兩個由5-硝基間苯二甲酸和雙咪唑基配體構(gòu)筑的錳、鈷配合物的合成及晶體結(jié)構(gòu)
李國峰 王亞男 王慶偉*, 李秀梅 紀(jì)建業(yè) 潘亞茹
(1吉林師范大學(xué)資產(chǎn)管理處,四平136000)
(2環(huán)境友好材料制備與應(yīng)用省部共建教育部重點實驗室,吉林師范大學(xué),四平136000)
(3通化師范學(xué)院化學(xué)學(xué)院,通化134002)
兩個由5-硝基間苯二甲酸和雙咪唑基配體構(gòu)筑的錳、鈷配合物的合成及晶體結(jié)構(gòu)
李國峰1,2王亞男2王慶偉*,2李秀梅*,3紀(jì)建業(yè)3潘亞茹3
(1吉林師范大學(xué)資產(chǎn)管理處,四平136000)
(2環(huán)境友好材料制備與應(yīng)用省部共建教育部重點實驗室,吉林師范大學(xué),四平136000)
(3通化師范學(xué)院化學(xué)學(xué)院,通化134002)
通過水熱法合成了2個新的配合物[Mn(NIPH)(mbix)]n(1)和[Co(NIPH)(mbix)(H2O)3]2n·2nH2O(2)(H2NIPH=5-硝基間苯二甲酸,mbix=1,3-雙(咪唑基-1-基)苯)。并對其進行了元素分析、紅外光譜、紫外光譜、熱重和X-射線單晶衍射測定。這兩個配合物通過氫鍵和π-π相互作用形成了三維超分子網(wǎng)狀結(jié)構(gòu)。
水熱合成;晶體結(jié)構(gòu);錳配合物;鈷配合物
0 Introduction
Recently,studies on the synthesis of coordination polymers(CPs)have received much attention in coordination chemistry because of their interesting moleculartopologiesandtremendouspotential applications in catalysis,molecular selection,nonlinear optics,ion exchange and microelectronics[1-5]. Generally speaking,the structural diversity of such crystalline materials dependent on many factors,suchas metal ion,templating agents,metal-ligand ratio,pH value,counteranion,and number of coordination sites provided by organic ligands[6-7].Among the strategies, the rational selection of organic ligands or coligands according to their length,rigidly,and functional groups is important for the assembly of structural controllable CPs,and a great deal of significant works have been done by using the strategy[8].Usually,the organic ligands with bent backbones,such as V-shaped, triangular,quadrangular,and so on,are excellent candidatesforbuildinghighlyhigh-connected, interpenetrating,or helical coordination frameworks due to their bent backbones and versatile bridging fashions[9-10].Besides,the carboxylate groups are good hydrogen-bond acceptor as well as donor,depending upon the degree of deprotonation.Among which, quadrangular polycarboxylic acids,such as 5-nitroisophthalic acid,3,3′,4,4′-benzophenone tetracarboxylic acid,4,4′-oxydibenzoic acid,are paid much attention due to their rich coordination modes[11].Apart from the carboxylate linkers,bis(imidazole)bridging ligands with different length andflexibly,suchas1,3-bis(imidazol-1-ylmethyl)benzene,1,4-bis(imidazol-1-ylmethyl)benzene,1,4-bis(imidazol-1-yl)-butaneare frequently used in the assembly process of CPs as bridging linkers[12].
Thus,these considerations inspired us to explore new coordination architectures with 5-nitroisophthalic acid(H2NIPH)and(bis)imidazolebridginglinkers (mbix).In this paper,we reported the synthesis and characterizations of two new CPs,namely,[Mn(NIPH) (mbix)]n(1)and[Co(NIPH)(mbix)(H2O)3]2n·2nH2O(2), 1 is two-dimensional(2D)network structure and 2 exhibits a zero-dimensional structure with NIPH-Combix-H2O-as building units.
1 Experimental
1.1 General procedures
All materials were commercially purchased and used without further purification.Infrared spectra (KBrpellets)weretakenonaNicolet6700 spectrometer and elemental analyses for C,H and N were performed on a Perkin-Elmer 240C analyzer. The TG studies were performed on a Perkin-Elmer TGA7 analyze.The UV spectrum was obtained on a Shimzu UV-250 spectrometer.
1.2 Synthesis
[Mn(NIPH)(mbix)]n(1):A mixture of Mn(OAc)2· 4H2O(0.5 mmol,0.12 g),H2NIPH(0.5 mmol,0.11 g), mbix(0.5 mmol,0.12 g)and H2O(18 mL)was sealed in a 30 mL Teflon-lined autoclave under autogenous pressure at 120℃for 5 d.After cooling to room temperature,yellow block crystals were collected by filtration and washed with distilled water in 32%yield (based on Mn).Anal.Calcd.(%)for C22H17MnN5O6:C, 52.60;H,3.41;N,13.94.Found(%):C,52.12;H, 3.05;N,13.21.IR(KBr,cm-1):3119w,1610s,1578w, 1561m,1522m,1447w,1432m,1370w,1346w,1290 w,1 236m,1 196w,1 160w,1 107w,1 088m,1 031m, 932m,851w,840w,791m,774w,730m,716w,656m, 634w,547w,524w,461w.
[Co(NIPH)(mbix)(H2O)3]2n·2nH2O(2):A mixture of Co(OAc)2·4H2O(0.5 mmol,0.12 g),H2NIPH(0.5 mmol,0.11 g),mbix(0.5 mmol,0.12 g)and H2O(18 mL)was sealed in a 30 mL Teflon-lined autoclave under autogenous pressure at 140℃for 5 d.After cooling to room temperature,purple block crystals were collected by filtration and washed with distilled water in 46%yield(based on Co).Anal.Calcd.(%)for C44H50Co2N10O20:C,45.68;H,4.36;N,12.11.Found (%):C,45.07;H,3.98;N,11.76.IR(KBr,cm-1):3 119 w,1 629s,1 561m,1 523m,1 456w,1 433w,1 346 m,1 237m,1 199w,1 157w,1 107w,1 087s,1 033 m,941w,866w,839w,791m,710w,729w,659m,634 w,557w,527w,456w,437w.
1.3 Structure determination
Single crystal diffraction data of 1 and 2 were respectively collected on a Bruker APEXⅡCCD diffractometer equipped with a graphite-monochromatic Mo Kα(λ=0.071 073 nm)radiation at room temperature. The structure was solved by direct methods with SHELXS-97 program[13]and refined byfull-matrix least-squares techniques on F2with SHELXL-97[14]. All non-hydrogen atoms were refined anisotropically and the hydrogen atoms of organic ligands were generated geometrically.Hydrogen atoms of watermolecule were located from the different Fourier maps.The selected bond parameters are given in Table 1.
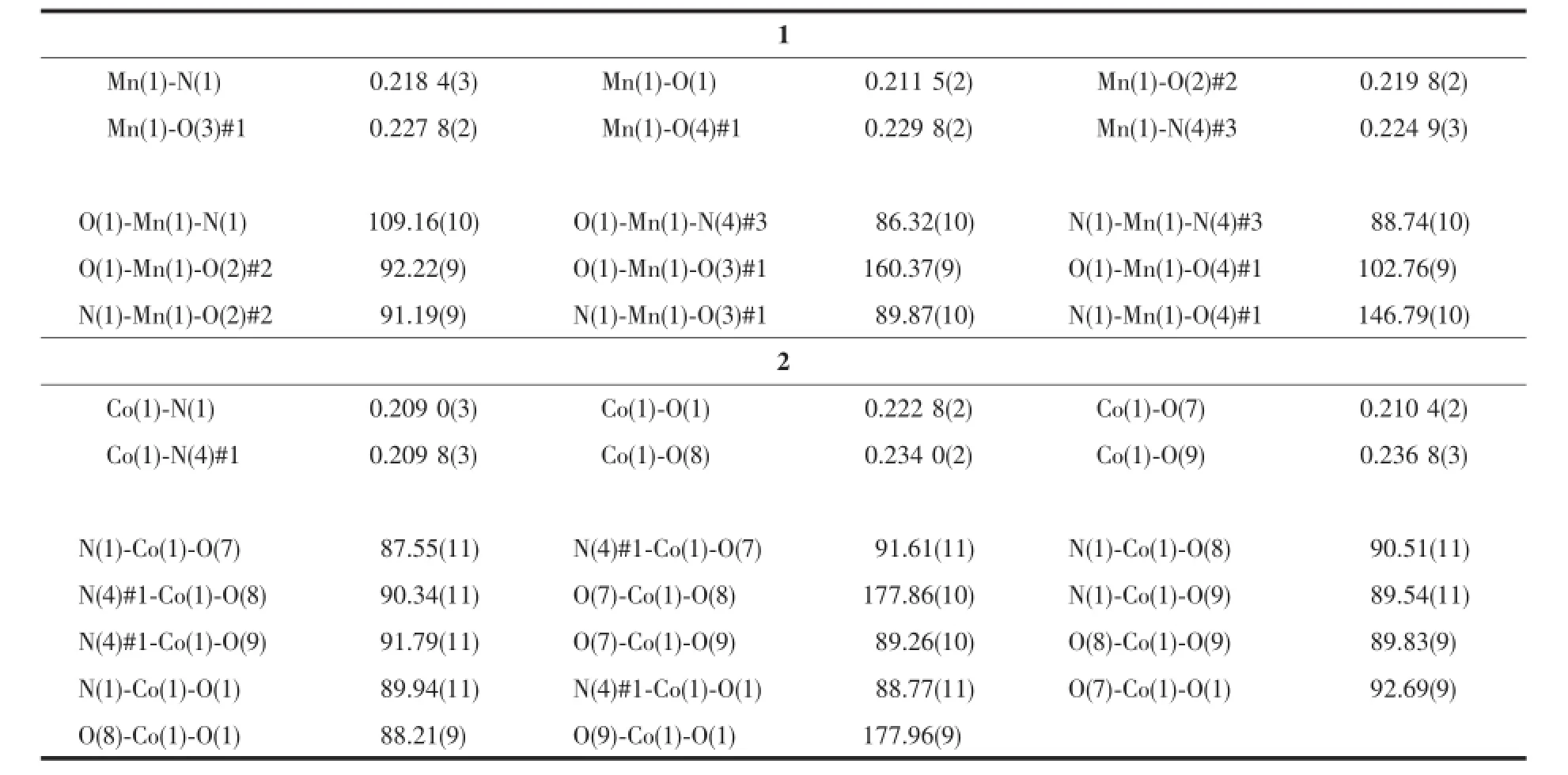
Table1 Selected bond lengths(nm)and bond angles(°)for 1 and 2
Crystaldatafor1:C22H17MnN5O6,triclinic, space group P1,Mr=502.35,a=0.841 59(7)nm,b= 1.040 95(9)nm,c=1.348 07(12)nm,α=76.082 0(10)°, β=72.112 0(10)°,γ=74.667(2)°,V=1.067 43(16)nm3, Z=2,F(000)=514,μ(Mo Kα)=0.670 mm-1,Dc=1.563 g ·cm-3,5 752 reflections measured,4 110 unique(Rint= 0.021 5),3 370 observed reflections with I>2σ(I),R= 0.048 0,wR=0.107 8,S=1.074.
Crystaldatafor2:C44H50Co2N10O20,triclinic, space group P1,Mr=1 156.80,a=0.865 73(8)nm,b= 1.195 78(11)nm,c=1.258 22(12)nm,α=69.494(2)°, β=81.480(2)°,γ=85.311(2)°,V=1.205 91(19)nm3,Z= 1,F(000)=598,μ(Mo Kα)=0.780 mm-1,Dc=1.593 g· cm-3,5 990 reflections measured,4 151 unique(Rint= 0.025 3),3 281 observed reflections with I>2σ(I),R= 0.050 9,wR=0.120 3,S=1.083.
CCDC:979616,1;1011701,2.
2 Results and discussion
2.1 IR spectrum
Complex 1:The COO-is coordinated with its asymmetric and symmetric stretching appearing at 1 610 cm-1(ν(OCO)assym)and 1 432 cm-1(ν(OCO)sym)[15], respectively.TheΔν(ν(OCO)assym-ν(OCO)sym)is 178 cm-1(<200),showing the presence of bidentate linkage of carboxylates in the dianions.Thus the carboxylates coordinate to the metal as bidentate ligands via the carboxylate groups[16].
Complex 2:The COO-is coordinated with its asymmetric and symmetric stretching appearing at 1 629 cm-1(ν(OCO)assym)and 1 346 cm-1(ν(OCO)sym)[15], respectively.The Δν(ν(OCO)assym-ν(OCO)sym)are 283 cm-1(>200),showing the presence of monodentate linkage of carboxylates in the dianions.Thus the carboxylates coordinate to the metal as monodentate ligands via the carboxylate groups[16].
In addition,X-ray diffraction analysis further indicatesthebidentatecoordinationmannersof carboxylate groups in 1 and monodentate coordination manners of carboxylate groups in 2.
2.2 Description of the structure
Complex 1 crystallizes in the triclinic space group P1 and features a two-dimensional(2D)network structure.The coordination environment of Mnin 1 is shown in Fig.1.There are one Mnion,one NIPH ligand and one mbix ligand in the asymmetric unit. Each Mnion is six-coordinated by four carboxylateoxygen atoms(O(1),O(2)#2,O(3)#1,O(4)#1)from three different NIPH ligands and two nitrogen donors (N(1)and N(4)#3)from two flexible mbix molecules to furnish a distorted octahedral coordination architecture. The bond distances of Mn-O in compound 1 fall in range of the 0.211 5(2)~0.229 8(2)nm,and Mn-N bond length fall in the range of 0.218 4(3)~0.224 9(3) nm,which are in the normal range and the coordination angles around Mn atom are in the range 57.71(8)°~178.42(9)°.In the coordination environment,the three carboxylate oxygen atoms(O(1),O(3)#1,O(4)#1)and one imidazole nitrogen atom(N(1))are located in the basal plane,whereas one carboxylate oxygen atom (O(2)#2)and one imidazole nitrogen atom(N(4)#3) occupy the axial positions from the opposite direction.
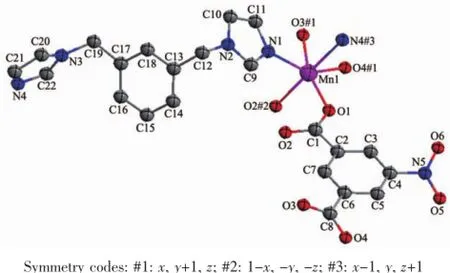
Fig.1 Coordination environment of the Mncenter
In 1,each NIPH ligand adopts μ3-η1∶η1∶η1∶η1coordination mode to bridge MnⅡatoms intoan infinite1Dchain,whichalternatelycontains8-membered rings and 16-membered rings with Mn…Mn distances of 0.462 3 and 0.766 7 nm,respectively (Fig.2a),and is similar to the reported complex [Cd(nip)(bix)0.5H2O]n[12g].Moreover,the mbix ligands adopt cis-conformation bridging mode with a dihedral angle between the two imidazole rings of 79.02°,and pillar these 1D chains into 2D coordination network (Fig.2b).Further investigation of the crystal packing of compound 1 suggests that there are one kinds of C-H…O hydrogen bonding interactions between carboxylate oxygen atom and carbon atoms of mbix ligands in complex 1(Table 2).Moreover,there are π-π interactions in complex 1 between benzene rings of mbixligands.Thecentroid-to-centroiddistance between adjacent ring is 0.361 7(2)nm for C13C14 C15C16C17C18 and C13′C14′C15′C16′C17′C18′(Symmetry codes:1-x,-y,-1+z)benzene rings.The perpendicular distance is 0.342 0(5)nm for C13C14 C15C16C17C18 and C13′C14′C15′C16′C17′C18′(Symmetry codes:1-x,-y,-1+z)benzene rings. Therefore,throughhydrogenbondsandπ-π interactions,the two-dimensional networks are further extendedintoathree-dimensionalsupramolecularframework(Fig.3).
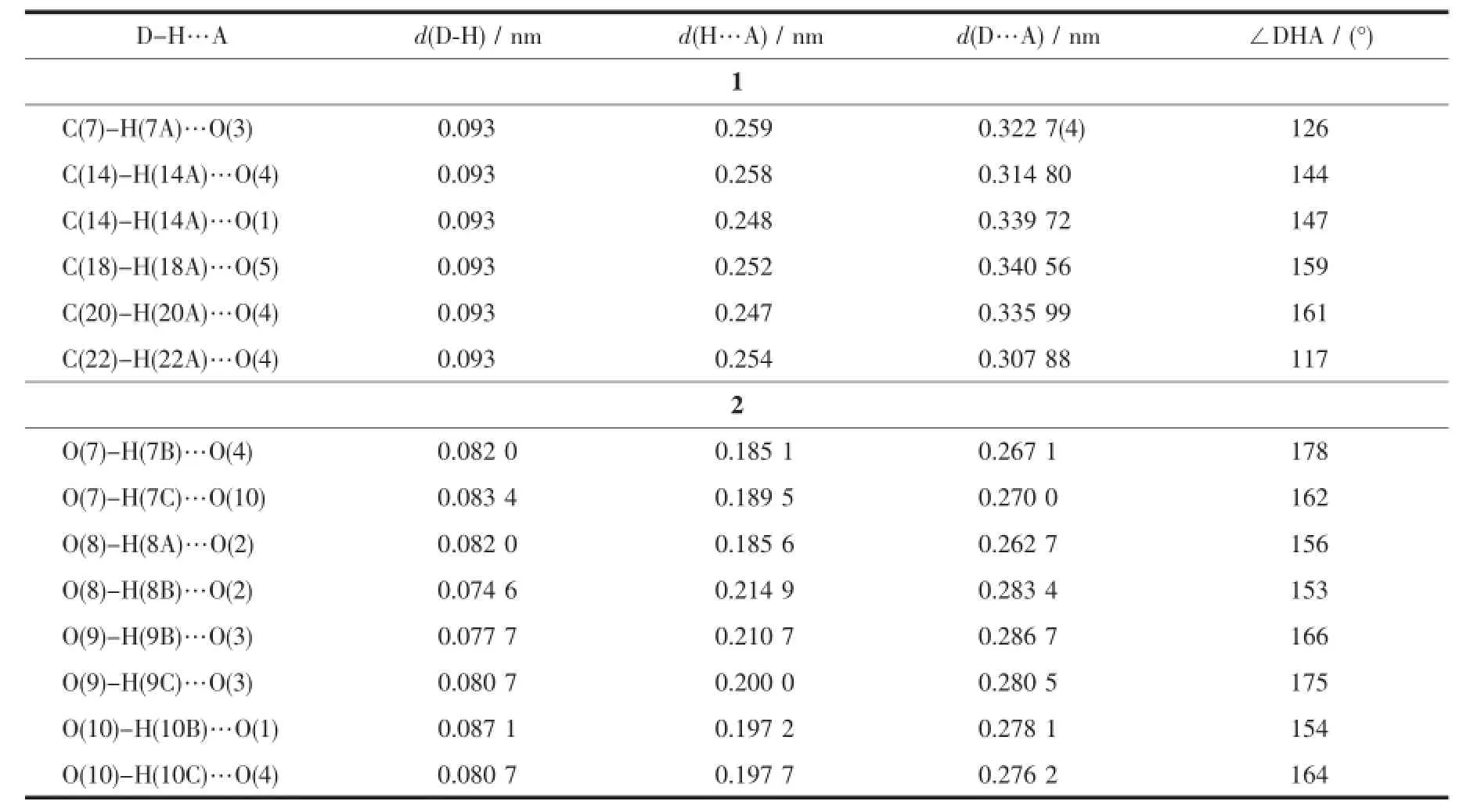
Table2 Hydrogen bonds for 1 and 2
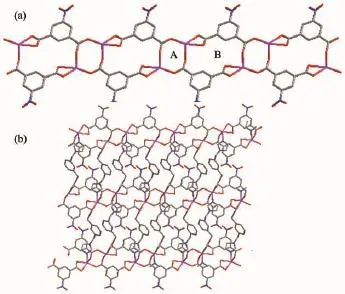
Fig.2 (a)1D chain structure of complex 1 with alternately contain 8-member(A)ring and 16-member(B)ring; (b)2D network structure of complex 1
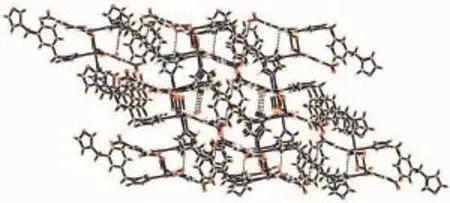
Fig.3 View of the 3D supramolecular architecture of 1 formed by hydrogen-bonding and π-π interactions
Complex 2 crystallizes in the triclinic space group P1 and exhibits a zero-dimensional structure. The molecular structure of compound 2 is shown in Fig.4.There are two coordination centers,Co(1)and Co(1)#1),in the crystal with the same coordination modes.TheCo(1)ion is six-coordinated by one carboxylate oxygen atoms(O(1))from NIPH ligand, two nitrogen atoms(N(1),N(4)#1)from two different mbix ligands and three coordinated water molecular (O(7),O(8),O(9)),showing a slightlydistorted octahedral geometry.The Co-O bond distances are in the range of 0.209 0~0.222 8(2)nm,and those of Co-N are from 0.209 0(3)to 0.209 8(3)nm.The N(O)-Co-O(N)angles fall in the range of 87.55(11)°~178.43(11)°.
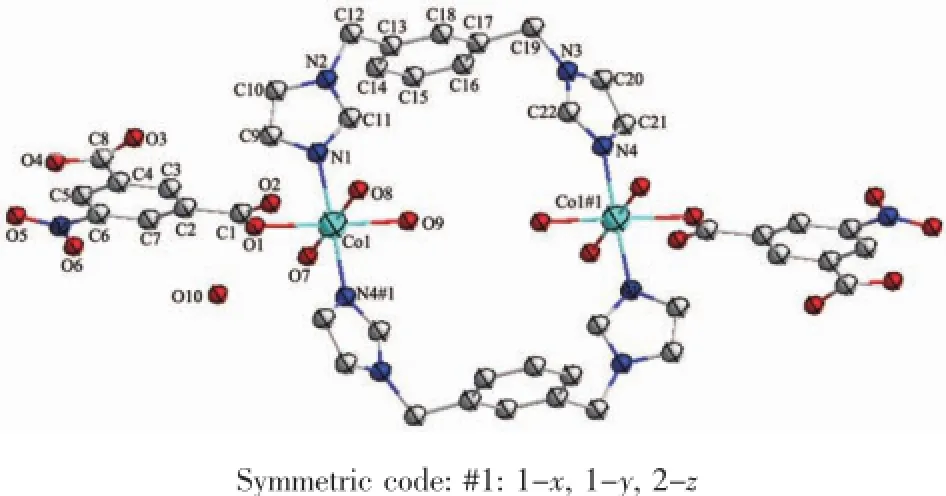
Fig.4 Coordination environment of Coin complex 2
One coordination mode of the NIPH ligand is present in the structure of compound 2,namely monodentate bridging mode.The mbix ligand exhibits cis-conformation bridging mode with a dihedral angle between the two imidazole rings of 51.32°,based on this,two Co(II)ions are linked by mbix ligands to yield a binuclear unit with 24-number ring.The Co…Co distance linked by mbix ligand is 0.799 4 nm.
There are persistent O-H…O hydrogen bonding interactions in the complex(Table 2),which play an important role in stabilizing the network structure. Moreover,there are π-π interactions in complex 2 between benzene rings of NIPH and mbix ligands (Table 3).Therefore,through hydrogen bonds and π-π interactions,thenetworkstructuresarefurther extendedintoathree-dimensionalsupramolecular framework(Fig.5).
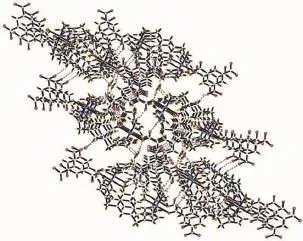
Fig.5 View of the 3D supramolecular architecture of 2 formed by hydrogen-bonding and π-π interactions
Toinvestigatewhethertheanalyzedcrystal structures are truly representative of the bulk materials, powder X-ray diffraction(PXRD)technologies have been performed for the complex 1 and 2 at room temperature(Fig.6).The main peak positions observed are in good agreement with the simulated ones.Although minor differences can be found in the positions,widths,and intensities of some peaks,it still can be considered that the bulk synthesized materials and the analyzed crystal are homogeneous. Thedifferencesmaybeduetothepreferred orientation of the powder samples[17-18].
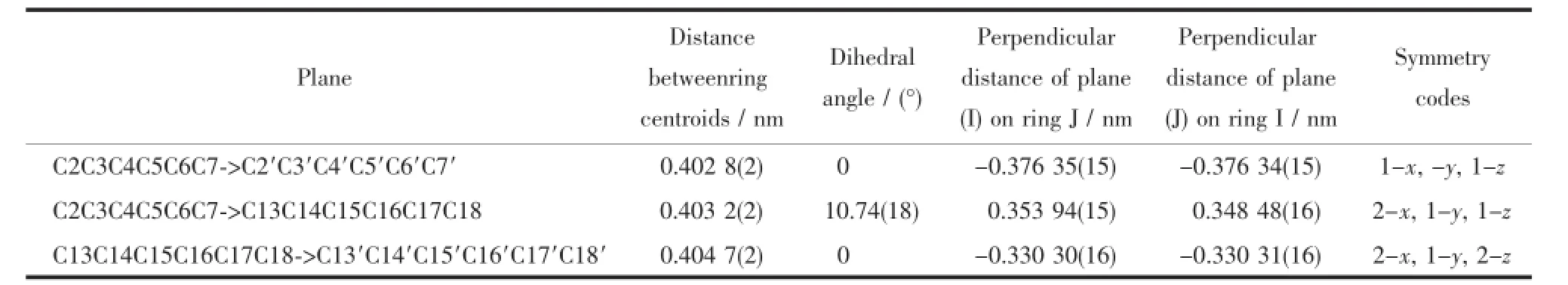
Table3 Parameters between the planes in 2
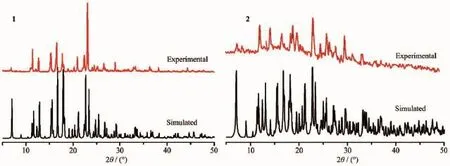
Fig.6 PXRD analysis of the complex
2.3 Thermal analysis
TG curve of 1(Fig.7)show that the first weight loss of 45.9%from 22 to 115℃corresponds to the removal of mbix ligand(Calcd.47.4%).Upon further heating,an obvious weight loss(39.6%)occurs in the temperature range of 115~550℃,corresponding to the release of NIPH ligand(Calcd.41.6%).After 550℃no weight loss is observed,which means the complete decomposition of 1.The residual weight should be MnO.
TG curve of 2(Fig.7)shows that the first weight loss of 50.03%from 37 to 414℃corresponds to the removal of water molecules and NIPH ligand(Calcd. 48.6%).Upon further heating,an obvious weight loss (40.1%)occurs in the temperature range of 414~809℃,corresponding to the release of mbix ligands (Calcd.41.2%).After 809℃no weight loss is observed,which means the complete decomposition of 1.The residual weight should be CoO.
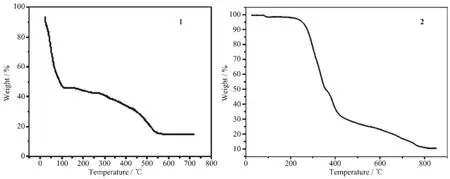
Fig.7 TG curve of complex 1 and 2
2.4 UV spectrum
The UV spectra for the compound 1,compound 2,H2NIPH and mbix ligands have been investigated in the solid state.H2NIPH has one absorption band at about 274 nm and mbix has one absorption band at about 277 nm,while the title compound 1 and 2 have oneabsorptionbandatabout279,344nm, respectively(Fig.9),which should be assigned to the n→π*transition of mbix and the charge transfer transition[19].
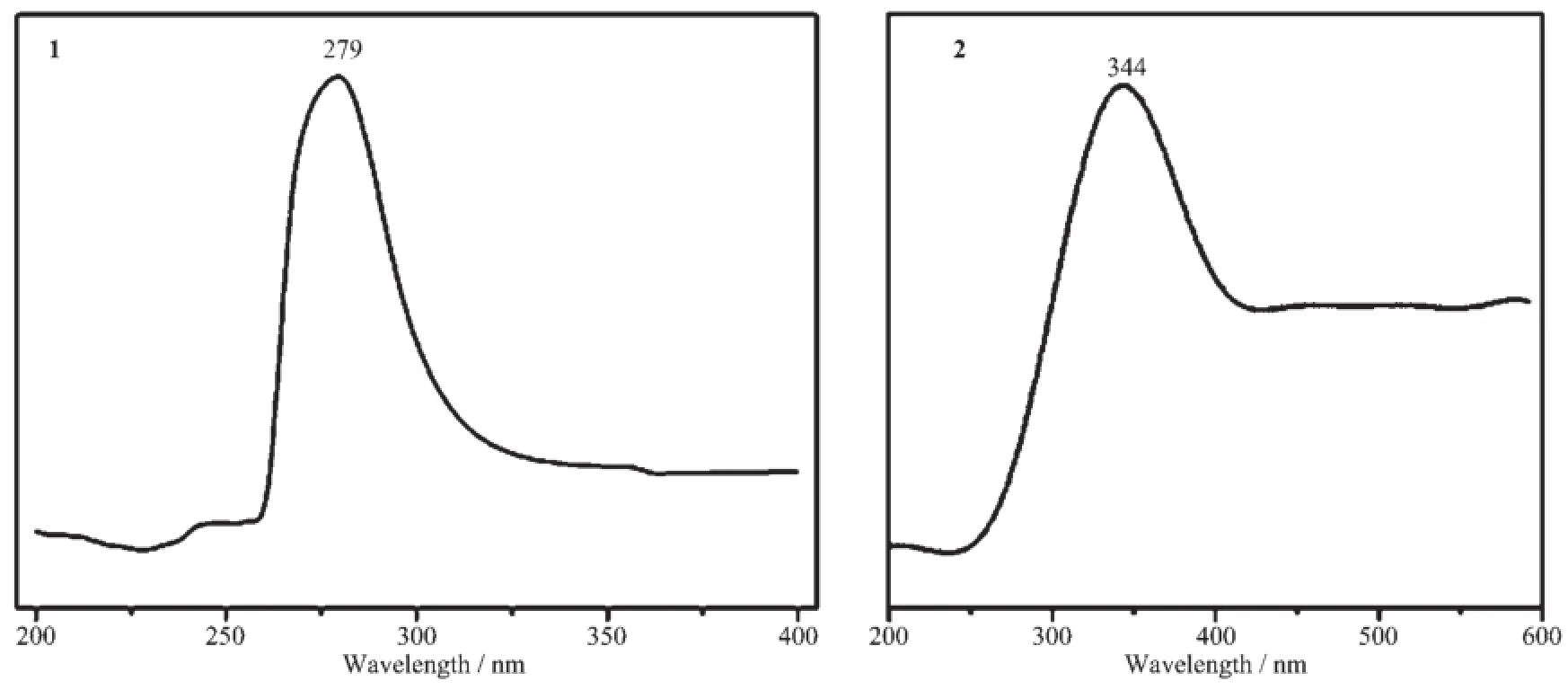
Fig.8 UV spectra of complexes 1 and 2
[1]Hagrman P J,Hagrman D,Zubieta J.Angew.Chem.Int. Ed.,1999,38:2638-2684
[2]Blake A J,Champness N R,Hubberstey P,et al.Coord.Chem. Rev.,1999,183:117-138
[3]Moulton B,Zaworotko M J.Chem.Rev.,2001,101:1629-1658
[4]Rao C N R,Natarajan S,Vaidhyanathan R.Angew.Chem. Int.Ed.,2004,43:1466-1496
[5]Kitagawa S,Kitaura R,Noro S I.Angew.Chem.Int.Ed., 2004,43:2334-2375
[6](a)Chen B L,Ockwig N W,Fronczek F R,et al.Inorg.Chem., 2005,44:181-183
(b)Yang S,Lin X,Blake A J,et al.Chem.Commun.,2008: 6108-6110
(c)Choi H S,Suh M P.Angew.Chem.Int.Ed.,2009,48:6865-6869
(d)Chen B L,Ockwig N W,Millward A R,et al.Angew. Chem.Int.Ed.,2005,44:4745-4749
(e)Liu J L,Lin W Q,Chen Y C,et al.Inorg.Chem.,2013, 52:457-463
(f)Guo P H,Liu J L,Zhang Z M,et al.Inorg.Chem.,2012, 51:1233-1235
[7](a)Bao X,Guo P H,Liu W,et al.Chem.Sci.,2012,3:1629-1633
(b)Zhao X L,Sun D,Yuan S,et al.Inorg.Chem.,2012,51: 10350-10355
(c)Wang S N,Peng Y Q,Wei X L.CrystEngComm,2011,13: 5313-5316
(d)Fan L M,Zhang X T,Li D C,et al.CrystEngComm, 2013,15:349-355
(e)Chen C X,Liu Q K,Ma J P,et al.J.Mater.Chem.,2012, 22:9027-9033
(f)Liu T,Wang S,Lu J,et al.CrystEngComm,2013,15:5476-5489
[8](a)Sun D,Li Y H,Hao H J,et al.Cryst.Growth Des.,2011, 11:3323-3327
(b)Li X J,Jiang F L,Wu M Y,et al.Inorg.Chem.,2012,51: 4116-4122
(c)Feng R,Chen L,Chen Q H,et al.Cryst.Growth Des., 2011,11:1705-1712
(d)Li D S,Zhang P,Zhao J,et al.Cryst.Growth Des.,2012, 12:1697-1072
[9](a)Suh M,Cheon Y,Lee E.Coord.Chem.Rev.,2008,252: 1007-1026
(b)Cui Y J,Yue Y F,Qian G D,et al.Chem.Rev.,2012, 112:1126-1162
(c)Silva C G,Corma A,Garcia H.J.Mater.Chem.,2010,20: 3141-3153
[10](a)Thirumurugan A,Natarajan S.J.Mater.Chem.,2005,15: 4588-4594
(b)Sun L B,Li Y,Liang Z Q,et al.Dalton Trans.,2012,41: 12790-12796
(c)Liu G,Li H.CrystEngComm,2013,15:6870-6878
(d)Wang J J,Wang T T,Tang L,et al.J.Coord.Chem.,2013,66:3979-3988
[11](a)LI Xiu-Mei(李秀梅),WANG Qing-Wei(王慶偉),LIU Bo (劉博),et al.Chinese J.Inorg.Chem.(無機化學(xué)學(xué)報), 2011,6:1207-1211
(b)Li X M,Dong Y H,Wang Q W,et al.Chinese J.Struct. Chem.,2007,26:1495-1498
(c)Lan Y Q,Li S L,Wu X M,et al.Dalton Trans.,2008,48: 6796-6807
[12](a)LI Xiu-Mei(李秀梅),JI Jian-Ye(紀(jì)建業(yè)),NIU Yan-Ling (牛艷玲),et al.Chinese J.Inorg.Chem.(無機化學(xué)學(xué)報), 2013,29:165-169
(b)ZHAN Pei-Ying(戰(zhàn)佩英),JI Jian-Ye(紀(jì)建業(yè)),NIU Yan-Ling(牛艷玲),et al.Chinese J.Inorg.Chem.(無機化學(xué)學(xué)報),2013,29(2):424-428
(c)LI Xiu-Mei(李秀梅),JI Jian-Ye(紀(jì)建業(yè)),NIU Yan-Ling (牛艷玲),et al.Chinese J.Inorg.Chem.(無機化學(xué)學(xué)報), 2013,6:1302-1306
(d)Wang Z T,Ji J Y,Li X M,et al.Chinese J.Struct. Chem.,2013,32:296-300
(e)Liu B,Guo J,Zhou S,et al.Chinese J.Struct.Chem., 2013,32:199-204
(f)Li X M,Ji J Y,Niu Y L,et al.Synth.React.Inorg.Met.-Org.Chem.,2014,44:891-894
(g)HU Zong-Zhi(胡宗志),ZHAO Jun(趙君),KE Xi-Jun(柯希俊),et al.Chinese J.Inorg.Chem.(無機化學(xué)學(xué)報), 2011,27:184-188
[13]Sheldrick G M.SHELXS 97,Program for the Solution of Crystal Structure,University of G?ttingen,Germany,1997.
[14]Sheldrick G M.SHELXS 97,Program for the Refinement of Crystal Structure,University of G?ttingen,Germany,1997.
[15]Devereux M,Shea D O,Kellett A.et al.Inorg.Biochem., 2007,101:881-892
[16]Bellamy L J.The Infrared Spectra of Complex Molecules. New York:Wiley,1958.
[17]Gilber A,Baggott J.Essentials of Molecular Photochemistry. Boca Raton,FL:CRC Press,1991.
[18]Han Z B,He Y K,Ge C H,et al.Dalton Trans.,2007:3020-3024
[19]Mohamed G G,El-Gamel N E A.Spectrochim.Acta,Part A, 2004,60:3141-3154
Syntheses and Crystal Structures of Two Complexes of Manganese, Cobalt Assembled by 5-Nitroisophthalic Acid and Bis(imidazol)Ligands
LI Guo-Feng1,2WANG Ya-Nan2WANG Qing-Wei*,2LI Xiu-Mei*,3JI Jian-Ye3PAN Ya-Ru3
(1Department of Assets Management,Jilin Normal University,Siping,Jilin 136000,China)
(2Key Laboratory of Preparation and Applications of Environmental Friendly Materials of Ministry of Education,Jilin Normal University,Siping,Jilin 136000,China)
(3Faculty of Chemistry,Tonghua Normal University,Tonghua,Jilin 134002,China)
Two new complex[Mn(NIPH)(mbix)]n(1)and[Co(NIPH)(mbix)(H2O)3]2n·2nH2O(2)(H2NIPH=5-nitroisophthalic acid,mbix=1,3-bis(imidazol-1-ylmethyl)benzene)have been hydrothermally synthesized and structurally characterized by elemental analysis,IR spectrum,UV spectrum,TG and single-crystal X-ray diffraction.They are further extended into a three-dimensional supramolecular network structure through hydrogen bonds and π-π interactions.CCDC:979616,1;1011701,2.
hydrothermal synthesis;crystal structure;manganese complex;cobalt complex
O614.71+1;O614.81+2
A
1001-4861(2015)01-0183-08
10.11862/CJIC.2015.034
2014-07-04。收修改稿日期:2014-08-30。
吉林省教育廳科學(xué)技術(shù)研究(吉教科合字[2013]第384號)、吉林省科技發(fā)展計劃(No.201205080)資助項目。*
。E-mail:wqw611223@163.com,lixm20032006@163.com

