電針對腦缺血再灌注模型大鼠血清白介素的影響
Wang Ping (王平), Mu Yan-yun (穆艷云), Cheng Jie (程潔), Shen Jie (沈潔), Shen Mei-hong (沈梅紅), Chen Xia (陳霞),
Li Qian (李茜)2, Sun Yong (孫永)2, Gong Mei-rong (龔美蓉)2
1 Department of Traditional Chinese Medicine, the People’s Hospital of Jimo, Qingdao, Shandong 266200, China
2 The Second Medical College of Nanjing University of Chinese Medicine, Jiangsu 210046, China
Interleukin (IL) is a group of cytokines that has gained much attention for its action in ischemic cerebrovascular disease[1-2]. So far, the family members such as IL-1β[3-5], tumor necrosis factor (TNF)-α[6-8], IL-6[9-10],IL-8[11-12], and IL-10[13-15]have been involved in relevant studies. Clinical studies have found that the level of serum IL is correlated with the severity of disease and neurologic deficit. IL-6 and IL-8 are proinflammatory factors, and IL-10 is an anti-inflammatory factor; but the current laboratory researches are not completely consistent with clinical studies. Some studies have shown that IL-6 and IL-10 possibly protect brain from damage in ischemic cerebral disorders[15-16]. A large amount of clinical and experimental studies have proven the effect of acupuncture in promoting the recovery of neurological function in ischemic cerebrovascular diseases. Based on our previous works[17-20], the current study was to observe the effect of electroacupuncture (EA) on the level of serum IL-6,IL-8 and IL-10 in the rat model of cerebral ischemiareperfusion injury, and to discover the mechanism of acupuncture in the recovery of ischemic brain injury.
1 Materials and Methods
1.1 Animals
Male Sprague Dawley (SD) rats of clean standard, 8 months, weighing (280±20) g, were purchased from Zhejiang Provincial Experimental Animal Center, license number: SCXK(Zhe)2008-0033. In the whole process of the experiment, the animals were treated in consistent with the requirements of the experimental animal ethics. The animals were applied to the experiment after 1 week of adaptive feeding at 25℃.
1.2 Chemicals and equipment
The G6805 EA apparatus (Shanghai Medical Equipment Factory, Shanghai, China); HH-4 digital thermostatic water bath (Guohua Electric Appliance Co.,Ltd., Changzhou, China); DG5033A enzyme reader(Huadong Electronics Medical Equpment Co., Ltd.,Nanjing, China); IL-6, IL-8, IL-10 Elisa kits (Jiancheng Bioengineering Company, Nanjing, China). The apparatuses were used strictly according to the instructions.
1.3 Animal grouping
The rats were randomized into a sham-operation (SO)group and a model control (MC) group. The rats in the SO group were then divided into a 6-hour SO group and a 24-hour SO group, 8 rats in each group. The rats that finished cerebral ischemia-reperfusion modeling process were scored for neurologic deficit according to the relevant literatures[21]. The rats scored over 2 points were randomized into a MC group and an EA group, and the two groups were also respectively subdivided into a 6-hour group and a 24-hour group, 9 in each group. The rats scored less than 2 points were sacrificed following the standard of experimental animal ethics.
1.4 Model preparation
The rat model of ischemia of middle cerebral artery(MCA) was developed using modified intraluminal suture occlusion method[22-23], the same way we adopted in the previous studies[16-18]. The rats were anesthetized using 10% chloral hydrate (0.35 mL/100 g)and then fixed supinely on a surgery table. After standard sterilization, the right common carotid artery(CCA), external carotid artery (ECA), and internal carotid artery (ICA) were isolated through a midline incision of about 2 cm long. A piece of suture of 0.25 mm in diameter, coated with silicon, was introduced into the ECA (0.4 cm away from the Y-section) to the opening of MCA [about (18±2) mm]. Then the incision was closed with 1 cm suture left outside. Two hours later, the suture was pulled out till the Y-section of the CCA (a slight resistance was felt), to achieve reperfusion. The neurologic deficit was scored 2 h after reperfusion.Neurologic deficit score ≥2 points indicated the success of modeling.
The scoring criteria of neurologic deficit are as follows.
0 point: Normal movement, no neurologic deficit.
1 point: Homer’s sign positive on the right side.
2 points: The tail suspended, and failure to extend left forepaw.
3 points: Autonomic movement, circling to the left(the hemiplegia side).
1.5 Interventions
1.5.1 SO group
1.5.2 MC group
Rats in the MC group were modeled but not given EA intervention.
1.5.3 EA group
Two hours after modeling, rats in the EA group started to receive EA treatment.
Acupoints: Dazhui (GV 14) and Baihui (GV 20).
Operation: The acupoints were located following the rat’s acupoint map in the Experimental Acupuncture Science[24]. After standard sterilization, filiform needles(Huatuo Brand, Suzhou, China) of 0.25 mm in diameter and 0.25 mm in length were adopted for treatment.Baihui (GV 20) was horizontally punctured with the needle tip towards the rat’s tail, and Dazhui (GV 14) was needled by 30° obliquely, both by 0.5 cun. The needles were twirled for 30 s for stimulation and then connected to the G6805 EA apparatus, with sparseintense wave, 2 Hz/15 Hz, 1-3 mA, and 1-3 V. The stimulation should cause a slight swing of the punctured area. The intervention lasted 30 min each time.
The EA group was subdivided into a 6-hour group and a 24-hour group. The 6-hour EA group only received one session of EA treatment, while the 24-hour EA group would receive another session before sacrifice.The two groups of rats were respectively sacrificed 6 h and 24 h after modeling.
1.6 Detecting parameters
1.6.1 Neurologic deficit scoring
After modeling, the 6 h and 24 h groups were scored according to the following scoring criteria of neurologic deficit[23].
Spontaneous activities (score 0-3): Observe the rats for 5 min to see their ability to approach the wall of cage and explore the environment.
Balance of the four paws (score 0-3): Lift up the rat’s tail to see whether the movements of the paws are balanced.
Balance of the two forepaws (score 0-3): Keep the rat on the edge of a table with its hind paws in the air to see the balance of the forepaws.
Climbing (score 1-3): Place the rat on the wall of cage to observe its climbing ability, and feel its clinging ability when removing the rat from the wall.
Proprioception (score 1-3): To observe the rat’s response to stimulation by touching the two sides of its body with a stick.
Response to whisker touching (score 1-3): To observe the rat’s response to touching its whisker with a stick from behind.
The result would be 3-18 points, and score 18 indicated normal activities. The lower the score, the severer the neurologic deficit.
1.6.2 Serum levels of IL-6, IL-8 and IL-10
When the rats were sacrificed, 5 mL blood was drawn from the abdominal aorta and placed for 10 min at room temperature. The supernatant was isolated after centrifugation at 4300 r/min and then kept in fridge at-20 ℃.
1.7 Statistical processing
The SPSS 16.0 version statistical software was adopted for analyses of all data. The measurement data were expressed by mean ± standard deviation (),and one-way ANOVA was chosen to analyze the variance. The least significance difference (LSD) was used for comparison of the data with equal variances;Dunnett’s T3 for the one with unequal variances.P<0.05 was considered a statistical significance.
2 Results
2.1 Comparison of neurologic deficit score
Six hours after the reperfusion, the MC group scored the lowest, and the neurologic deficit score of the EA group was significantly higher than that in the MC group(P<0.01); the scores of the MC and EA groups were significantly different from the score of the SO group(both P<0.01). Twenty-four hours after the reperfusion,the scores in the MC and EA groups both increased; the MC group remained the lowest score, and it’s markedly different from the scores of SO group and EA group(both P<0.01); there was a significant difference in comparing the score between the EA group and the SO group (P<0.01), (Table 1).
2.2 Comparison of serum IL-6 level
Six hours after the reperfusion, the MC group had the highest level of serum IL-6, and it’s significantly different from the level in the SO group (P<0.01), while it’s insignificantly different from that in the EA group; there was no significant difference in comparing the serum IL-6 level between the SO and EA groups. Twenty-four hours after the reperfusion, every group showed a decrease in the serum IL-6 level, though the MC group remained the highest level, it’s insignificantly different from the level in the SO group but it’s significantly different from that in the EA group (P<0.05), and there was no significant difference between the SO group and the EA group. There were significant differences in comparing the serum IL-6 level between the 6-hour MC group and 24-hour MC group and between the 6-hour EA group and 24-hour EA group (both P<0.01), while there was no significant difference between the 6-hour SO group and the 24-hour SO group (Table 2).
Table1.Comparison of neurologic deficit score (, point)

Table1.Comparison of neurologic deficit score (, point)
Note: Compared with the SO group, 1) P<0.01; compared with the MC group, 2) P<0.01
Group n 6 h 24 h SO 8 17.38±0.52 17.50±0.53 MC 9 5.11±1.361) 8.56±1.671)EA 9 8.67±1.121)2) 13.11±1.051)2)
Table2.Comparison of serum IL-6 level (, ng/L)

Table2.Comparison of serum IL-6 level (, ng/L)
Note: Compared with the SO group, 1) P<0.01; intra-group comparison with the 6-hour group, 2) P<0.01; compared with the MC group of the same period, 3) P<0.05
Group n 6 h 24 h SO 8 176.79±21.83 165.67±18.95 MC 9 209.47±3.421) 180.70±14.112)EA 9 194.52±23.37 151.43±33.12)3)
2.3 Comparison of serum IL-8 level
Six hours after the reperfusion, the MC group had the highest level of serum IL-8, which was significantly different from the level of SO group (P<0.01) and the level of EA group (P<0.05); there was no significant difference between the SO group and the EA group.Twenty-four hours after the reperfusion, the level of serum IL-8 dropped in each group. The MC group still had the highest level, which was significantly different from that in the EA group (P<0.05), while insignificantly different from that in the SO group, and there was no significant difference between the SO and EA groups. There were significant differences in comparing the IL-8 level between the 6-hour MC group and the 24-hour MC group and between the 6-hour EA group and the 24-hour EA group (P<0.01, P<0.05).There was no significant difference in comparing the level between the 6-hour SO group and 24-hour SO group (Table 3).
Table3.Comparison of serum IL-8 level (, ng/L)
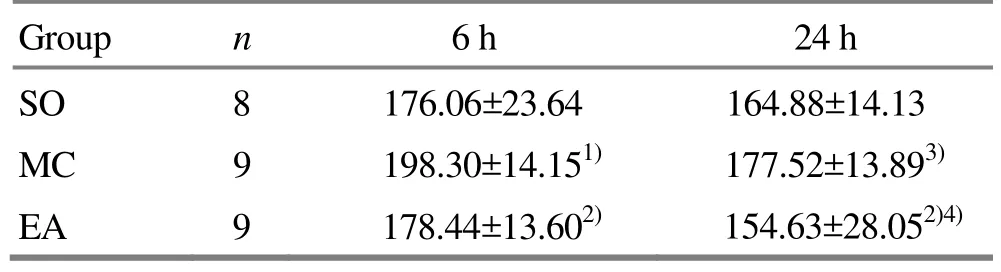
Table3.Comparison of serum IL-8 level (, ng/L)
Note: Compared with the SO group, 1) P<0.05; compared with the MC group of the same period, 2) P<0.05; intra-group comparison with the 6-hour group, 3) P<0.01, 4) P<0.05
Group n 6 h 24 h SO 8 176.06±23.64 164.88±14.13 MC 9 198.30±14.151) 177.52±13.893)EA 9 178.44±13.602) 154.63±28.052)4)
2.4 Comparison of serum IL-10 level
Six hours after the reperfusion, the MC group had the highest level of serum IL-10, which was significantly different from the level in the SO group (P<0.05), but not significantly different from that in the EA group;there was a significant difference between the SO and EA groups (P<0.05). Twenty-four hours after the reperfusion, the level of serum IL-10 dropped in each group, and there were no significant differences among the three groups. There were no significant differences in comparing the IL-10 level between each two subgroups (Table 4).
Table4.Comparison of serum IL-10 level (, ng/L)

Table4.Comparison of serum IL-10 level (, ng/L)
Note: Compared with the SO group, 1) P<0.05
Group n 6 h 24 h SO 8 92.31±16.06 87.22±5.13 MC 9 106.00±11.841) 98.88±15.29 EA 9 105.02±8.321) 90.76±21.30
3 Discussion
It’s believed that the levels of serum IL-6, IL-8 and IL-10 are correlated with the severity of cerebral ischemic injury, climbing high in the acute stage while declining with time. Therefore, the levels of serum IL-6,IL-8 and IL-10 are considered the marker for acute cerebral infarction. IL-6 and IL-8 are proinflammatory factors and IL-10 is an anti-inflammatory factor. The three cytokines can also be used for evaluating the severity and prognosis of cerebral infarction. The change of serum interleukin level suggests that cerebral ischemic injury can evoke the general immune response,which is regarded as a sub-clinical inflammatory state[25-26].
The results showed that the EA group had a better neurologic function than the MC group[27-29], which is in consistence with the previous studies[17-19], indicating that EA intervention be effective in protecting the cerebral function.
The levels of the three serum cytokines in the MC group were higher than that in the SO group 6 h after the reperfusion, indicating that cerebral ischemia causes the increase of the cytokine levels, and the central ischemia triggers a general reaction; the levels in the EA group were lower than that in the MC group,suggesting that early intervention of EA helps regulate the levels of the three cytokines. Twenty-four hours after the reperfusion, the levels of the three cytokines dropped in each group, and the levels of IL-6 and IL-8 in the EA group were lower than that in the MC group; the differences between the two subgroups of the MC and EA groups were statistically significant. It suggests that the intervention of EA can regulate the levels of serum IL-6, IL-8 and the general immune response;comparatively speaking, EA produces a more significant effect in inhibiting IL-6 and IL-8 than promoting IL-10.
Although IL-6 is considered a proinflammatory factor,many experimental studies in animals have proven its action in protecting cerebral nerve cells[30-33], which doesn’t match up with the results of clinical studies. Our previous studies showed that the IL-6 level in brain tissues went down from 6 h till 24 h after the reperfusion, while the level in the EA group was higher than that in the MC group[34]. The current study showed that the serum IL-6 level also went down, but the level in the 24-hour EA group was lower than that in the MC group. The expression of IL-6 in brain tissues is not conforming to that in serum, which may be because that brain tissues are directly injured during cerebral ischemia and the reaction of brain tissues is not as same as the general reaction. Therefore, the change of cytokines in serum can’t represent the change of cytokines in brain tissues, and the serum cytokine level cannot be taken as the absolute evidence to prove the damaging effect of IL-6 to brain.
Our past studies have discovered that EA can produce a content effect in protecting cerebral function in cerebral ischemia[17-19]. The current study revealed a more significant effect of EA in inhibiting IL-6, IL-8 and an insignificant effect in regulating IL-10. It suggests that regulation of serum interleukin level may possibly be connected with the effect of EA in protecting brain, but the details still expect further studies.
Conflict of Interest
The authors declared that there was no potential conflict of interest.
This work was supported by National Natural Science Foundation of China (No. 81102633, No. 81373748).
Statement of Informed Consent
The treatment of animals conformed to the ethical criteria in this experiment.
[1]Persson J, Folkersen L, Ekstrand J, Helleberg J, Gabrielsen A, Lundman P, Hedin U, Paulsson-Berne G. High plasma adiponectin concentration is associated with all-cause mortality in patients with carotid atherosclerosis.Atherosclerosis, 2012, 225(2): 491-496.
[2]Zeng L, Wang Y, Liu J, Wang L, Weng S, Chen K, Domino EF, Yang GY. Pro-inflammatory cytokine network in peripheral inflammation response to cerebral ischemia.Neurosci Lett, 2013, 548: 4-9.
[3]Chen XD, Zhen J, Zhao Y, Feng YL, Ma XL. Study on serum IL-6 and IL-8 in patients with cerebral infarction.Inner Mongolia Med J, 2012, 44(11): 1358-1359.
[4]Di ZL, Wan Q, Lai HA, Wang HD. The role of IL-1β and IL-6 in inflammation of endothelial cell after global cerebral ischemia reperfusion. Xi’an Yike Daxue Xuebao,2001, 22(5): 432-434.
[5]Guo YM, Liang XR, Du YH, Guo YT, Shi XM. The influence of brain-activating acupuncture on the IL-1β content of brain tissues and serum in rats with cerebral focal ischemia. Shanghai Zhenjiu Zazhi, 2004, 23(8):35-37.
[6]Zhou W, Wang LP, Liu H, Bian Y. Influence of scalp acupuncture on serum tumor necrosis factor in patients with acute cerebral infarction. Shanghai Zhenjiu Zazhi,2002, 21(1): 11-12. [4]
[7]Xu XJ, You C, Gao JJ, Liao SC. The expression and function of TNF-α in different time span of cerebral ischemia reperfusion injury. Sichuan Yixue, 2006, 27(7):670-672.
[8]Wu J, Liu KD, Su ZQ, Rao ML, Zhang SQ. Tunor necrosis factor-α expression in ischemic neurons. Zhongfeng Yu ShenjingJibing Zazhi, 2000, 17(2): 77-78.
[9]Ye F, Luo JQ, Chen J, Ye SH, Wu RD. Serum NSE and IL-6, IL-8 and acute cerebral infarction. Zhejiang Shiyong Yixue, 2012, 17(3): 192-193.
[10]Qian LL, Jia K. Brain protection of Xing Nao Jing injection for patients with acute cerebral infarction and its effect on IL-6 and IL-8. Zhongchengyao, 2013, 35(8):1633-1636.
[11]Jin ML, Yang GZ. Discovery of the correlation between plasma IL-8 and acute cerebral infarction. Zhongguo Yixue Chuangxin, 2012, 9(14): 56-57.
[12]Cao QY, Pan XD. The change of the level of interleukin-8 in focal cerebral ischemia and ischemia-reperfusion injury in rats. Chin J Neuroimmunol & Neurol, 2001, 8(1): 20-22.
[13]Chen BL, Li XB, Li J, Ma L. Attack of transient cerebral ischemia and IL-6, IL-8 and IL-10. Nao Yu Shenjing Jibing Zazhi, 2013, 21(4): 247-250.
[14]Zhang CG, Qu CH, Yang H, Liu WH. Dynamic change of serum IL-17 and IL-10 in patients with acute cerebral infarction. Chin J Appl Physiol, 2014, 30(1): 36-37.
[15]Ma J, Yan FL. Role of IL-10 in stroke-induced immunodepression syndrome. Chin J Cerebrovas Dis:Electronic Edition, 2011, 5(5): 49-54.
[16]Zhang Q, Wang YF, Zhang YB, Li JM. Inflammatory biomarkers of stroke. Chin J Stroke, 2013, 8(4): 276-279.
[17]Mu YY, Shen MH, Cheng J, Xia YB, Liu XH, Xiang XR.Effect of electroacupuncture on interleukin-6 expression in hippocampus of cerebral ischemia. Liaoning Zhongyi Zazhi, 2013, 40(4): 797-800.
[18]Shen MH, Xiang XR, Li Y, Pan JL, Ma C, Li ZR. Effect of electroacupuncture on expression of γ-glutamylcysteine synthetase protein and mRNA in cerebral cortex in rats with focal cerebral inchemia-reperfusion. Zhen Ci Yan Jiu,2012, 37(1): 25-29.
[19]Shen MH, Li C, Li ZR. Effect of electroacupuncture on the concentration of GSH and activation of GSH-Px and GR in rats with cerebral ischemia-reperfusion injury. Nanjing Zhongyiyao Daxue Xuebao, 2011, 27(2): 137-139.
[20]Wu WZ, Li ZR, Cheng J, Shen MH, Zhou JL, Wu XL.Effect of electroacupuncture on serum iNOS and HO-1 in patients with acute cerebral infarction of wind-phlegm obstructing collaterals type. Shanghai Zhenjiu Zazhi, 2012,31(12): 858-859.
[21]Kuluz JW, Prado RJ, Dietrich WD, Schleien CL, Watson BD. The effect of nitric oxide synthase inhibition on infarct volume after reversible focal cerebral ischemia in conscious rats. Stroke, 1993, 24(12): 2023-2029.
[22]Longa EZ, Weinstein PR, Carlson S, Cummins R.Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke, 1989, 20(1): 84-91.
[23]Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats statistical validation.Stroke, 1995, 26(4): 627-634.
[24]Li ZR. Experimental Acupuncture Science. Beijing: China Press of Traditional Chinese Medicine, 2005: 255-257.
[25]Senn JJ, Klover PJ, Nowak IA, Zimmers TA, Koniaris LG,Furlanetto RW, Mooney RA. Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependentinsulin resistance in hepatocytes. J Biol Chem,2003, 278(16): 13740-13746.
[26]Krogh-Madsen R, Plomgaard P, Keller P, Keller C,Pedersen BK. Insulin stimulates interleukin-6 and tumor necrosis factor-alpha gene expression in human subcutaneous adipose tissue. Am J Physiol Endocrinol Metab, 2004, 286(2): E234-E238.
[27]Ge LB, Fang C, Xu MS, Xu J, Li CZ. Effects of electroacupuncture on the ability of learning and memory in rats with ischemia-reperfusion injury. J Acupunct Tuina Sci, 2009, 7(1): 3-7.
[28]Dong ZH, Fang JQ, Shao XM. Advances in the study of mechanisms for acupuncture-moxibustion prevention and treatment of ischemia-reperfusion injury. Shanghai Zhenjiu Zazhi, 2011, 30(12): 877-880.
[29]Liu CY, Xu MS, Ge LB. Effects of electroacupuncture plus dopamine D1 receptor antagonist on somatosensory evoked potentials and behavioral changes in rats with cerebral ischemia-reperfusion. J Acupunct Tuina Sci, 2012,10(3): 133-137.
[30]Gertz K, Kronenberg G, K?lin RE, Baldinger T, Werner C,Balkaya M, Eom GD, Hellmann-Regen J, Kr?ber J, Miller KR, Lindauer U, Laufs U, Dirnagl U, Heppner FL, Endres M. Essential role of interleukin-6 in post-stroke angiogenesis. Brain, 2012, 135(Pt 6): 1964-1980.
[31]Nakamachi T, Tsuchida M, Kagami N, Yofu S, Wada Y,Hori M, Tsuchikawa D, Yoshikawa A, Imai N, Nakamura K, Arata S, Shioda S. IL-6 and PACAP receptor expression and localization after global brain ischemia in mice. J Mol Neurosci, 2012, 48(3): 518-525.
[32]Suzuki S, Tanaka K, Suzuki N. Ambivalent aspects of interleukin-6 in cerebral ischemia: inflammatory versus neurotrophic aspects. J Cereb Blood Flow Metab, 2009,29(3): 464-479.
[33]Gravante G, Ong SL, Metcalfe MS, Sorge R, Sconocchia G,Orlando G, Lloyd DM, Dennison AR. Cytokine response to ischemia/reperfusion injury in an vivo perfused porcine liver model. Transplant Proc, 2009, 41(4): 1107-1112.
[34]Mu YY, Shen HM, Cheng J, Xia YB, Liu XH, Xiang XR.Effect of electroacupuncture on interleukin-6 expression in hippocampus of cerebral ischemia-reperfusion rats.Liaoning Zhongyi Zazhi, 2013, 40(4): 797-799.
 Journal of Acupuncture and Tuina Science2015年1期
Journal of Acupuncture and Tuina Science2015年1期
- Journal of Acupuncture and Tuina Science的其它文章
- 復式針刺補瀉對臀大肌攣縮術后髖關節和膝關節屈伸角度的影響
- 隔藥灸天樞和氣海對慢性炎性內臟痛大鼠痛行為和痛情緒的影響
- Clinical observation on warm needling in canicular days for knee osteoarthritis
- Observation on clinical effect of electroacupuncture plus pricking-cupping bloodletting therapy for herpes zoster
- Observation on clinical effect of acupuncture plus Zi Shen Tiao Gan Decoction for perimenopausal insomnia
- Observation on the efficacy of acupoint massage plus moxibustion for refractory insomnia
