ZnO/大孔碳復合材料的一步合成及其光催化性能(英文)
曲玲玲 韓婷婷 施鶴飛 等
摘要以硝酸鋅和葡萄糖酸鈉為原料,通過一步法合成納米ZnO/大孔碳復合材料.葡萄糖酸鈉不同官能團之間的協同作用在ZnO/大孔碳復合材料的生成過程中起到了重要作用.利用XRD,SEM,TEM,Raman和TGA對產品的物相、形貌和結構進行了表征.以亞甲基藍(MB)為探針分子考察ZnO/大孔碳復合材料的光催化活性,結果表明,與商用ZnO粉末相比,ZnO/大孔碳復合材料具有更好的光催化活性.ZnO/大孔碳復合材料具有較高催化活性的可能原因是多孔碳具有優良的接受和傳導電子性能,抑制了ZnO光生電子空穴的復合幾率,從而提高了光催化活性.
關鍵詞大孔碳;氧化鋅;光催化劑; 復合物
Semiconductor photocatalysis has become more and more attractive and important since it is one of the promising processes to solve environmental problems via the photochemical decomposition of pollutants and hazardous materials[12]. Zinc oxide (ZnO), as a potential semiconductor with direct wide band gap (3.37 eV), is close to being one of the ideal photocatalysts because of its relatively inexpensive and provide photogenerated holes with high oxidizing power due to its wide band gap energy[34].
In the photocatalytic oxidation process of semiconductors, the photocatalytic efficiency depends on the fate of photogenerated holeelectron pairs under irradiation.The electronhole recombination has faster kinetics than surface redox reactions and greatly reduces the quantum efficiency of photocatalysis. Therefore, to enhance the photocatalysis efficiency, it is essential to retard the recombination of the charge carriers[56]. Many works have been devoted to reduce the recombination of charge carriers by coupling the ZnO with carbon materials, such as activated carbon[7], carbon nanotube[8], and grapheme[910]. Generally, the fabrication of ZnO/carbon composites require two steps, the synthesis of ZnO and the subsequent mixing with carbon materials, which make the synthesis route complicated and render the catalysts too expensive for widespread industrial use[3, 8, 10].
In the present study, we develop a facile onestep method to construct nano ZnO decorated macroporous carbon (ZnO/MPC), which only needs two reagents, the Zn(NO3)2·6H2O as the zinc source and sodium gluconate. Different functional groups of sodium gluconate play synergetic roles in the formation of ZnO/MPC. ZnO/MPC photocatalyst showed enhanced photocatalytic activity for the degradation of organic dye. Photoluminescence (PL) is employed to study the excited states of ZnO/MPC and comfirm that MPC hybridized ZnO inhibits the recombination of electrons and holes on ZnO/MPC successfully, which make ZnO/MPC possess a significantly enhanced photocatalytic activity over the commercial ZnO powder.
1Experimental
1.1Preparation of nano ZnO decorated macroporous carbon (ZnO/MPC)
ZnO/MPC was synthesized by heating a mixture of Zn(NO3)2·6H2O and sodium gluconate after milling in the mass ratio of 1∶3 at 900 ℃ for 3 h in N2 atmosphere. After cooling down at room temperature, the black products were washed several times by deionized water and absolute ethanol. Finally, the washed precipitation was dried in vacuum oven at 60 ℃ for 24 h.
湖南師范大學自然科學學報第38卷第5期曲玲玲等:ZnO/大孔碳復合材料的一步合成及其光催化性能1.2Characterization
Xray powder diffraction (XRD) analysis was performed on a Bruker D8 diffractometer with highintensity Cu Kα radiation (λ=1.540 6 ) for the phase composition of samples. The fieldemission scanning electron microscope (FESEM) measurements were characterized with a Hitachi S4800 operating at 15 kV. The samples used for FESEM were prepared by dispersing of some products in ethanol, then placing a drop of the solution onto the surface of Al column and Au was sprayed on them to improve their surface conductive. Raman spectra were obtained using a Renishaw Raman system model 2 000 spectrometer. The BET surface area of the powders was determined from BrunauerEmmettTeller (BET) measurements using a ASAP 2020 surface area and porosity analyzer. Room temperature photoluminescence spectra (PL) of the samples were measured on a Varian Cary Eclipse fluorescence spectrophotometer at an excitation wavelength of 325 nm.
1.3Photochemical experiments
The photocatalytic activity of ZnO/MPCs was evaluated by the degradation of MB at room temperature. A 125 W highpressure mercury lamp with the strongest emission at 365 nm was used as light source. The experiments were carried out in a 250 mL beaker, opening to air and the distance between the lamp and the solution is about 12 cm. A mixture containing a powdered catalyst (50 mg) and a fresh aqueous solution of MB (100 mL, 10 mg/L) was magnetically stirred in the dark for about 1 h to establish an adsorptiondesorption equilibrium. The suspensions were kept under constant airequilibrated conditions before and during illumination. At certain time intervals, 4 mL aliquots were sampled and remove the photocatalyst particles. The filtrates were analyzed by recording variations of the maximum absorption band (665 nm), using a UVVis spectrophotometer (Shimadzu Corporation, UV2450).
2Results and discussion
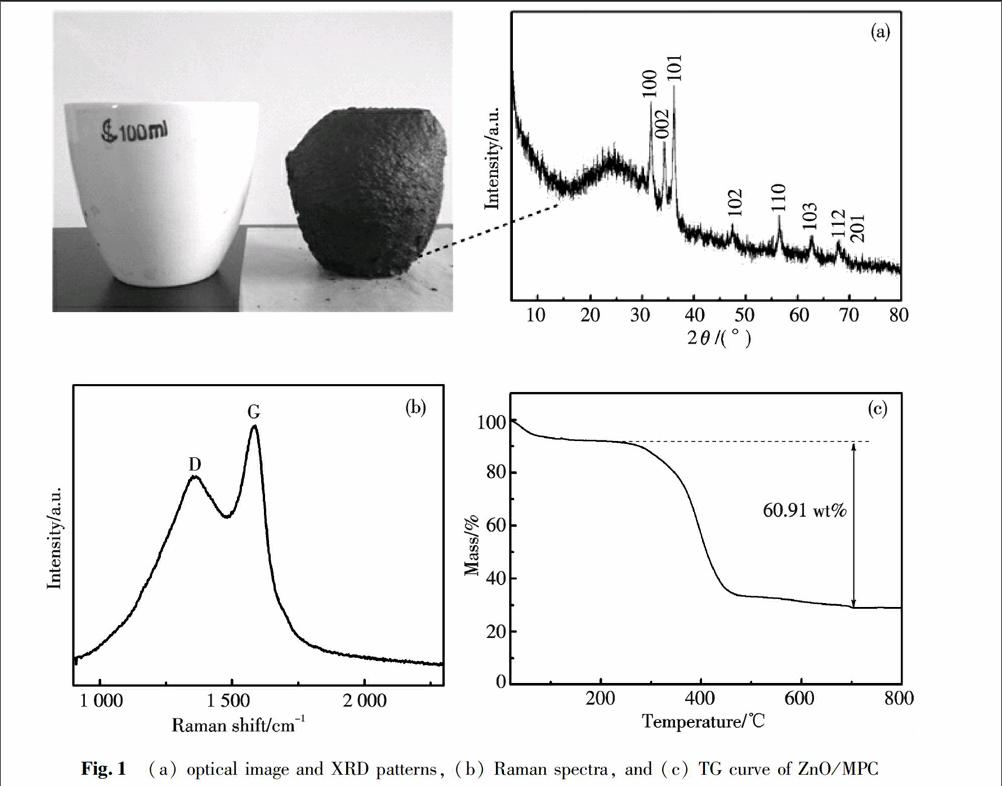
The phase and composition of products were identified by Xray diffraction (XRD). Fig.1a shows the optical image of product and its typical XRD pattern. All of the observed peaks in the patterns can be indexed to the standard wurtzite structure of ZnO (JCPDS card No. 361451). The intensive peaks reveal that the hexagonal ZnO possess highly crystalline through the low temperature carbonization process. The Raman spectra (Fig.1b) of ZnO/MPC shows three primary peaks including a D band at ~1 360 cm-1, a G band at ~1 583 cm-1, respectively, typical for amorphous carbons. The large ID/IG value (0.78) indicates the low degree of graphitization of ZnO/MPC. The carbon content is determined by thermogravimetric analysis (TGA, Fig.1c). It can be noticed that the mass loss below 220 ℃ could be probably attributed to the evaporation of the adsorbed gaseous molecules or moisture, and the major mass loss takes place at 220 ℃ and completes at 600 ℃. The estimations based on the TG curves indicate that the carbon content in the ZnO/MPC is about 60.91 wt%.
Fig.1(a) optical image and XRD patterns, (b) Raman spectra, and (c) TG curve of ZnO/MPCThe morphology and microstructure of the products are characterized by SEM and TEM. Fig.2a and Fig.2c present the panoramic SEM image and TEM image of the ZnO/MPC, respectively. SEM image in Fig.2a shows the ZnO/MPC has an open structure with interconnected macropores. The macroporous cores exhibit a foamlike morphology surrounded by thin walls. The sizes of most macropores are about several hundred nanometers, and the thickness of the walls around them is less than 100 nm (Fig.2b). From the Fig.2d, the image clearly indicates that a number of ZnO nanoparticles attached to the carbon wall of macropores and these ZnO nanoparticles are less than 50 nm. These ZnO nanoparticles are dispersed well into the carbon materials. The nitrogen adsorption and desorption measurements were performed to explore their inner structures. Fig.3 is the typical nitrogen adsorption/desorption isotherm of the ZnO/MPC, which belongs to the type Ⅳ isotherm according to the IUPAC classification. BET (BrunauerEmmettTeller) surface areas, calculated from nitrogen adsorption isotherms, show that the surface area of ZnO/MPC is 79.4 m2·g-1.
Fig.2SEM (a,b) and TEM (c,d) images of the ZnO/MPCFig.3Nitrogen adsorption/desorption isotherm of the ZnO/MPCGenerally, the fabrication of metal oxide/carbon composites requires two steps, the synthesis of metal or metal oxide and the subsequent mixing of carbon materials, which make the synthesis route complicated and render the catalysts too expensive for widespread industrial use.ZnO/MPC composites can be fabricated by onestep synthesis which should attribute to the synergetic roles of different functional groups of sodium gluconate. Different functional groups of sodium gluconate play synergetic roles in the formation process of ZnO/MPC. Firstly, the strong coordinating ability of carboxylate group to metal cation make the gluconate can strongly bond with the Zn2+ and form the zinccarboxylate complex[11], ZnⅡ(RCOO)2-nn; Secondly, when heating up to specified temperature, ZnⅡ(RCOO)2-nn can thermally decompose into ZnO and CO2 gas. Specially, CO2 produced in situ by carboxylate group pyrolysis can serve as the template to form the macroporous carbon. In the gasification, both the porosity and specific surface area of the carbon are increased[1214]; Thirdly, sodium gluconate as the derivative of glucose could form the carbon materials via high temperature carbonization reaction[1516].
The photocatalytic activity of the present ZnO/MPC was evaluated with the photodegradation of MB in aqueous solution. In the presence of the ZnO/MPC, MB was almost completerly degraded after 90 min of UV light irradiation (Fig.4a). Further experiments were carried out to compare the photocatalytic activities of ZnO/MPC and commercial ZnO powder (Fig.4b). As illustrated in Fig.4b, a blank experiment in the absence of the photocatalyst but under UV light irradiation shows that only a small quantity of MB was degraded. In the presence of commercial ZnO powder, about 38% of MB was degraded after 90 min of UV light irradiation. It is obvious that ZnO/MPC show a significant improvement in MB photodegradation activity over the commercial ZnO powder.
Fig.4(a) UVVisible spectra of MB vs. photoreaction time in the presence of ZnO/MPC; (b) the photocatalytic degradation of MB over the ZnO/MPC and commercial ZnO powderFig.5Photoluminescence spectra of commercial pure ZnO powder and ZnO/MPCPhotoluminescence (PL) is often employed to study surface structure and excited states of semiconductor. With electronhole pair recombination after a photocatalyst is irradiated, photons are emitted, resulting in photoluminescence[1718]. It was reported that ZnO typically exhibits UV band edge emission and broad visible emissions at green and yellow bands at room temperature. The PL peak at 391 nm is due to the recombination of a photogenerated hole with an electron occupying the oxygen vacancies in the ZnO[19]. As the ZnO nanoparticles were attached on MPCs, the PL emission intensity of ZnO/MPC at 391 nm decreased dramatically compared with that of commercial pure ZnO powder (Fig.5). The results indicate that attachment of ZnO nanoparticles on MPCs inhibits the recombination of electrons and holes on ZnO/MPC successfully. The low recombination rate of electrons and holes is also an indispensable reason for the enhanced photocatalytic activity of ZnO/MPC.
3Conclusion
In summary, we demonstrate a feasible synthetic route for the synthesis and fabrication of ZnO/MPC. During the whole construction process, different functional groups of sodium gluconate play synergetic roles in the formation of ZnO/MPC. The hybridization with ZnO on the surface of MPC could significantly increase the photocatalytic efficiency of ZnO. The intimate contact between MPC and ZnO nanoparticles is beneficial for efficient electron transfer, which is supposed to be responsible for reducing the recombination of charge carriers. The enhancement of photocatalytic activity was attributed to the high migration efficiency of photoinduced electrons and the inhibited charge carriers recombination due to the electronic interaction between ZnO and MPC.
References:
[1]CHEN C C, MA W H, ZHAO J C, Semiconductormediated photodegradation of pollutants under visiblelight irradiation[J]. Chem Soc Rev, 2010,39(11):42064219.
[2]TANG H J, HAN T T, LUO Z J, et al. Magnetite/Ndoped carboxylaterich carbon spheres: Synthesis, characterization and visiblelightinduced photocatalytic properties[J]. Chin Chem Lett, 2013,24(1):6366.
[3]XU T G, ZHANG L W, CHENG H Y, et al. Significantly enhanced photocatalytic performance of ZnO via graphene hybridization and the mechanism study[J]. Appl Catal B: Environ, 2011,101(34):382387.
[4]WANG Y X, LI X Y, WANG N, et al. Controllable synthesis of ZnO nanoflowers and their morphologydependent photocatalytic activities[J]. Sep Purif Technol, 2008,62(3):727732.
[5]YAO Y, LI G H, CISTON S, et al. Photoreactive TiO2 /carbon nanotube composites: synthesis and reactivity[J]. Environ Sci Technol, 2008,42(13):49524957.
[6]TIAN L H, YE L Q, LIU J Y, et al. Solvothermal synthesis of CNTsWO3 hybrid nanostructures with high photocatalytic activity under visible light[J]. Catal Commun, 2012,17:99103.
[7]MELIN E P, DAZ O G, RODRGUEZ J M D, et al. ZnO activation by using activated carbon as a support: Characterisation and photoreactivity[J]. Appl Catal A: Gen, 2009,364(12):174181.
[8]SALEH T A, GONDAL M A, DRMOSH Q A, et al. Enhancement in photocatalytic activity for acetaldehyde removal by embedding ZnO nano particles on multiwall carbon nanotubes[J]. Chem Eng J, 2011,166(1):407412.
[9]FU D Y, HAN G Y, CHANG Y Z, et al. The synthesis and properties of ZnOgraphene nano hybrid for photodegradation of organic pollutant in water[J]. Mater Chem Phys, 2012,132(23):673681.
[10]FAN H G, ZHAO X T, YANG J H, et al. ZnOgraphene composite for photocatalytic degradation of methylene blue dye[J]. Catal Commun, 2012,29:2934.
[11]WANG J, GAO Z, LI Z S, et al. Green synthesis of graphene nanosheets/ZnO composites and electrochemical properties[J]. J Solid State Chem, 2011,184(6):14211427.
[12]GHULE A V, GHULE K, CHEN C Y, et al. In situ thermoTOFSIMS study of thermal decomposition of zinc acetate dehydrate[J]. J Mass Spectrom, 2004,39(10):12021208.
[13]GUO S H, PENG J H, LI W, et al. Effects of CO2 activation on porous structures of coconut shellbased activated carbons[J]. Appl Surf Sci, 2009,255(20):84438449
[14]CHEN X Y, SONG H, ZHANG Z J, et al. A rational template carbonization method for producing highly porous carbon for supercapacitor application[J]. Electrochim Acta, 2014,117:5561.
[15]TITIRICI M M, ANTONIETTI M, Chemistry and materials options of sustainable carbon materials made by hydrothermal carbonization[J]. Chem Soc Rev, 2010,39(1):103116.
[16]LUO Z J, TANG H J, QU L L, et al. A visiblelightdriven solid state photoFenton reagent based on magnetite/carboxylaterich carbon spheres[J]. Cryst Eng Comm, 2012,14(18):57105713.
[17]YU J G, MA T T, LIU S W, Enhanced photocatalytic activity of mesoporous TiO2 aggregates by embedding carbon nanotubes as electrontransfer channel[J]. Phys Chem Chem Phys, 2011,13(8):34913501.
[18]WEI A, XIONG L, SUN L, et al. Onestep electrochemical synthesis of a grapheneZnO hybrid for improved photocatalytic activity[J]. Mater Res Bull, 2013,48(8):28552860.
[19]SUN J H, DONG S Y, WANG Y K, et al. Preparation and photocatalytic property of a novel dumbbellshaped ZnO microcrystal photocatalyst[J]. J Hazard Mater, 2009,172(2/3):15201526.
(編輯楊春明)