石墨烯還原度對P25/石墨烯復合材料光催化活性的影響
王 劍, 王 猛, 熊吉如, 陸春華
石墨烯還原度對P25/石墨烯復合材料光催化活性的影響
王 劍1,2, 王 猛2, 熊吉如2, 陸春華1
(1.南京工業大學材料化學工程國家重點實驗室,材料科學與工程學院,江蘇南京210009;
2.南京倍立達新材料系統工程股份有限公司,江蘇南京211100)
采用高溫熱剝離和溶劑熱過程分別還原氧化石墨和氧化石墨制備出石墨烯,進一步使用所合成的石墨烯與P25通過一步水熱過程合成出石墨烯/P25復合材料。樣品的光催化活性通過可見光下降解羅丹明B進行評測,其中P25和熱剝離還原得到的石墨烯復合比P25和溶劑熱還原的石墨烯復合顯示出更優異的光催化活性,這是由于熱剝離還原的石墨烯具有更高的還原度和更強的電子—空穴分離效率所致。進一步在不同溫度下通過熱剝離法制備了還原石墨烯,探討的石墨烯/ P25復合材料的光催化活性。較高的剝離溫度有利于石墨烯還原程度的改善,導致光催化活性的提高。
光催化;降解;P25;熱剝離;石墨烯還原度
1 Introduction
Visible-light-driven photocatalytic degradation of organic pollutants have attracted more attention in recent years to solve the environmental contamination problems[1,2].The TiO2-based materials,as a kind of stable,nontoxic and inexpensive semiconductor, are the most promising candidates for photocatalytic decontamination[3,4].At present,different TiO2compounds were prepared and modified by many methods,and showed good photocatalytic activities[5].In particular,a combination of TiO2and carbon materials is being considered as one of the most effective materials for the purification of air/water.Various carbon materials,such as activated carbons[6]and carbon nanotubes[7,8],have been widely investigated asthe supportof TiO2 owing to their stable light-absorption properties and good electronic conductivity.
Graphene,one-atom-thick two-dimensional sheet with high surface area,high electrical conductivity and superior mechanical properties[9,10],has also been widely applied to form composites with TiO2[11,12]. The photogenerated electrons would be expected to transfer from the conduction band of TiO2into the graphene surface fast in the composites of graphene and TiO2,leading to an improvementof photocatalytic efficiency.The TiO2/graphene composites can be formed by an in-situ growth of the semiconductor material on graphene[13],or by reduction of graphene oxide(GO)previously deposited on the semiconductor[14,15].In the latter case,the graphene was usually prepared by reduction of graphene oxide under hydrothermal[11,15,16]or light-irradiation conditions[17].The photocatalytic activity of the composite photocatalysts strongly depends on the preparation method.However,there are few studies about the influence of the graphene which was prepared by different reduction methods on the photocatalytic activity of the TiO2/ graphene composites.
At present,solvothermal and high-temperature thermal exfoliation are two of the most common methods for preparing reduced graphenes.Meanwhile,it has been reported that the graphene prepared by a thermal exfoliation method showed a much better conductivity and higher reduction degree than the direct solvothermal reduction[18].In the present work,the graphene oxide was reduced by the thermalexfoliation and solvothermal methods for synthesis of the graphene,and the commercial TiO2(P25)was further supported on the graphene as photocatalyst via a onestep hydrothermal method.The P25/graphene photocatalysts from hydrothermal method exhibited different photocatalytic activity for photodegradation of Rhodamine B,which may be caused by the difference of the graphene reduction degree.The graphene was further synthesized by thermal exfoliation method at different temperatures,and the corresponding photocatalytic activity of the P25/graphene composites was also investigated.
2 Experimental
2.1Synthesis of the P25/graphene composites
Graphite oxide was purchased from Nanjing XFNANO Materials Tech Co.,Ltd.All the chemicals were of analyticalgrade and were used withoutfurther purification.The thermal exfoliated graphite was prepared according to the following method[19].200 mg of graphite oxide powder was transferred into a crucible and subjected to thermal exfoliation at 600, 800,and 1 000℃for 30 s in a tube furnace,and subsequently pulled out fast and cooled to room temperature,which were named as TG600,TG800 and TG1000 respectively.The solvothermal reduced graphene(SG)was as follows.A total of 35 mL of 0.5 mg/mL graphene oxides aqueous solution was transferred to a Teflon-lined autoclave and heated at 180℃for 6 h.
The P25/graphene composites were prepared via a hydrothermal method.Briefly,2 mg of graphene and 0.2 g of P25 was dissolved in a solution of distilled H2O(20 mL)and ethanol(10 mL)by ultrasonic treatment for 2 h to get a homogeneous suspension.The suspension was then placed in a 40 mL Teflon-sealed autoclave and maintained at 120℃for 3 h. Finally,the resulting composite was washed by deionized water,and dried at 40℃.The composites prepared from graphenes via the thermal exfoliation and solvothermal reduction were named as P25/TG and P25/SG,respectively.
2.2Characterizations
X-ray diffraction(XRD)was performed on a ARL X’TRA X-ray diffractometer at room temperature,using Cu Kαradiation(λ=0.154 06 nm). The morphology of the products was characterized by transmission electron microscopy(TEM,JEM-2010, 200 kV)and field emission scanning electron microscopy(FESEM,S-4800,15 kV).The FT-IR spectra were recorded on a Vector-22 FT-IR spectrometer in the range of 4 000-400 cm-1.UV-vis diffuse reflectance spectra were recorded with a 3101 spectrometer. Photoluminescence(PL)emission spectrum was recorded on a FL3-221 fluorescence spectrophotometer equipped with a 450 W xenon lamp as the excitation source at room temperature(excitation wavelength λex=290 nm).The Brunauer-Emmett-Teller(BET) surface area were measured by a nitrogen adsorption technique at 77 K using an ASAP2020 M automated gas-sorption system(America).The electrical conductivity of the SG and TG which was prepared to be a film in advance was measured by a four point probe method(SB100 A/2,Qianfeng).The SG and TG films were prepared by the vacuum filtration method, using a cellulose ester membrane(50 mm in diameter,220 nm pore size,Shenghemo)as a filter.After being dried at60℃in a vacuum desiccator for 3 d, paper-like SG and TG films were obtained.
2.3Photocatalytic degradation of Rhodamine B
The photocatalytic activities of the composites were evaluated by photodegradation of Rhodamine B. Before irradiation,0.1 g photocatalysts were added into 50 mL Rhodamine B aqueous solution with aconcentration of 10 mg/L and stirred in the dark for 30 min.During the photoreaction,the suspension was irradiated by a 300 W mercury lamp with a 420 nm filter under magnetic stirring.Approximately 4 mL of aqueous solution was collected at regular intervals and centrifuged.The concentration of Rhodamine B in the centrifuged aqueous solution was determined by measuring the absorption of Rhodamine B at550 nm on a UV-Vis spectrophotometer,from which the photocatalytic activity was evaluated.
3 Results and discussion
3.1Structure and morphology of the thermal exfoliated graphene and the P25/graphene composites
Fig.1(a)shows the XRD patterns of the graphite oxide and the thermal exfoliated graphene prepared under different temperatures.The XRD pattern of graphite oxide shows a typical(002)peak located at 12.3°,corresponding to an interlayer spacing of 0.776 nm.After the high-temperature treatment,the sharp peak around 12°disappears,indicating that the graphite oxide transfer into the graphene by the thermal reduction.The XRD patterns of the P25-SG,P25-TG600,P25-TG800 and P25-TG1000 are shown in Fig.1(b).Allof the P25/graphene composites have a similar XRD pattern to the pure P25,and no diffraction peaks for carbon species are observed,which might be due to the low amount of graphene in the composites.

Fig.1 XRD patterns of(a)the graphite oxide and thermal exfoliated graphene and(b)the P25/graphene composites.
The morphologies of the obtained TG and the P25/TG samples were observed by the SEM and TEM(Fig.2).Fig.2(a)exhibits the SEM image of the graphene synthesized by the thermal exfoliation at 1 000℃,which shows a presence of agglomerates of graphene nanosheets.Fig.2(b)is the SEM magnification image of P25/TG1000.Lots of TiO2nanoparticles and some graphenes can be observed.In order to investigate the morphology ata high magnification, the TEM images of the TG1000 and P25/TG1000 are shown in Fig.2(c)and 2(d),respectively.A twodimensional sheet structure with micrometers-long wrinkles can be found in Fig.2(c),which is an obvious feature of the graphene.Some P25 nanoparticles were well dispersed on the graphene in Fig.2(d). However,due to the little content of graphene,there is also a lot of TiO2nanoparticles that are not loaded on the surface of graphene.

Fig.2 (a),(b)SEM and(c),(d)TEM images of the TG1000 and P25/TG1000 samples.
3.2Photocatalytic activity of the P25/graphene composites
To investigate the optical properties of the P25/ graphene composites,the UV-vis absorption spectra of the samples were further performed.As shown in Fig.3,there is not any absorption above 400 nm for the pure P25,however,the band edges of the P25/ SG and P25/TG1000 have an obvious red shift,which means thata more efficientutilization of the solar spectrum could be achieved.The possible reason may be due to the formation of Ti-O-C bond between P25 and graphene,similar to the case of carbon-doped TiO2composites[20,21].In order to prove this,the FT-IR spectra of the pure P25 and the P25/ TG1000 composite were characterized.Fig.4 shows the FT-IR spectra of the pure P25 and the P25/ TG1000 composite in the range of 3 000~450 cm-1with different magnifications.After the introduction of graphene,the absorption peak corresponding to Ti -O-Ti of P25 is blue-shifted to a high wavenumber. The blue shift was attributed to a combination of the vibration of Ti-O-Ti and Ti-O-C bonds[20].The FTIR results confirmed the formation of Ti-O-C bonds between P25 and graphene.

Fig.3 UV-vis absorption spectra of the P25,P25/SG and P25/TG1000.

Fig.4 FTIR spectra of P25 and P25/TG 1000 composite with different magnifications in the range of 3 000-450 cm-1.
The photocatalytic activities of the P25/graphene composites were further measured by the photodegradation of Rhodamine B as model reaction under visible light irradiation.As shown in Fig.5,there is little decrease in concentration of Rhodamine B for blank test without photocatalysts.The P25/SG composite shows a better activity than the pure P25.More than 60%of the initial dye was decomposed by the P25/ SG composites,butnearly 90%of the initial dye still remained in the solution after the same time period for the bare P25 due to its limited photoresponding range. Moreover,it also can be found that all of the P25/TG composite show a larger improvement in the photodegradation rate of the dye than the P25/SG composite.The photocatlytic degradation rate of P25/TG composites increase with the thermal exfoliation temperature.
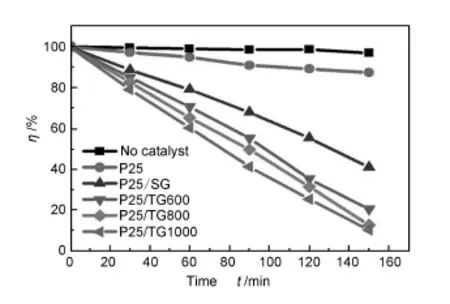
Fig.5 Photocatalytic degradation of RhodamineB under visible-light irradiation for different photocatalysts.
3.3Influence of reduction degree of graphene on the photocatalytic activity
It has been reported that three factors,including the adsorption of contaminantmolecules,the lightabsorption,and the charge transportation and separation,are crucial in photodegradation reactions.The absorption ability of two different P25/graphene composites was first studied.Fig.6(a)shows the nitrogen absorption-desorption isotherms of the P25/graphene composites.The BET surface areas of the P25/ TG1000 and the P25/SG composites are 42.7 and 39.7 m2/g,respectively.The little difference in BET surface area suggests that the adsorption of the dye molecule for these two composites is almost the same during the photocatalytic degradation.From the light absorption spectra in Fig.3,it can be found that the distinction of light-absorption ability is also small for the two P25/graphene composites.
For the P25/graphene composites,the graphene was mainly used as a good electron acceptor to promote the migration of photogenerated electron from the semiconductor to graphene.For that,the PL excitation of the P25/TG1000 and P25/SG composites was further researched.As shown in Fig.6(b),all of the P25,P25/TG1000 and P25/SG composites at 370 nm show a strong fluorescence emission peak. However,the fluorescence intensities of the P 25/TG1000 and P25/SG significantly decreased compared with the pure P25 nanoparticles,and the P25/TG1000 composite shows a lower emission intensity than the P25/SG composite,which suggessa much better photogenrated electron transfer ability from the conduction band of P25 into the graphene for the P25/TG1000 composite than P25/SG composite.

Fig.6 (a)Nitrogen adsorption-desorption isotherms of P25/SG and P25/TG1000,(b)PL emission of P25,P25-SG and P25-TG1000.
Based on the fact that the graphene was prepared by different reductive methods,it is easy to understand that the reduction degree of graphehe is responsible for the different separation ability of photogenerated electron and hole pairs.The XPS was employed to analyze the reduction degree of the graphene.The C 1s XPS spectra of the different kinds of graphene are shown in Fig.7.

Fig.7 C 1s XPS spectra of the graphene reduced by the thermalexfoliation:(a)TG600,(b)TG800,(c)TG1000 and(d)SG via solvothermal method.
The binding energies of 284.6,286.5 and 288.5 eV are attributed to the C—C bonds,the C—O and C= O functional groups,respectively. The changes of the C/O ratio of graphene indicate the different reduction degrees and are summarized in Table 1.It can be seen that the TG1000 has a larger C/O ratio than the SG,indicating that the high-temperature is helpful for improving the reduction degree of graphene.For the graphene prepared by the thermal exfoliation method under different temperatures, the morphology was further characterized by SEM (Fig.8).It can be seen that the TG600 and TG800 exhibite a similar graphene layer structure to the TG1000(Fig.2(a)),which suggests that the morphology of thermalexfoliated graphene under different conditions has no obvious changes.However,therelative ratios of C/O increased with the thermalexfoliation temperature,so the reductive degrees of thermal exfoliated graphene also increase.

Table 1 Relative ratios of C/O and electrical conductivities of the SG and TG.

Fig.8 SEM images of TG600 and TG800.
Moreover,it is easy to understand that the graphene which has a high reduction degree would have little defects and a high electrical conductivity.So the electrical conductivity of the graphene was further measured for graphene films and the average values of the electrical conductivity are shown in Table 1.The electrical conductivities of the SG,TG600,TG800 and TG1000 were about5.3,169.8,182.4 and 198. 6 S·m-1,respectively.Therefore,the photogenrated electron on the TG surface transfers faster than that on the SG,leading to the improvement in photocatalytic activity.It can also be speculated from the electrical conductivity of TG that high thermal exfoliation temperature is beneficialfor improving the electrical conductivity and reduction degree,and therefore the photocatalytic activity.Based on the above analysis,it can be concluded that when the graphene as a supporter of the P25 nanoparticle,itcan suppress the recombination of photogenerated electron-hole pairs to improve its photocatalytic quantum efficiency.Further, the graphene prepared by the high-temperature treatment has a high reduction degree and electrical conductivity,which is helpfulfor the photogenrated electron transfer on its surface,leading to the enhancement of photoinduced carriers’separation and photocatalytic activity.
4 Conclusions
Graphene sample has been prepared by the thermal exfoliation and solvothermal method.The P25/TG and P25/SG composites exhibit similar absorption for dye and visible-light responding ability. The P25/TG composites exhibit the better photocatalytic activity than that of the P25/SG composite,which may be caused by a high reduction degree of the as-prepared graphene by the thermal exfoliation method.This work is anticipated to open a new possibility for enhancing the photocatalytic activity of graphene-based materials by improving the reduction degree of the graphene.
[1] Di Paola A,García-Lópeza E,Marcìa G,et al.A survey of photocatalytic materials for environmental remediation[J].J Hazard Mater,2012,211:3-29.
[2] Kudo A,Miseki Y.Heterogeneous photocatalyst materials for water splitting[J].Chem Soc Rev,2009,38(1):253-278.
[3] Linsebigler A L,Lu G,Yates Jr J T.Photocatalysis on TiO2surfaces:principles,mechanisms,and selected results[J]. Chem Rev,1995,95(3):735-758.
[4] Mor G K,Varghese O K,Paulose M,etal.A review on highly ordered,vertically oriented TiO2nanotube arrays:Fabrication, material properties,and solar energy applications[J].Sol Energ Mater&Sol C,2006,90(14):2011-2075.
[5] Bavykin D V,Friedrich J M,Walsh F C.Protonated titanates and TiO2nanostructured materials:synthesis,properties,and applications[J].Adv Mater,2006,18(21):2807-2824.
[6] Huang B,Saka S.Photocatalytic activity of TiO2crystallite-activated carbon composites prepared in supercritical isopropanol for the decomposition of formaldehyde[J].J Wood Sci,2003,49 (1):79-85.
[7] Yu Y,Yu J C,Yu J G,et al.Enhancement of photocatalytic activity of mesoporous TiO2by using carbon nanotubes[J].Appl Catal A:Gen,2005,289(2):186-196.
[8] Woan K,Pyrgiotakis G,Sigmund W.Photocatalytic carbonnano-tube-TiO2composites[J].Adv Mater,2009,21(21):2233-2239.
[9] Neto A C,Guinea F,Peres N,etal.The electronic properties of graphene[J].Rev Mod Phys,2009,81(1):109.
[10] Geim A K,Novoselov K S.The rise of graphene[J].Nature Mater,2007,6(3):183-191.
[11] Liang Y,Wang H,Casalongue H S,et al.TiO2nanocrystals grown on graphene as advanced photocatalytic hybrid materials [J].Nano Res,2010,3(10):701-705.
[12] Kim H I,Moon G H,Monllor-Satoca D,et al.Solar photoconversion using graphene/TiO2composites:Nanographene shell on TiO2core versus TiO2nanoparticles on graphene sheet [J].J Phys Chem C,2012,116(1):1535-1543.
[13] Zhang H,Xu P,Du G,et al.A facile one-step synthesis of TiO2/graphene composites for photodegradation of methyl orange[J].Nano Research,2011,4(3):274-283.
[14] Dreyer D R,Park S,Bielawski C W,et al.The chemistry of graphene oxide[J].Chem Soc Rev,2010,39(1):228-240.
[15] Shen J,Yan B,Shi M,et al.One step hydrothermal synthesis of TiO2-reduced graphene oxide sheets[J].J Mater Chem, 2011,21(10):3415-3421.
[16] Perera S D,Mariano R G,Vu K,et al.Hydrothermalsynthesis of graphene-TiO2nanotube composites with enhanced photocatalytic activity[J].ACS Catal,2012,2(6):949-956.
[17] Williams G,Seger B,Kamat P V.TiO2-graphene nanocomposites.UV-assisted photocatalytic reduction of graphene oxide [J].ACS Nano,2008,2(7):1487-1491.
[18] Luo D,Zhang G,Liu J,et al.Evaluation criteria for reduced graphene oxide[J].J Phys Chem C,2011,115(23):11327-11335.
[19] Mcallister M J,Li J L,Adamson D H,etal.Single sheetfunctionalized graphene by oxidation and thermal expansion of graphite[J].Chem Mater,2007,19(18):4396-4404.
[20] Sakthivel S,Kisch H.Daylight photocatalysis by carbon-modified titanium dioxide[J].Angewandte Chemie International E-dition,2003,42(40):4908-4911.
[21] Ren W,Ai Z,Jia F,et al.Low temperature preparation and visible light photocatalytic activity of mesoporous carbon-doped crystalline TiO2[J].Appl Catal B-Environ,2007,69(3): 138-144.
Enhanced photocatalytic activity of a TiO2/graphene composite by improving the reduction degree of graphene
WANG Jian1,2, WANG Meng2, XIONG Ji-ru2, LU Chun-hua1
(1.StateKeyLaboratoryofMaterials-OrientedChemicalEngineering, CollegeofMaterialsScienceandEngineering,NanjingTechUniversity,Nanjing210009,China;2.NanjingBeilidaNewMaterialsSystemEngineeringCO.Ltd.,Nanjing211100,China)
Two kinds of graphene prepared by a high-temperature exfoliation and a solvothermal method were used as supports of a TiO2catalyst(P25)from Degussa,Inc to prepare TiO2/graphene composites.The photocatalytic activities of the composites were evaluated by their degradation of Rhodamine B in aqueous solutions under visible light.Results indicate that the composites prepared by high-temperature exfoliation have much higher photocatalytic activities than those produced by the solvothermal method or the unsupported P25.Both the adsorption capacity of Rhodamine B on the composites and their light absorption characteristics are independent of the kind of graphene used.The activity increases with exfoliation temperature and reduction degree of the graphene regardless of the methods and conditions used,indicating that a high degree of reduction of graphene can inhibit the recombination of electron-hole pairs generated by light irradiation by increasing electron transfer from TiO2to the graphene layer.
Photocatalytic;Degradation;P25;Thermal exfoliation;Graphene reductive degree
LU Chun-hua,E-mail:chhlu@njtech.edu.cn
TB332
A
中國博士后基金(2014M551577).
陸春華.E-mail:chhlu@njtech.edu.cn
王 劍,博士.E-mail:wangjian@sxicc.ac.cn
1007-8827(2015)04-0357-07
Received date:2015-03-16;Revised date:2015-08-10
Foundation item:China Postdoctoral Science Foundation(2014M551577).
Author introduction:WANG Jian,Ph.D.E-mail:wangjian@sxicc.ac.cn
English edition available online ScienceDirect(http://www.sciencedirect.com/science/journal/18725805).
10.1016/S1872-5805(15)60195-0

