重組新城疫病毒rl-RVG抑制人肺腺癌A549系細胞遷移及相關機制
金 惠,步雪峰,王穆彬,蘇春香,嚴玉蘭
(江蘇大學 1.附屬人民醫院 呼吸科 江蘇 鎮江 212002; 2.臨床醫學院,江蘇 鎮江 212001;3.附屬人民醫院 普外科, 江蘇 鎮江 212002)
?
研究論文
重組新城疫病毒rl-RVG抑制人肺腺癌A549系細胞遷移及相關機制
金 惠1, 2,步雪峰3,王穆彬2,蘇春香2,嚴玉蘭1*
(江蘇大學 1.附屬人民醫院 呼吸科 江蘇 鎮江 212002; 2.臨床醫學院,江蘇 鎮江 212001;3.附屬人民醫院 普外科, 江蘇 鎮江 212002)
目的觀察表達狂犬病毒糖蛋白的重組新城疫病毒(rl-RVG)對人肺癌細胞系A549的遷移影響,初步探討其可能的機制。方法重組新城疫病毒rl-RVG直接感染A549細胞為rl-RVG實驗組,新城疫病毒LaSota株處理的A549細胞組及未感染病毒的A549細胞組作為對照組。Western blot法檢測NDV-HN蛋白及狂犬病毒糖蛋白(RVG),細胞增殖實驗測定新城疫病毒使用的最佳作用濃度;劃痕實驗及Transwell法測A549遷移;Western blot法及免疫熒光法檢測E-cadherin,MMP2蛋白表達。結果空白對照組無NDV、rl-RVG表達,rl-RVG僅在感染rl-RVG細胞組有表達,NDV在感染rl-RVG細胞組及感染NDV細胞組皆有表達;與空白對照組相比,rl-RVG及NDV稀釋濃度大于5×10-5對細胞的增殖有抑制作用(P<0.05);rl-RVG組遷移的距離及細胞數明顯減少(P<0.05)。與空白對照組及NDV感染組相比,rl-RVG組E-cadherin蛋白表達水平上調(P<0.05),MMP2蛋白表達水平減弱(P<0.05)。結論重組新城疫病毒rl-RVG能抑制人肺癌細胞系A549的遷移,并可能通過影響肺腺癌A549上皮細胞-間質轉化(EMT)過程中的調控因子E-cadherin,MMP2蛋白而對細胞遷移而作用。
重組新城疫病毒RVG;肺癌;遷移;上皮細胞-間質轉化
重組新城疫病毒(recombinant avirulent NDV LaSota strain expressing the rabies virus glycoproteinrl-RVG)作為基因治療腫瘤的載體,插入外源基因不影響NDV的結構,也不影響病毒對腫瘤特異性抗腫瘤作用,對人正常細胞無不良反應[1]。前期的研究中發現表達狂犬病毒糖蛋白的重組新城疫病毒(recombinant avirulent NDV LaSota strain expressing the rabies virus glycoprotein,rl-RVG)[2]對于人類肺腺癌A549細胞具有較強的生長抑制作用,rl-RVG[2]對人肺腺癌A549細胞系有抑制增殖并促進其凋亡作用[3],提示rl-RVG可能會引起人肺腺癌的遷移及轉移活性減弱。但是rl-RVG對人肺腺癌A549細胞遷移的影響及可能機制無相關報道。所以本實驗將表達狂犬病毒糖蛋白的重組新城疫病毒疫苗感染A549肺癌細胞,通過觀察A549細胞的遷移能力,進一步探索 rl-RVG對肺腺癌細胞的作用。
1 材料與方法
1.1 材料
新城疫病毒LaSota系、rl-RVG和雞抗NDV血清一抗(中國農業科學院哈爾濱研究所饋贈);小鼠抗狂犬病毒ERA系G蛋白一抗、羊抗兔抗體β-actin(Santa Cruz公司)。MTT試劑盒(Sigma公司); 兔抗E-cadherin,兔抗MMP2抗體(博士德公司); HRP標記的山羊抗兔IgG二抗(康為世紀公司)。人肺腺癌A549 細胞為江蘇大學基礎醫學研究所保存;DMEM及胎牛血清(維森特公司)。
1.2 細胞增殖實驗(MTT)檢測A549細胞增殖
LaSota系NDV病毒液及rl-RVG病毒液的滴度都在雞胚半數感染量(50% egg infective doses,EID50)為109.8EID50/mL 左右[4]。用無血清DMEM將病毒原液稀釋至以下濃度: 1×10-3、1×10-4、5×10-5和1×10-6。對數增殖期A549細胞接種于96孔板上(5×104個/mL),每孔100 μL過夜培養后換含20 mL/L胎牛血清的DMEM維持液,在NDV LaSota組和rl-RVG組加入病毒液,陰性空白對照組為PBS;每組設5個平行孔,DMEM完全培養基為調零孔。每孔過夜培養后加入20 μL MTT再培養4 h,后加入150 μL二甲基亞砜溶解,酶標儀490 nm上測定吸光度(A)值,實驗重復3 次,并用以下公式計算細胞活力值。細胞活力值=(實驗組平均A值/空白對照組平均A值)×100%。
1.3 Western blot法檢測RVG、NDV HN和E-cadherin、MMP2蛋白的表達
對數增殖期A549細胞種植于6孔板上(5×104個/mL),NDV-LaSota組和rl-RVG組加入10-6稀釋度病毒液,陰性對照為PBS組。培養24 h提取細胞蛋白經120 g/L SDS-PAGE后轉膜; 50 g/L脫脂奶粉封閉1 h,分別用小鼠抗RVG抗體、雞抗NDV 抗體(1∶300)、羊抗兔抗體β-actin(1∶10 000),4 ℃孵育過夜; 緩沖液(TBST)洗3 次,加入HRP標記的山羊抗小鼠二抗和HRP標記的兔抗雞二抗、室溫孵育2 h; TBST洗3次,ECL發光劑在Typhoon9400掃描儀上掃描。E-cadherin、MMP2檢測方法同上,一抗分別為兔抗E-cadherin抗體(1∶300),兔抗MMP2抗體(1∶300),二抗為山羊抗兔IgG(1∶10 000)。內參為羊抗兔抗體β-actin,EC顯色后掃描,Image J圖片軟件處理數據。
1.4 細胞劃痕遷移實驗
接種對數增殖期A549細胞于24孔板中(1×105個/孔),用含10%胎牛血清的高糖培養基(DMEM)培養液培養24 h,用無菌200 μL槍頭在細胞層中縱向劃線,形成寬度均勻一致的無細胞傷口模型;加入空白對照組(DMEM培養基)和NDV、rl-RVG(10-6稀釋度)分別感染24 h;光鏡下觀察劃痕的寬度。
1.5 Transwell實驗
用無血清DMEM調整對數增殖期A549細胞濃度為1.0×105個/mL,按100 μL/孔細胞量接種于放入24孔培養板中Transwell上室,下室加入含LaSota系NDV(10-6稀釋度)和rl-RVG(10-6稀釋度) 的DMEM 600 μL(對照組加入DMEM 600 μL),37 ℃培養24 h。用棉簽擦去Transwell上室聚碳酸酯膜表面的細胞,PBS洗2遍后,將上室置于4%多聚甲醛中固定15 min。PBS洗2遍后,結晶紫染色20 min,再用PBS洗2遍后,在倒置熒光顯微鏡下對5個不同視野的穿過膜細胞計數(×200),求平均值。
1.6 免疫熒光法檢測蛋白表達
取24孔板中NDV,rl-RVG處理24 h后的A549細胞,棄培養液,甲醛固定6 h,TBS洗滌,加triton X- 100、牛血清白蛋白400 μL, 37 ℃保持1 h,后加入E-cadherin,MMP2一抗, 4 ℃孵育24 h, TBS洗滌3遍,加二抗,室溫下孵育45 min,TBS洗滌3 遍。加核熒光抗體,照相。Image J圖片軟件處理數據。
1.7 統計學分析
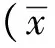
2 結果
2.1 Western blot法檢測RVG及NDV-HN蛋白表達
感染A549細胞24 h后,發現RVG蛋白在NDV LaSota組和PBS組沒有表達,RVG蛋白可以在感染rl-RVG的A549細胞中穩定表達,并且能促進NDV-HN蛋白在A549細胞中的表達(P<0.05)(圖1)。
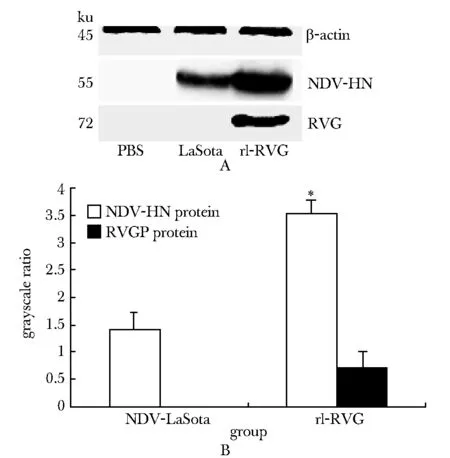
2.2 rl-RVG病毒對A549細胞生長的影響
細胞增殖實驗(MTT)檢測A549細胞增殖:不同濃度NDV和rl-RVG作用A549細胞24 h后,細胞活力值隨溶度增加而升高,與空白對照組相比,rl-RVG及NDV稀釋濃度大于5×10-5對細胞的增殖有抑制作用(P<0.05) (圖2),故選擇稀釋溶度為106倍的rl-RVG及NDV,作用于A549細胞24 h,對細胞增殖無明顯影響。
A.Westren blot; B.grayscale ratio; *P<0.05 compared with NDV-LaSota group圖1 rl-RVG感染A549細胞24 h后RVG蛋白和NDV蛋白表達Fig 1 The expression of RVG protein and NDV protein in A549 cells after infected by rl-RVG(n=5)
*P<0.05 compared with PBS control group圖2 不同溶度rl-RVG,NDV對肺癌A549細胞生長的影響Fig 2 The cell growth of lung adenocarcinoma A549 was influenced after infected by different solubilities of rl-RVG and NDV
2.3 rl-RVG對肺腺癌A549 細胞遷移能力的影響
2.3.1 細胞劃痕遷移實驗:A549細胞劃痕后經rl-RVG、NDV處理24 h,由劃痕邊緣向中央遷移后兩側距離分別為(465±39.9)μm,(778.6±6.7)μm,與對照組劃痕邊緣向中央遷移后兩側距離為(1 405±12.6)μm相比,rl-RVG感染后的A549細胞由劃痕邊緣向中央遷移的兩側距離較大(P<0.05) (圖3)。
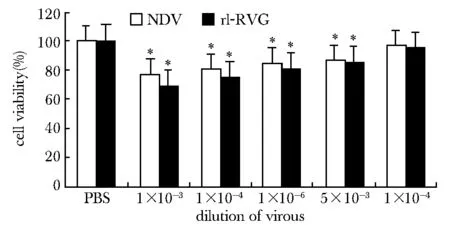
2.3.2 Transwell實驗:Transwell 實驗檢測,與對照組相比,rl-RVG處理組細胞穿過Transwell小室的細胞數明顯減少(P<0.05) (圖4)。
2.4 Western blot法及免疫熒光法檢測rl-RVG感染A549細胞后E-cadherin、MMP2的表達量
rl-RVG感染A549細胞后E-cadherin表達量增加,3組吸光度比值有統計學意義(P<0.05),MMP2 66 ku/72 ku表達減弱,3組吸光度比值有統計學意義 (P<0.05)。免疫熒光法示rl-RVG組細胞膜上及細胞質中的E-cadherin熒光強度升高,3組細胞熒光強度有統計學意義(P<0.05);rl-RVG組細胞膜上及細胞質中的MMP2熒光強度減弱(P<0.05);NDV感染A549細胞,E-cadherin表達量及熒光強度也增加(P<0.05),但弱于rl-RVG組,MMP2表達量及熒光強度也減弱(P<0.05),但強于rl-RVG組(圖5)。
3 討論
國內外大量研究表明NDV具有抗腫瘤作用[5-7],主要通過對腫瘤細胞的特異性溶瘤的直接細胞毒作用[5,8- 10],引起腫瘤細胞增殖抑制并促進其凋亡[3]。本研究中,劃痕實驗及Transwell實驗表明rl-RVG感染后的A549細胞組遷移明顯抑制。而Western blot法檢測結果顯示rl-RVG組NDV HN蛋白表達量較優于NDV組,證明了RVG 能夠促進NDV在細胞間的傳播,與報道實驗結果一致。因此,rl-RVG抗腫瘤作用除NDV特異性溶瘤特性外,還可以通過抑制腫瘤細胞遷移得以實現。
而腫瘤細胞的遷移和對周圍組織和血管的侵襲是腫瘤細胞轉移的關鍵步驟[11],上皮細胞-間質轉化(epithelial mesenchymalt ransition,EMT)與腫瘤細胞的原位侵襲和遠處轉移有著密切的關系。EMT是多細胞生物胚胎發生中的基礎過程,也存在于多種慢性疾病以及腫瘤的發生發展過程中, 它以上皮細胞極性喪失為主要特征。而參與EMT的重要調控因子有E-cadherin和MMP2[12- 13]。E-cadherin 是一類主要介導細胞間相互黏附的單鏈I型鈣依賴性跨膜蛋白,激活鈣黏蛋白, 促進橋粒連接形成,維持正常上皮細胞的完整性及分化[14],故而E-cadherin的異常表達被認為與EMT的產生直接相關,在癌癥侵襲中起著重要作用。MMP2是具有Zn2+依賴性的最主要的降解型膠原酶蛋白水解酶,參與腫瘤新生血管形成、腫瘤細胞的侵襲和轉移灶的形成,與腫瘤EMT的發生密切相關, MMP2在易發生早期轉移的小細胞肺癌中表達增高[15]。

圖3 通過劃痕實驗檢測rl-RVG及NDV感染對A549細胞遷移能力影響
Arrow pointed to A549 cell; *P<0.05 compared with PBS group
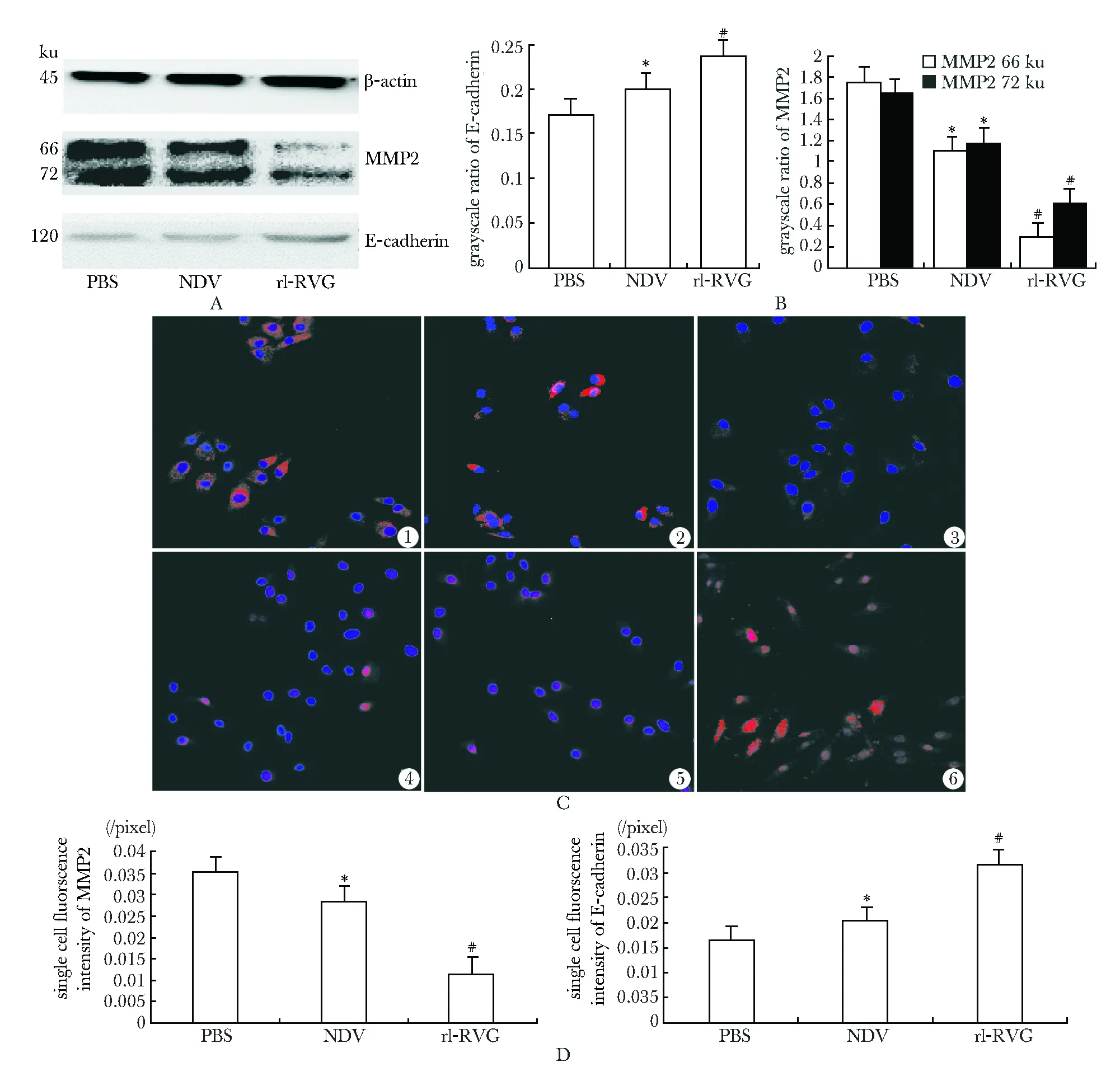
A:Western blot; B:the grayscale ratio of MMP2, E-cadherin;C:immunofluorescence of MMP2 and E-cadherin, ①~③.MMP2 of PBS, NDV and rl-RVG group,respectively; ④~⑥.E-cadherin of PBS, NDV and rl-RVG group,respectively; D:single cell fluorsence intensity of MMP2,E-cadherin;*P<0.05 compared with PBS group;#P<0.05 compared with PBS and NDV group
圖5 rl-RVG及NDV 感染A549 細胞24 h 后的MMP2、E-cadherin蛋白Western blot及免疫熒光表達
Fig 5 Western blot and Immunofluorescence analysis of MMP2, E-cadherin protein expression in A549 cells after infected by rl-RVG and NDV on 24 hours
本研究Western blot結果表明,感染rl-RVG后E-cadherin表達增多、MMP2降低,提示rl-RVG抑制A549細胞遷移能力較強,rl-RVG對人肺癌細胞A549中E-cadherin、MMP2的合成和分布起一定的調節作用。rl-RVG可能通過增加A549細胞中的E-cadherin的合成, 減少MMP2的合成增加抑制肺癌細胞的遷移;這一推測與劃痕愈合實驗,Transwell實驗的結果一致。
綜上所述,rl-RVG 感染A549后能抑制細胞生長及遷移,并可能通過影響E-cadherin,MMP2的表達實施EMT而起作用,但關于rl-RVG對肺腺癌抗遷移的影響與肺腺癌EMT的相關性,還需體內實驗的進一步證實。通過這次實驗,為rl-RVG在臨床上用于肺癌患者術后的復發和轉移的治療提供了一定的實驗依據。
[1] Janke M,Peeters B, de Leeuw O,etal.Recombinant Newcastle disease virus (NDV) with inserted gene coding for GM-CSF as a new vector for cancer immunogene therapy[J]. Gene Therapy, 2007, 14: 1639- 1649.
[2] Ge J, Wang X, Tao L,etal.Newcastle disease virus-vectored rabies vaccine is safe, highly immunogenic, and provides long-lasting protection in dogs and cats.[J]. J Virol, 2011, 85: 8241- 8252.
[3] 嚴玉蘭,梁冰,張金,等.rl-RVG 體外抑制肺癌細胞增殖并促進其凋亡[J].細胞與分子免疫學雜志,2014,30:449- 453.
[4] Kim YJ,Lee SA,Myung SC,etal.Radicicol,an inhibitor of Hsp90,enhances TRAIL-induced apoptosis in human epithelial ovarian carcinoma cells by promoting activation of apoptosis-related proteins[J]. Mol Cell Biochem,2012,359: 33- 43.
[5] Pecora AL, Rizvi N, Cohen GI,etal.Phase I trial of intravenous administration of PV701, an oncolytic virus, in patients with advanced solid cancers.[J]. J Clin Oncol, 2002, 20: 2251- 2266.
[6] Freeman AI, Zakay-Rones Z, Gomori JM,etal.Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme.[J]. Mol Ther, 2006, 13: 221- 228.
[7] Fu F, Zhao M, Yang YJ,etal. Antiproliferative effect of newcastle disease virus strain D90 on human lung cancer cell line A549[J]. Oncol Res, 2011, 19: 323- 333.
[8] Phuangsab A, Lorence RM, Reichard KW,etal. Newcastle disease virus therapy of human tumor xenografts: antitumor effects of local or systemic administration.[J]. Cancer Lett, 2001, 172: 27- 36.
[9] Elankumaran S,Rockemann D,Samal SK.Newcastle disease virus exerts oncolysis by both intrinsic and extrinsic caspasE-dependent pathways of cell death[J]. J Virol,2006,80: 7522- 7534.
[10] Hrabak A,Csuka I,Bajor T,etal.The cytotoxic anti-tumor effect of MTH-68 /H,a live attenuated Newcastle disease virus is mediated by the induction of nitric oxide synthesis in rat peritoneal macrophagesinvitro[J]. Cancer Lett,2006,231: 279- 289.
[11] Sahai E. Mechanisms of cancer cell invasion.[J]. Curr Opin Genet Dev, 2005, 15: 87- 96.
[12] Huber MA, Kraut N, Beug H,etal.Molecular requirements for epithelial-mesenchymal transition during tumor progression[J].Curr Opin Cell Biol, 2005,17:548- 558.
[13] Ray ME, Mehra R, Sandler HM,etal. E-cadherin protein expression predicts prostate cancer salvage radiotherapy outcomes [J]. J Urol, 2006, 176: 1409- 1414.
[14] Schmalhofer O,Brabletz S,Brabletz T. E-cadherin,beta-catenin,and ZEB1 in malignant progression of cancer[J].Cancer Meta Rev,2009,28:151- 166.
[15] Ylisirni? S, H?yhty? M, Turpeenniemi-Hujanen T. Serum matrix metalloproteinases -2, -9 and tissue inhibitors of metalloproteinases -1, -2 in lung cancer-TIMP-1 as a prognostic marker[J].Anticancer Res, 2000, 20: 1311- 1316.
Recombinant avirulent newcastle disease virus rl-RVG inhibits migration of lung adenocarcinoma A549 and related mechanism
JIN Hui1,2, BU Xue-feng3, WANG Mu-bin2, SU Chun-xiang2, YAN Yu-lan1*
(1.Dept. of Respiratory Medicine,the Affiliated People’ s Hospital of Jiangsu University, Zhenjiang 212002;2.Clinical Medicine College of Jiangsu University, Zhenjiang 212001;3.Dept. of General Surgery,the Affiliated People’ s Hospital of Jiangsu University,Zhenjiang 212002,China)
Objective Observe the effects of rl-RVG on migration of lung adenocarcinoma A549cells, preliminarily explore potential mechanism.Methods The group infected with the rl-RVG was experimental group, The group infected with NDV and the group not infected with virous were control groups.The experimental detected for the expressions of RVG and NDV-HN proteins by Western blot.Cell growth experiment determined the best active concentrations of newcastle disease virus and rl-RVG;Observe the effects of rl-RVG and NDV on migration of lung adenocarcinoma A549 cells by scratch assay and Transwell method.The expression of E-cadherin and MMP2 was observed by Western blot and immunofluorescence.The LaSota strain of NDV was used as control group and PBS was the blank control. Results Neither RVG nor NDV proteins didn’t expressed in blank control group.RVG protein expressed in rl-RVG group and NDV protein expressed in both rl-RVG group and NDV group. Cell proliferation was inhibited in rl-RVG group and NDV group more significantly as compared with the blank control group(P<0.05). After infected with rl-RVG, migration distance and number of cells significantly reduced(P<0.05). After A549 cells were infected with rl-RVG, the expression of E-cadherin was enhanced(P<0.05)and the expression of MMP2 was decreased as compared with the blank control group and NDV group(P<0.05).Conclusions Recombinant avirulent newcastle disease virus can inhibit the migration of A549 cellsinvitro, which may be attributed to regulatory factors of E-cadherin and MMP2 in the procession of epithelial mesenchymal transition EMT.
rl-RVG; lung cancer; migration; epithelial mesenchymal transition
2014- 10- 09
2015- 01- 04
鎮江市社會發展基金(2013041)
1001-6325(2015)04-0508-06
R734.2
A
*通信作者(corresponding author):ylyan2005@163.com

