苷類衍生物家族的研究進(jìn)展
何靜仁,鄺敏杰,齊敏玉,劉 剛,李書藝,吳 鬧,祝振洲,郭 瑩,梁 征
(1.武漢輕工大學(xué)食品科學(xué)與工程學(xué)院,湖北省農(nóng)產(chǎn)品加工與轉(zhuǎn)化重點(diǎn)實(shí)驗(yàn)室,湖北 武漢 430023;2.武漢輕工大學(xué),農(nóng)產(chǎn)品加工湖北省協(xié)同創(chuàng)新中心,湖北 武漢 430023)
?
吡喃花色苷類衍生物家族的研究進(jìn)展
何靜仁1,2,鄺敏杰1,齊敏玉1,劉 剛1,2,李書藝1,吳 鬧1,祝振洲1,郭 瑩1,梁 征1
(1.武漢輕工大學(xué)食品科學(xué)與工程學(xué)院,湖北省農(nóng)產(chǎn)品加工與轉(zhuǎn)化重點(diǎn)實(shí)驗(yàn)室,湖北 武漢 430023;2.武漢輕工大學(xué),農(nóng)產(chǎn)品加工湖北省協(xié)同創(chuàng)新中心,湖北 武漢 430023)
摘 要:吡喃花色苷是近些年發(fā)現(xiàn)于果酒(如紅葡萄酒)中的新型花色苷衍生物,是酒體最重要的呈色物質(zhì)之一。吡喃花色苷家族具有第四個(gè)吡喃環(huán)的基本特征,是在發(fā)酵、陳釀過(guò)程中由漿果花色苷與葡萄糖發(fā)酵代謝的中間產(chǎn)物及(或)漿果中其他酚類成分經(jīng)環(huán)加合反應(yīng)形成的一系列天然色素物質(zhì)。本文將系統(tǒng)介紹吡喃花色苷家族及其第二代衍生物家族的種類、分子結(jié)構(gòu)、形成機(jī)制、穩(wěn)定性與色度特征、抗氧化及抗腫瘤等生物活性。多種不同類型的吡喃花色苷家族不僅是重要呈色物質(zhì),而且具有較高的穩(wěn)定性、良好的色澤特征及較強(qiáng)的功能活性,本文為進(jìn)一步開展吡喃花色苷類衍生物的結(jié)構(gòu)與其穩(wěn)定性、色澤和功能性質(zhì)關(guān)系的研究及其在葡萄酒產(chǎn)業(yè)和食品加工業(yè)中的應(yīng)用提供有益的參考。
關(guān)鍵詞:花色苷;吡喃花色苷家族;第二代花色苷衍生物;陳釀果酒
花色苷(anthocyanins)是一類廣泛存在于果蔬類植物中的水溶性天然色素,屬類黃酮多酚化合物,且是紅葡萄酒或櫻桃、草莓等發(fā)酵果酒中最重要的呈色物質(zhì),具有重要生物活性和生理功能。在發(fā)酵和陳釀過(guò)程中,酒體顏色的逐步變化主要是由漿果花色苷與葡萄糖發(fā)酵代謝的中間產(chǎn)物及漿果中的其他多酚類成分發(fā)生環(huán)化、加成或濃縮、聚合等多種反應(yīng),形成更為穩(wěn)定的呈色物質(zhì)(花色苷衍生物)造成的[1]。不同類型、不同色調(diào)的花色苷衍生物家族的形成對(duì)葡萄酒體顏色的重要性已越來(lái)越引起葡萄與葡萄酒產(chǎn)業(yè)科研人員的興趣和重視。
吡喃花色苷[2](pyranoanthocyanins)是葡萄酒體重要呈色物質(zhì)中的一種新型花色苷衍生物,它的基本結(jié)構(gòu)是在原花色苷結(jié)構(gòu)的基礎(chǔ)上,在花色苷的C4位與C5位的羥基之間經(jīng)環(huán)加合反應(yīng)形成另外的第四個(gè)吡喃環(huán)D[3-4]。到目前為止,在葡萄酒類發(fā)酵果酒或果汁飲料中已分離和鑒定了許多家族的吡喃花色苷類化合物。不同種類吡喃花色苷家族及其第二代衍生物鑒定方法、呈色特征和光譜特征等如表1所示,主要有Vitisins型吡喃花色苷、甲基吡喃花色苷、酚基吡喃花色苷、黃烷醇-吡喃花色苷、Portisins型吡喃花色苷、吡喃花色苷二聚體和Oxovitisins型吡喃花色苷。吡喃花色苷與花色苷在許多理化性質(zhì)方面存在差異,相比于紅色的花色苷,羧基吡喃花色苷、酚基吡喃花色苷、黃烷醇-吡喃花色苷等的最大吸收波長(zhǎng)發(fā)生部分藍(lán)移[5-6],它們的顏色多為橙紅色,其中甲基吡喃花色苷吸收波長(zhǎng)偏移較大,顯示為橙黃色;而第二代吡喃花色苷衍生物的最大吸收波長(zhǎng)則發(fā)生紅移,大部分顯示更深的藍(lán)色[7-9]。
國(guó)內(nèi)在涉及花色苷衍生物方面的研究還相對(duì)較少,可能與我國(guó)葡萄酒工業(yè)發(fā)展的歷史背景及葡萄酒化學(xué)的研究現(xiàn)狀有關(guān)。由于吡喃花色苷類衍生物具有較高的穩(wěn)定性及良好的色澤特性,因而它對(duì)天然花色苷本身存在的結(jié)構(gòu)穩(wěn)定性問(wèn)題研究及其發(fā)展應(yīng)用于葡萄酒類果酒加工業(yè)等均具有重要意義。本文結(jié)合作者在國(guó)外對(duì)葡萄酒化學(xué)研究領(lǐng)域中葡萄酒色素的多年研究工作積累,重點(diǎn)闡述吡喃花色苷衍生物家族的種類、結(jié)構(gòu)表征、形成機(jī)制、理化性質(zhì)及功能性質(zhì),為花色苷的反應(yīng)活性研究以提高其在食品與農(nóng)產(chǎn)品加工、貯藏和應(yīng)用中色澤或功能的穩(wěn)定性提供參考依據(jù)。
1 吡喃花色苷及第二代花色苷衍生物家族的形成機(jī)制
1.1吡喃花色苷的形成機(jī)制
在葡萄酒釀造過(guò)程中,漿果花色苷與糖代謝的許多中間產(chǎn)物(如丙酮酸、乙醛、乙酰乙酸)發(fā)生一系列的反應(yīng)生成許多吡喃花色苷[5,10-11]。羧基吡喃花色苷是其中最重要的一類,由烯醇化的丙酮酸與花色苷反應(yīng)生成[5],Vitisin B是由乙醛和錦葵花色苷形成的化學(xué)加合物,甲基吡喃花色苷則是由漿果花色苷和酵母發(fā)酵的代謝產(chǎn)物乙酰乙酸反應(yīng)形成。它們的形成機(jī)制相似,以羧基吡喃花色苷的形成機(jī)制為例(圖1),在一定的酸性條件下,丙酮酸的羰基可烯醇化,隨后具有電負(fù)性的甲基與花色苷的C4位(具正電性)發(fā)生加成縮合,而后經(jīng)脫水和氧化形成另外的一個(gè)吡喃環(huán)[12]。甲基吡喃花色苷的不同之處則是要再經(jīng)過(guò)脫羧才能形成。
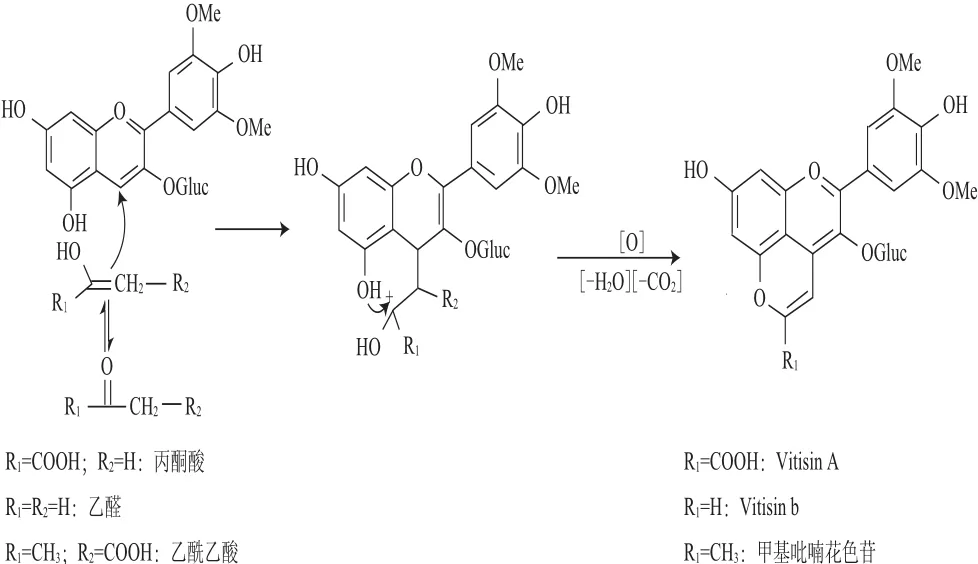
圖1 Vitisins型吡喃花色苷及甲基吡喃花色苷的形成機(jī)制Fig.1 Proposed formation mechanisms of vitisin-type pyranoanthocyanins and methyl pyranoanthocyanins
酚基吡喃花色苷[13]是在1996年從紅酒中經(jīng)錯(cuò)流微濾聚合膜分離得到的最早被確認(rèn)的第一個(gè)吡喃花色苷,并通過(guò)模擬實(shí)驗(yàn)表明該化合物是葡萄錦葵花色苷與乙烯酚的反應(yīng)產(chǎn)物,而乙烯酚是香豆酸的脫羧產(chǎn)物,可通過(guò)釀酒酵母的肉桂酸脫羧酶形成[14]。黃烷醇-吡喃花色苷是葡萄酒中發(fā)現(xiàn)的另一家族吡喃花色苷,F(xiàn)rancia-Aricha等[6]于
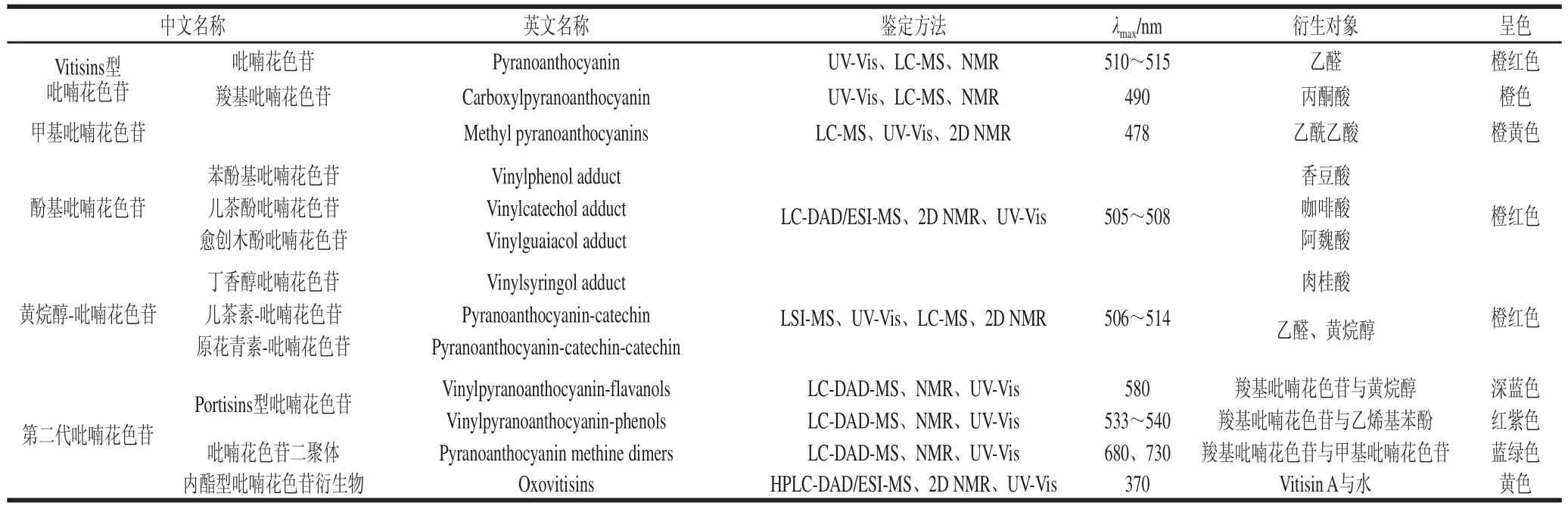
表1 吡喃花色苷家族的類型、鑒定方法、衍生對(duì)象、光譜表征及呈色特征Table 1 Classification and identification, derived object, spectral and chromatic properties of pyranoanthocyanins
1997年首次在模擬酒溶液中發(fā)現(xiàn)花色苷與兒茶素、表兒茶素、原花青素B2的環(huán)加合反應(yīng)產(chǎn)物。黃烷醇-吡喃花色苷衍生物在酒中的形成來(lái)源于花色苷與乙烯基黃烷醇的環(huán)加合反應(yīng)[15-16],而乙烯基黃烷醇可能來(lái)自于已被證實(shí)存在于酒中的黃烷醇-烷基-黃烷醇聚合物的斷裂,亦可能來(lái)自于黃烷醇和乙醛在酸性條件下加成并脫水反應(yīng)形成的中間產(chǎn)物[17]。酚基吡喃花色苷與黃烷醇-吡喃花色苷衍生物的形成機(jī)制相似(如圖2所示,以黃烷醇-吡喃花色苷為例),乙烯酚上的乙烯基與錦葵花色苷的C4位和C5位上的羥基之間發(fā)生環(huán)加合反應(yīng),隨后經(jīng)氧化生成第四個(gè)芳香環(huán)[14]。

圖2 黃烷醇-吡喃花色苷的形成過(guò)程Fig.2 Proposed formation mechanism of pyranoanthocyanin-flavanols
1.2第二代吡喃花色苷
第一代吡喃花色苷是在果酒發(fā)酵陳釀過(guò)程中由花色苷與小分子反應(yīng)衍生形成,如Vitisins型吡喃花色苷、酚基吡喃花色苷和黃烷醇-吡喃花色苷,而在陳釀后期,由花色苷形成的第一代吡喃花色苷會(huì)繼續(xù)與小分子反應(yīng)生成更為復(fù)雜的衍生物,這些復(fù)雜的衍生物稱為第二代吡喃花色苷衍生物,如Portisins型吡喃花色苷、吡喃花色苷二聚體和Oxovitisins型吡喃花色苷。
1.2.1 Portisins型吡喃花色苷衍生物
Mateus等[18]于2003年在Port葡萄酒中發(fā)現(xiàn)了一類新的吡喃花色苷的衍生物家族并命名為Portisins。Portisins型吡喃花色苷又可分為兩種類型:乙烯黃烷醇基-吡喃花色苷(Portisins A)和乙烯酚基-吡喃花色苷(Portisins B),它們的結(jié)構(gòu)是吡喃花色苷單體與黃烷醇或羥基酚中間以乙烯鍵橋連接組成的[19]。Portisins A是由羧基吡喃花色苷(如Vitisin A)與乙烯基黃烷醇反應(yīng)形成的,后者可能是由中間以乙基鍵橋連接的黃烷醇寡聚物裂解形成或者由乙基鍵連接的花色苷-黃烷醇的衍生物裂解形成的,這是首次發(fā)現(xiàn)葡萄酒中花色苷和黃烷醇單體不直接充當(dāng)前體物質(zhì)的反應(yīng),其形成機(jī)理如圖3所示。烯醇化的乙烯基黃烷醇與Vitisin A上的C10發(fā)生親核反應(yīng),經(jīng)去甲酸基、氧化形成乙烯基鍵連接的Portisins A衍生物[20]。通過(guò)LC-DAD-MS和NMR分析可以得出Portisins的結(jié)構(gòu)特征[20](表1),這種色素具有獨(dú)特的光譜學(xué)特征,它的最大吸收波長(zhǎng)(λmax在580 nm左右)與其他花色苷相比發(fā)生紅移,在酸性環(huán)境下呈現(xiàn)藍(lán)色[21]。
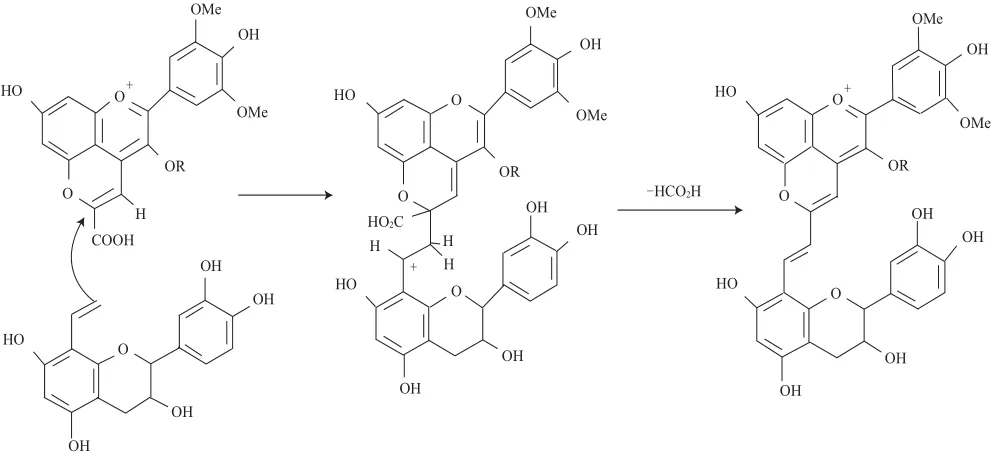
圖3 Portisins A型吡喃花色苷在陳釀Port紅酒中的形成機(jī)制Fig.3 Formation of vinylpyranoanthocyanin-flavanols (portisins A) in aged Port red wine
Portisins B是在陳釀Port葡萄酒中發(fā)現(xiàn)的另一種類型的吡喃花色苷衍生物,其結(jié)構(gòu)和形成機(jī)制見(jiàn)圖4,它們是由羧基吡喃花色苷與乙烯基苯酚或葡萄中的羥基肉桂酸類化合物經(jīng)多步反應(yīng)縮合而成,與Portisins A型形成機(jī)制相似,不同的是最后脫去羧基[22]。Portisins B型的呈色特征與Portisins A型不同,其最大可見(jiàn)吸收波長(zhǎng)在533~540 nm,呈現(xiàn)紅紫色[22]。Portisins B型色素溶液(酸性條件下)在結(jié)冰過(guò)程中能呈現(xiàn)出特殊的從紅色到藍(lán)色的改變,這可能是由于它的電子及振動(dòng)特性使其發(fā)生可逆的物理與化學(xué)之間的轉(zhuǎn)變[23]。
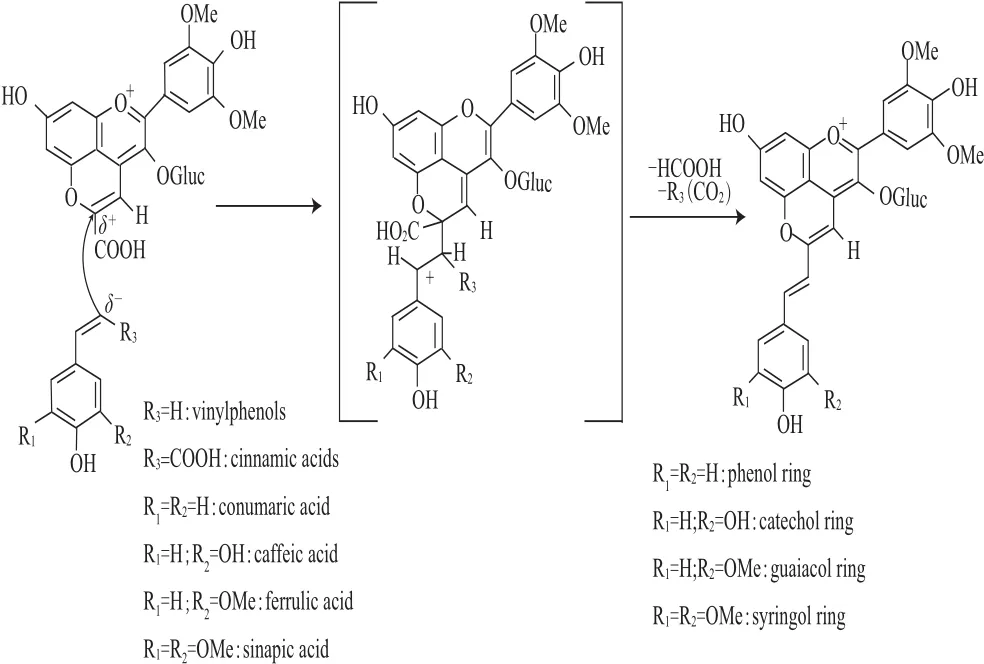
圖4 Portisins B型吡喃花色苷在陳釀Port紅酒中的形成機(jī)制Fig.4 Formation of vinylpyranoanthocyaninphenols (portisins B) in aged Port red wine
1.2.2 吡喃花色苷二聚體
Oliveira等[7]于2010年在Port紅葡萄酒及其酒渣中發(fā)現(xiàn)了一種新的吡喃花色苷衍生物,即吡喃花色苷二聚體。這種色素的結(jié)構(gòu)是兩個(gè)吡喃花色苷單體之間以甲基-次甲基鍵橋連接構(gòu)成,經(jīng)LC-DAD-MS和1H和13C NMR確認(rèn)了吡喃花色苷二聚體的正確結(jié)構(gòu),其最大可見(jiàn)吸收波長(zhǎng)在680、730 nm附近,分子離子峰m/z為1 059,主要碎片離子峰的m/z為897和735(糖苷配基),在酸性條件下呈現(xiàn)天藍(lán)色[24]。吡喃花色苷二聚體在葡萄酒中的形成來(lái)源于羧基吡喃花色苷和甲基吡喃花色苷的反應(yīng),其形成機(jī)制有兩種途徑(圖5):1)甲基吡喃花色苷上的甲基經(jīng)去質(zhì)子化在C10位形成以雙鍵連 接的亞甲基結(jié)構(gòu),然后對(duì)羧基吡喃花色苷上的C10位進(jìn)行親核攻擊,再經(jīng)去甲酸基形成以甲基-次甲基鍵橋連接的吡喃花色苷二聚體。2)甲基吡喃花色苷與羧基吡喃花色苷的芳香環(huán)之間經(jīng)復(fù)雜的電子轉(zhuǎn)移形成穩(wěn)定的π-π共軛體系,隨后經(jīng)離子或自由基反應(yīng)在兩者之間形成甲基-次甲基鍵橋,最后經(jīng)去甲酸基形成吡喃花色苷二聚體。研究表明第2種反應(yīng)機(jī)制更可能發(fā)生,因?yàn)榻?jīng)NMR測(cè)定甲基吡喃花色苷中的質(zhì)子轉(zhuǎn)移反應(yīng)發(fā)生在pH>11的溶液中而非pH≈3.6的葡萄酒中[7]。
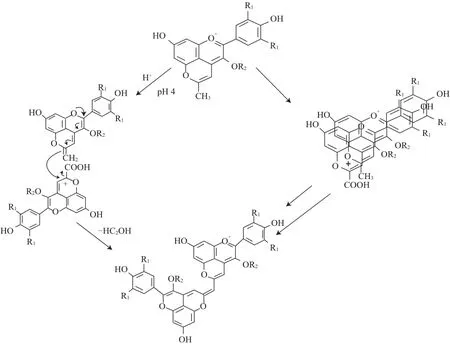
圖5 吡喃花色苷二聚體在陳釀Port酒中的兩種形成機(jī)制Fig.5 Two putative pathways for the formation of pyranoanthocyanin dimers in aged Port red wine
1.2.3 吡喃花色苷衍生物Oxovitisins
作者與所在團(tuán)隊(duì)于2010年在陳釀的葡萄酒中首次發(fā)現(xiàn)了一類新的吡喃花色苷的衍生物家族并命名為Oxovitisins[25],經(jīng)LC-DAD-MS和1H和13C NMR對(duì)其結(jié)構(gòu)進(jìn)行鑒定,確認(rèn)了Oxovitisins為具有2-吡喃酮結(jié)構(gòu)(內(nèi)酯型)的非氧鎓離子衍生物,并證實(shí)了該類化合物由羧基吡喃花色苷經(jīng)水合和微氧化反應(yīng)形成的機(jī)制。這是由紅色花色苷(鎓鹽)衍生轉(zhuǎn)化而成非氧鎓離子的中性化合物的首次報(bào)道。Oxovitisins最大可見(jiàn)吸收波長(zhǎng)在酸性條件下為373 nm(pH 2),連同黃酮類可以代表一類對(duì)陳釀葡萄酒中黃色色調(diào)具有重要貢獻(xiàn)的化合物[26]。Oxovitisins在葡萄酒中的形成來(lái)源于Vitisin A與水的反應(yīng),其機(jī)制見(jiàn)圖6,首先水對(duì)Vitisin A上的C 10進(jìn)行親核攻擊形成半縮醛形式,隨后經(jīng)脫羧、氧化和脫水,生成一個(gè)具有吡喃酮結(jié)構(gòu)的花色苷衍生物。該親核攻擊發(fā)生的很緩慢,是由Vitisins碳正離子不可逆形成中性吡喃花色苷的第一步[27]。Oxovitisins的形成機(jī)制為以羧基吡喃花色苷為前體物質(zhì)形成其他衍生物色素提供了新的途徑。
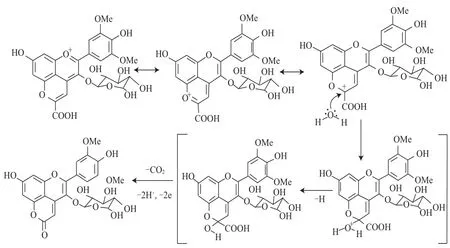
圖6 Oxovitisins的形成機(jī)理Fig.6 Proposed mechanisms for the formation of pyranoneanthocyanins (oxovitisins)
2 吡喃花色苷的理化性質(zhì)
2.1吡喃花色苷的穩(wěn)定性
由吡喃花色苷衍生物的結(jié)構(gòu)特征可知,它們具有比原花色苷更穩(wěn)定的特性。針對(duì)吡喃花色苷類衍生物的穩(wěn)定性研究已有報(bào)道,作者研究了pH值、SO2和貯藏時(shí)間對(duì)黃烷醇-吡喃花色苷穩(wěn)定性的影響[28],結(jié)果表明:相同pH值條件下,黃烷醇-吡喃花色苷的穩(wěn)定性比原花色苷高(圖7),且對(duì)SO2有很強(qiáng)的抵抗作用(圖8),在貯藏6個(gè)月后,黃烷醇-吡喃花色苷的穩(wěn)定性比原花色苷要高很多。Bakker等[5]證實(shí)了Vitis ins類型花色苷衍生物比花色苷具有更強(qiáng)的抵抗SO2漂白的作用,并且在弱酸性至中性pH值環(huán)境下表現(xiàn)出比花色苷類色素更深的顏色。另外,Oliveira等[9]針對(duì)pH值和SO2等因素對(duì)Portisins的影響進(jìn)行了研究,結(jié)果證明它具有很強(qiáng)的抵抗水和酸性亞硫酸鹽親核攻擊的能力,特別是與錦葵花色苷相比,在不同pH值溶液中具有很強(qiáng)的色澤穩(wěn)定性,在pH 3.6和pH 1.0的溶液中色澤幾乎相同。另外,錦葵花色苷在弱酸性或中性條件下色度明顯降低,這是因?yàn)榘l(fā)生水化平衡,使花色苷形成無(wú)色的半縮醛結(jié)構(gòu)[29-30],而吡喃花色苷能抵抗水的親核攻擊避免形成無(wú)色的半縮醛結(jié)構(gòu)[27]。在果酒的發(fā)酵或陳釀過(guò)程中,原花色苷的含量逐漸降低,形成更加穩(wěn)定的衍生色素和聚合色素,并與其他酚類成分一起賦予酒體持久而穩(wěn)定的顏色。
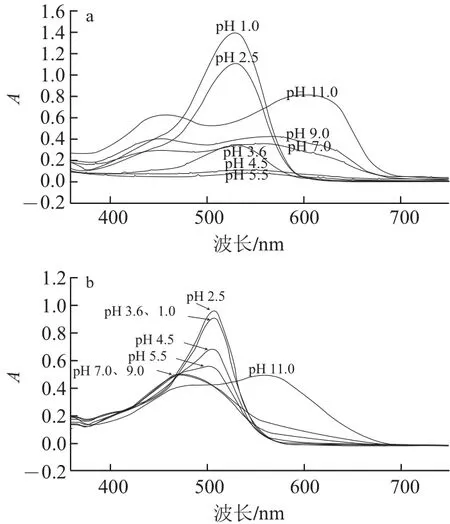
圖7 錦葵花色苷與兒茶素-吡喃錦葵花色苷在不同pH值(1.0~11.0)條件下的紫外-可見(jiàn)吸收光譜Fig. 7 Absorption spectra of malvidin-3-glucoside (Mv) and pyranoMv-(+)-catechin at different pH values (1.0–11.0)
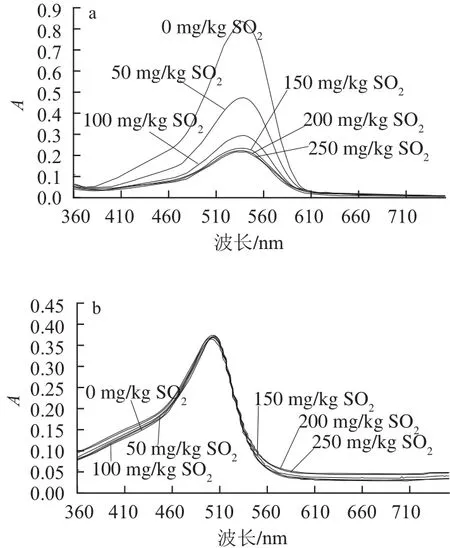
圖8 錦葵花色苷和兒茶素-吡喃錦葵花色苷在不同SO2含量模擬酒溶液(pH 3.6)中的紫外-可見(jiàn)吸收光譜Fig.8 Effect of bisulfite on the visible spectra of malvidin-3-glucoside (Mv) and pyranoMv-(+)-catechin in model wine (pH 3.6)
2.2吡喃花色苷的色度特征
吡喃花色苷對(duì)葡萄酒釀造中酒體顏色的改變起著很重要的作用,大部分吡喃花色苷比原花色苷更顯橙紅色調(diào),因此使酒體的顏色發(fā)生從新釀酒的紫紅色到陳釀酒的磚紅色轉(zhuǎn)變[31]。在葡萄酒溶液中吡喃花色苷的色澤穩(wěn)定性比原花色苷要高很多,當(dāng)溶液的pH值從1.5增加到3.6時(shí),錦葵花色苷的摩爾消光系數(shù)有大幅度降低,而吡喃花色苷衍生物的摩爾消光系數(shù)則變化很小或者有小幅度升高[28,32](表2)。有研究表明寡聚黃烷醇-吡喃花色苷衍生物在pH 3.6的酒體中最大吸光度比在pH 1.0時(shí)要高30%~50%,這種在弱酸性條件下的增色效應(yīng)是黃烷醇-吡喃花色苷分子內(nèi)輔助成色使其摩爾消光系數(shù)增大造成的,對(duì)酒體色澤起重要作用[28]。
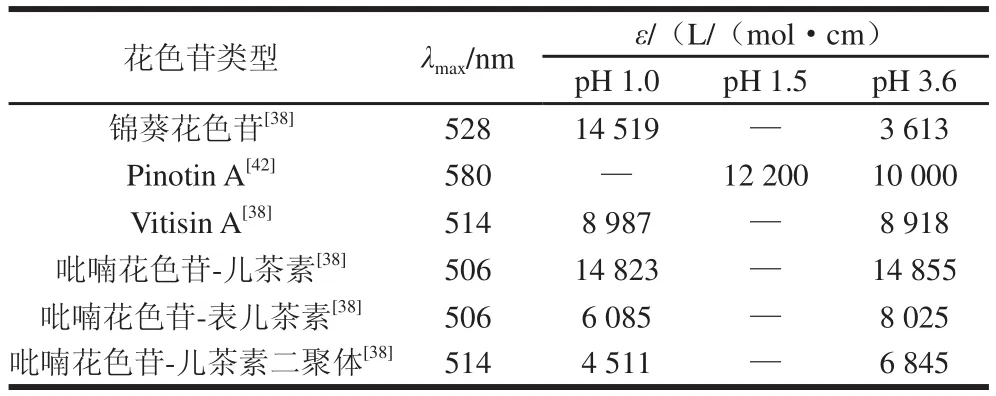
表2 模擬葡萄酒溶液酸性pH值條件下錦葵花色苷及吡喃花色苷的最大吸收波長(zhǎng)及摩爾消光系數(shù)Table 2 Wavelengths of maximum absorbance (λmax) and molar extinction coefficients (ε) for malvidin 3-glucoside and pyranoanthocyanins in model wine with acidic pH
吡喃花色苷衍生物比原花色苷具有抗變色特性的最主要原因是在它們的分子結(jié)構(gòu)C4位上增加了吡喃環(huán),可以保護(hù)分子不受水的親核攻擊(被水親核攻擊的位置一般在C2和C4位)。在不同pH值水溶液中吡喃花色苷經(jīng)質(zhì)子轉(zhuǎn)移反應(yīng)能快速達(dá)到平衡形成醌式堿形式,pH值在3~12之間的酸堿平衡能形成3 種醌式堿形式(以黃烷醇-吡喃花色苷為例,如圖9所示):中性A、陰離子A-、二價(jià)陰離子A2-,有 研究表明不同的吡喃花色苷酸度系數(shù)pKa值:pKa1為4.20~5.35;pKa2為7.82~8.34;pKa3為9.49~10.28[27,33]。含有酰基化基團(tuán)的吡喃花色苷的pKa值比非酰基化的稍微高,可能是由于酰基化基團(tuán)形成的空間位阻使質(zhì)子很難轉(zhuǎn)移造成的。
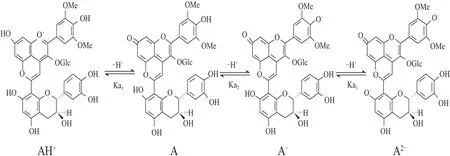
圖9 pH 3~12的水溶液中黃烷醇-吡喃花色苷的平衡形式Fig.9 Equilibrium forms of pyranoanthocyanin and flavanols in aqueous solutions in the pH range of 3–12
3 吡喃花色苷的功能活性
隨著吡喃花色苷類衍生物的發(fā)現(xiàn),它們的功能活性研究也相繼被報(bào)道。表3總結(jié)了幾種吡喃花色苷類衍生物在清除自由基、抗氧化、抗乳腺癌和抗腫瘤方面的生物活性及其功能評(píng)價(jià)方法與體系。Gou py等[34]研究了酚基吡喃花色苷和Vitisin A對(duì)1,1-二苯基-2-三硝基苯肼(1,1-diphenyl-2-picrylhyd razyl,DPPH)自由基的清除能力和抑制亞鐵血紅素誘導(dǎo)亞油酸過(guò)氧化反應(yīng),結(jié)果表明:Vitisin A和由錦葵花色苷形成的酚基吡 喃花色苷對(duì)DPPH自由基的清除能力都高于原錦葵花色苷,而由矢車菊花色苷形成的酚基吡喃花色苷對(duì)DPPH自由基的清除能力則低于原矢車菊花色苷,酚基吡喃花色苷對(duì)亞油酸過(guò)氧化反應(yīng)的 抑制作用高于原花色苷,而Vitisin A則略低于原花色苷。采用鐵離子還原法(ferric reducing antioxidant power,F(xiàn)RAP)、總抗氧化能力(trolox equivalent antioxidant capacity,TEAC)法和氧自由基吸收能力(oxygen radical absorbance capacity,ORAC)法對(duì)Vitisin A的抗 氧化活性測(cè)定,結(jié)果表明Vitisin A具有很強(qiáng)的抗氧化能力[35-38],采用電子自旋共振波譜法對(duì)Vitisin A的抗氧化活性進(jìn)行測(cè)定,結(jié)果表明其對(duì)超氧陰離子自由基有很強(qiáng)的清除能力,而對(duì)羥自由基不起作用[39]。另外,Garcia-Alonso等[36]采用酶聯(lián)免疫吸附法研究了Vitisin A對(duì)腫瘤壞死因子的抑制作用,結(jié)果表明Vitisin A在一定程度上能抑制腫瘤。有研究采用DPPH法和FRAP法測(cè)定Portisins的抗氧化活性及Portisins對(duì)大豆脂質(zhì)過(guò)氧化的抑制作用[38,40],發(fā)現(xiàn)Portisins具有很強(qiáng)的清除DPPH自由基及還原鐵離子的能力,而由飛燕草花色苷形成的Portisins的抗氧化能力略小于原飛燕草花色苷,由錦葵花色苷形成的Portisins的鐵離子還原能力最強(qiáng),Portisins具有很好的抵制大豆脂質(zhì)過(guò)氧化反應(yīng)的效果,特別是由矢車菊花色苷形成的Portisins型吡喃花色苷。采用電子順磁共振光譜法對(duì)Portisins進(jìn)行測(cè)定,結(jié)果表明Portisins具有很強(qiáng)的抗氧化能力[41]。另有研究表明Portisins具有抵抗人乳腺癌細(xì)胞增殖的作用[42]。
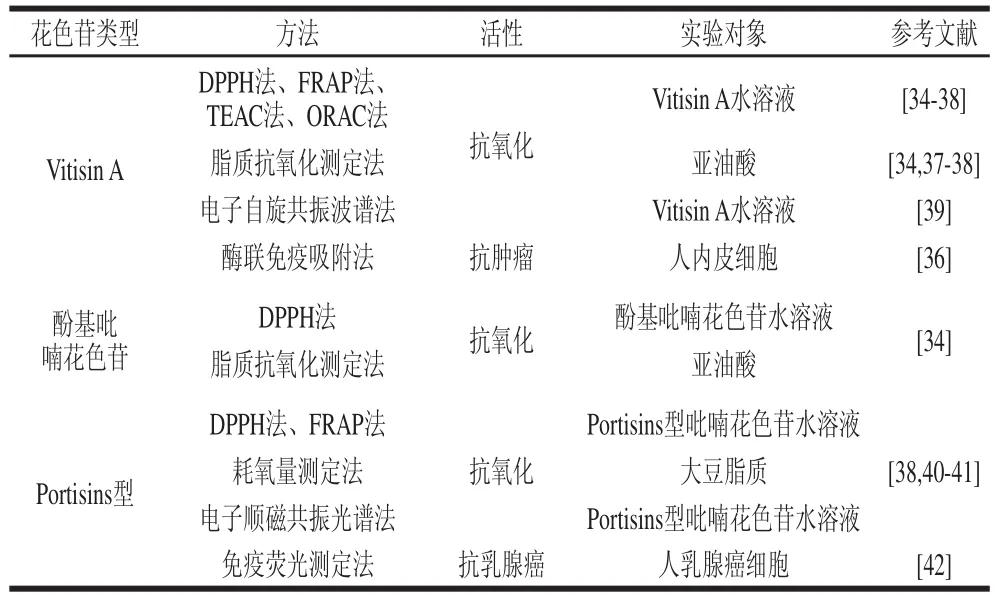
表3 不同吡喃花色苷功能活性的測(cè)定方法及實(shí)驗(yàn)對(duì)象Table 3 Methods and test objects for functional ac tivity assay of different pyranoanthocyanins
4 結(jié) 語(yǔ)
吡喃花色苷類衍生物是紅葡萄酒中最重要的呈色物質(zhì),明確它們的形成機(jī)理、呈色特征、理化穩(wěn)定性及生物活性和生理功能對(duì)穩(wěn)定紅葡萄酒色澤有關(guān)的果酒釀造工藝技術(shù)研究,以及深入理解果酒陳釀過(guò)程中化學(xué)成分的變化與酒體色澤的關(guān)系及陳釀果酒的健康作用具有重要意義。花色苷可與果酒釀造中糖代謝的許多中間產(chǎn)物以及漿果中含有的其他酚類物質(zhì)(如羥基肉桂酸類,黃烷醇類等)反應(yīng)形成不同家族的吡喃花色苷類衍生物。由于大部分吡喃花色苷是在酵母發(fā)酵過(guò)程中形成的,因此,酵母的種類及菌株、溫度、pH值、發(fā)酵過(guò)程中SO2的濃度等因素對(duì)吡喃花色苷的形成有很大的影響。盡管在葡萄酒中檢測(cè)到的一些吡喃花色苷類色素是微量的,但由于其家族結(jié)構(gòu)多樣性及獨(dú)特的光譜特性,在一定條件下它們對(duì)葡萄酒整體色澤同樣起到至關(guān)重要的作用。
相對(duì)于花色苷,吡喃花色苷衍生物具有更高的穩(wěn)定性、良好的色澤特征及較強(qiáng)的抗氧化活性,作為天然著色劑和膳食功能成分在食品行業(yè)具有廣闊應(yīng)用前景。然而,對(duì)吡喃花色苷衍生物的發(fā)現(xiàn)和結(jié)構(gòu)確認(rèn)及其形成機(jī)制的認(rèn)識(shí)較晚,有關(guān)吡喃花色苷的高效制備、理化性質(zhì)、功能評(píng)價(jià)及其在食品與農(nóng)產(chǎn)品加工中應(yīng)用的相關(guān)研究還很缺乏,隨著色譜、質(zhì)譜和光譜技術(shù)及其他分析程序的建立,吡喃花色苷類衍生物的相關(guān)評(píng)價(jià)數(shù)據(jù)將更加充實(shí)。作為一類特殊的多酚類化合物,吡喃花色苷類衍生物與葡萄酒類果酒中的其他成分對(duì)葡萄酒口感、風(fēng)味的影響還有待探索,這是深入認(rèn)識(shí)花色苷的化學(xué)活性和進(jìn)一步研究花色苷衍生物的變化及其對(duì)陳釀果酒品質(zhì)影響 的主要方向。
參考文獻(xiàn):
[1]GARCIA-PUENTE E R, ALCALDE-EON C, SANTOS-BUELGA C, et al. Behaviour and characterization of the colour during red wine making and maturation[J]. Analytica Chimica Acta, 2006, 563(1/2): 215-222.
[2]Wikipedia. Pyranoanthocyanin[DB/OL]. [2014-02-17]. http://en.wikipedia.org/wiki/Pyranoanthocyanin.
[3]de FREITAS V, MATEUS N. Formation of pyranoanthocyanins in red wines: a new and diverse class of anthocyanin derivatives[J]. Analytical and Bioanalytical Chemistry, 2011, 401(5): 1463-1473.
[4]RENTZSCH M, SCHWARZ M, WINTERHALTER P. Pyranoanthocyanins-an overview on structures, occurrence, and pathways of formation[J]. Trends in Food Science & Technology, 2007, 18(10): 526-534.
[5]BAKKER J, TIMBERLAKE C F. Isolatio n, identification and characterization of new color-stable anthocyanins occurring in some red wines[J]. Journal of Agricultural and Food Chemistry, 1997, 45(1): 35-43.
[6]FRANCIA-ARICHA E M, GUERRA M T, RIVAS-GONZALO J C, et al. New anthocyanin pigments formed after condensation with flavanols[J]. Journal of Agricultural and Food Chemistry, 1997, 45(6): 2262-2266.
[7]OLIVEIRA J, AZEVEDO J, SILVA A M S, et al. Pyranoanthocyanin dimers: a new family of turquoise blue anthocyanin-derived pigments found in port wine[J]. Journal of Agricultural and Food Chemistry, 2010, 58(8): 5154-5159.
[8]MATEUS N, OLIVEIRA J, HAETTICH-MOTTA M, et al. New family of bluish pyranoanthocyanins[J/OL]. Journal of Biomedicine and Biotechnology, 2004. http://dx.doi.org/10.1155/S1110724304404033.
[9]OLIVEIRA J, SANTOS-BUELGA C, SILVA A M S, et al. Chromatic and structural features of blu e anthocyanin-derived pigments present in port wine[J]. Analytica Chimica Acta, 2006, 563(1/2): 2-9.
[10]HE Jingren, SANTOS-BUELGA C, SILVA A M S, et al. Isolation and structural characterization of new anthocyanin-derived yellow pigments in aged red wines[J]. Journal of Agricultural and Food Chemistry, 2006, 54(25): 9598-9603.
[11]HAVASAKA Y, ASENSTORFER R E. Screening for potential pigments derived from anthocyanins in red wine using nanoelectrospray tandem mass spectrometry[J]. Journal of Agricultural and Food Chemistry, 2002, 50(4): 756-761.
[12]FULCRAND H, BENABDELJALIL C, RIGAUD J, et al. A new class of wine pigments generated by reaction between pyruvic acid and grape anthocyanins[J]. Phytochemistry, 1998, 47(7): 1401-1407.
[13]CAMEIRA-dos-SANTOS P J, BRILLOUET J M, CHEYNIER V, et al. Detection and partial characterisation of new anthocyaninderived pigments in wine[J]. Journal of the Science of Food and Agriculture, 1996, 70(2): 204-208.
[14]FULCRAND H, CAMEIRA DOS SANTOS P J, SAMI-MANCHADO P, et al. Structure of new anthocyanin-derived wine pigments[J]. Journal of the Chemical Society Perkin Transactions, 1996. doi: 10.1039/P19960000735.
[15]MATEUS N, SILVA A M, SANTOS-BUELGA C, et al. Identification of anthocyanin-flavanol pigments in red wines by NMR and mass spectrometry[J]. Journal of Agricultural and Food Chemistry, 2002, 50(7): 2110-2116.
[16]MATEUS N, CARVALHO E, CARVALHO A R, et al. Isolation and structural characterization of new acylated anthocyanin-vinylflavanol pigments occurring in aging red wines[J]. Journal of Agricultural and Food Chemistry, 2003, 51(1): 277-282.
[17]CRUZ L, TEIXEIRA N, SILVA A, et al. The role of vinylcatechin in the formation of pyranomalvidin-3-glucoside-(+)-catechin[J]. Journal of Agricultural and Food Chemistry, 2008, 56(22): 10980-10987.
[18]MATEUS N, SILVA A M, RIVAS-GONZALO J C, et al. A new class of blue anthocyanin-derived pigments isolated from red wines[J]. Journal of Agricultural and Food Chemistry, 2003, 51(7): 1919-1923.
[19]MATEUS N, OLIVEIRA J, SANTOS-BUELGA C, et al. NMR structure characterization of a new vinylpyrano anthocyanincatechin pigment (a portisin)[J]. Tetrahedron Letters, 2004, 45(17): 3455-3457.
[20]MATEUS N, OLIVEIRA J, PISSARRA J, et al. A new vinylpyranoanthocyanin pigment occurring in aged red wine[J]. Food Chemistry, 2006, 97(4): 689-695.
[21]CARVALNO A R F, OLIVEIRA J, de FREITAS V, et al. A computational study of vinylpyranoanthocyanin-phenolic pigments (portisins)[J]. Journal of Molecular Structure: THEOCHEM, 2010, 946(1/3): 113-118.
[22]OLIVEIRA J, de FREITAS V, SILVA A, et al. Reaction between hydroxycinnamic acids and anthocyanin-pyruvic acid adducts yielding new portisins[J]. Journal of Agricultural and Food Chemistry, 2007, 55(15): 6349-6356.
[23]CARVALHO A, OLIVEIRA J, de FREITAS V, et al. Unusual color change of vinylpyranoanthocyanin-phenolic pigments[J]. Journal of Agricultural and Food Chemistry, 2010, 58(7): 4292-4297.
[24]OLIVEIRA J, MATEUS N, RODRIGUEZ-BORGES J E, et al. Synthesis of a new pyranoanthocyanin dimer linked through a methylmethine bridge[J]. Tetrahedron Letters, 2011, 52(23): 2957-2960.
[25]Wikipedia. Oxovitisin[DB/OL]. [2014-03-12]. http://en.wikipedia.org/wiki/Oxovitisin.
[26]HE Jingren, OLIVEIRA J, SILVA A M S, et al. Oxovitisins: a new class of neutral pyranone-anthocyanin derivatives in red wines[J]. Journal of Agricultural and Food Chemistry, 2010, 58(15): 8814-8819.
[27]OLIVEIRA J, MATEUS N, SILVA A M S, et al. Equilibrium forms of vitisin B pigments in an aqueous system studied by NMR and visible spectroscopy[J]. Journal of Physical Chemistry B, 2009, 113(32): 11352-11358.
[28]HE Jingren, CARVALHO A, MATEUS N, et al. Spectral features and stability of oligomeric pyranoanthocyanin-flavanol pigments isolated from red wines[J]. Journal of Agricultural and Food Chemistry, 2010, 58(16): 9249-9258.
[29]ASENSTORFER R E, JONES G P. Charge equilibria and pK values of 5-carboxypyranomalvidin-3-glucoside (vitisin A) by electrophoresis and absorption spectroscopy[J]. Tetrahedron Letters, 2007, 63(22): 4788-4792.
[30]SCHWARZ M, WINTERHALTER P, A novel synthetic route to substituted pyranoanthocyanins with unique colour properties[J]. Tetrahedron Letters, 2003, 44(41): 7583-7587.
[31]CARVALHO A, OLIVEIRA J, de FREITAS V, et al. A theoretical interpretation of the color of two classes of pyranoanthocyanins[J]. Journal of Molecular Structure: THEOCHEM, 2010, 948(1/3): 61-64.
[32]HAKANSSON A E, PARDON K, HAYASAKA Y, et al. Structures and colour properties of new red wine pigments[J]. Tetrahedron Letters, 2003, 44(26): 4887-4891.
[33]CRUZ L, PETROV V, TEIXEIRA N, et al. Establishment of the chemical equilibria of different types of pyranoanthocyanins in aqueous solutions: evidence for the formation of aggregation in pyranomalvidin-3-O-coumaroylglucoside-(+)-catechin[J]. Journal of Physical Chemistry B, 2010, 114(41): 13232-13240.
[34]GOUPY P, BAUTISTA-ORTIN A B, FULCRAND H, et al. Antioxidant activity of wine pigments derived from anthocyanins: hydrogen transfer reactions to the DPPH radical and inhibition of the heme-induced peroxidation of linoleic acid[J]. Journal of Agricultural and Food Chemistry, 2009, 57(13): 5762-5770.
[35]JORDHEIM M, AABY K, FOSSEN T, et al. Molar absorptivities and reducing capacity of pyranoanthocyanins and other anthocyanins[J]. Journal of Agricultural and Food Chemistry, 2007, 55(26): 10591-10598.
[36]GARCIA-ALONSO M, RIMBACH G, RIVAS-GONZALO J C, et al. Antioxidant and cellular activities of anthocyanins and their corresponding vitisins A-studies in platelets, monocytes, and human endothelial cells[J]. Journal of Agricultural and Food Chemistry, 2004, 52(11): 3378-3384.
[37]MUSELIK J, GARCIA-ALONSO M, MARTIN-LOPEZ M P, et al. Measurement of antioxidant activity of wine catechins, procyanidins, anthocyanins and pyranoanthocyanins[J]. International Journal of Molecular Sciences, 2007, 8(8): 797-809.
[38]FARIA A, OLIVEIRA J, NEVES P, et al. Antioxidant properties of prepared blueberry (Vaccinium myrtillus) extracts[J]. Journal of Agricu ltural and Food Chemistry, 2005, 53(17): 6896-6902.
[39]GARCIA-ALONSO M, RIMBACH G, SASAI M, et al. Electron spin resonance spectroscopy studies on the free radical scavenging activity of wine anthocyanins and pyranoanthocyanins[J]. Molecular Nutrition & Food Research, 2005, 49(12): 1112-1119.
[40]AZEVEDO J, FERNANDES I, FARIA A, et al. Antioxidant properties of anthocyanidins, anthocyanidin-3-glucosides and respective portisins[J]. Food Chemistry, 2010, 119(2): 518-523.
[41]PIRKER K P, OLIVEIRA J, FREITAS V, et al. Antiradical properties of red wine portisins[J]. Journal of Agricultural and Food Chemistry, 2011, 59(21): 118 33-11837.
[42]FERNANDES I, FARIA A, AZEVEDO J, et al. Influence of anthocyanins, derivative pigments and other catechol and pyrogalloltype phenolics on breast cancer cell proliferation[J]. Journal of Agricultural and Food Chemistry, 2010, 58(6): 3785-3792.
Recent Progress in Research on Pyranoanthocyanins Derivatives
HE Jingren1,2, KUANG Minjie1, QI Minyu1, LIU Gang1,2, LI Shuyi1, WU Nao1, ZHU Zhenzhou1, GUO Ying1, LIANG Zheng1
(1. Hubei Key Laboratory for Processing and Transformation of Agricultural Products, College of Food Science and Engineering, Wuhan Polytechnic University, Wuhan 430023, China; 2. Hubei Collaborative Innovation Center for Processing of Agricultural Products, Wuhan Polytechnic University, Wuhan 430023, China)
Abstract:Pyranoanthocyanins are new anthocyanin-derived pigments found in fruit wines such as red wine in recent years, and they are one class of important coloring substances in grape wine. The pyranoanthocyanin family possesses an additional fourth pyran ring attached to anthocyanins. They consist of a series of natural pigment formed by cycloaddition reaction between berry anthocyanins and intermediates produced from glucose metabolization during fermentation and aging, and other phenolic compounds in fruits. The present paper summarizes the classification, molecular structural properities, formation mechanism, stability, chromatic features and functional bioactivities including antioxidation and antitumor of pyranoanthocyanins and second generation derivatives. Many different types of pyranoanthocyanins are not only important coloration substances, but also have high stability, good color characteristics and a strong functional activity, which will offer beneficial references for further investigating the relationships between the structures of pyranoanthocyanin derivatives and stability, coloring and functional properties for their applications in grape wine and food procesing industries.Key words: anthocyanins; pyranoanthocyanin family; second-generation anthocyanin derivatives; aged fruit wines
中圖分類號(hào):TS202.3
文獻(xiàn)標(biāo)志碼:A
文章編號(hào):1002-6630(2015)07-0228-07
doi:10.7506/spkx1002-6630-201507042
作者簡(jiǎn)介:何靜仁(1974—),男,教授,博士,研究方向?yàn)樯攀彻π镔|(zhì)基礎(chǔ)與分子營(yíng)養(yǎng)。E-mail:jingrenh@yahoo.com
基金項(xiàng)目:國(guó)家國(guó)際科技合作專項(xiàng)(2014DFG32310);國(guó)家自然科學(xué)基金面上項(xiàng)目(31371727);湖北省教育廳科學(xué)技術(shù)研究重點(diǎn)項(xiàng)目(D20121803);武漢市科技人才培育晨光計(jì)劃項(xiàng)目(2013070104010023)
收稿日期:2014-04-29

