Preparation and characterization of monodisperse zirconia spherical nanometer powder via lamellar liquid crystal template method
Weiyan He,Jinrong Liu,Zhenzhu Cao,Caihong Li,Yanfang Gao
School of Chemical Engineering,Inner Mongolia University of Technology,Hohhot 010051,China
Keywords:Zirconia Spherical nanometer powder Lamellar liquid crystal template
ABSTRACT Cubic phase sphericalzirconia nano-powderwas prepared by a directtemplate route in the lamellar liquid crystal formed by polyoxyethylene tert-octylphenyl ether(Triton X-100)/sodium dodecyl sulfate(SDS)/H2O.The precursor powder and zirconia powder were characterized by XRD,FT-IR,TG/DSC,TEM,and SEM methods.Results show that the stability of the lamellar liquid crystal is controlled by NH3·H2O concentration.The size of nanoparticles is greatly affected by NH3·H2Oand ZrOCl2·8H2Oconcentrations.The zirconia nanoparticles shownarrow particle size distribution of 10-30 nm.
1.Introduction
Extensive researches have been carried out to control the morphology of zirconia[11,12].Monodisperse spherical zirconia particle has been prepared by sol-gel reaction between zirconium isopropoxide and zirconium-chloride at 340°C[13].However,the high reaction temperature limits its large-scale production.Thermalhydrolysis of zirconium slat solution in a bulk solution is another way to produce colloidal particles.For example,Tai et al.have prepared spherical zirconia particles in the thermal hydrolysis of zirconyl nitrate solution at 95°C in a stable microemulsion[14].Microemulsion solution is a transparent,isotropic and thermodynamically stable system with nanosized droplets dispersed in a continuous oilphase[15].Recently,sphericalzirconia nanoparticles are successfully produced in a microemulsion[16,17].Generally,liquid synthesis routes of ZrO2mainly involve in microemulsion[18].Emulsions are dispersions of one liquid in another,both liquids being mutually immiscible,so emulsions are thermodynamically unstable.Since non-equilibrium nature of emulsions determines its lifetime,droplet size and polydispersity are highly dependent on the preparation method.Moreover,the structure of dispersed and continuous phases is a topic recently for the production of specialty materials[19].
Compared with microemulsion,lamellar liquid crystal is a suitable candidate for template and self-assembling of nanoscale materials due to its order and mobility at the molecular level[20,21].Much scienti fic and technological effort has been dedicated to the preparation of zirconia by lytropic liquid crystal template.Ding et al.synthesized monodisperse zinc gluconate nanoparticles using the lamellar liquid crystal formed by Triton X-100/n-C10H21OH/H2O system[22]and showed that the shape and size of nanoparticle are nearly independent of the composition of lamellar liquid crystal.Freitas et al.reported directional growth of macroscopic ceramic needles by combining a thermoresponsive sulfated hydroxy zirconyl hydrosol with a swollen hexagonal liquid crystal[23].Santos et al.reported the synthesis of zirconia microneedles by direct nucleation of particles inside a hexagonal swollen liquid crystal[24].However,little attention has been paid to the stability and instability effects of precursor on the lamellar phase.
In this work,a lamellar liquid crystal composed of polyoxyethylene tert-octylphenyl ether(Triton X-100)/sodium dodecyl sulfate(SDS)/H2O is used to prepare monodisperse spherical zirconia nano-powder via the reaction between ammonia water and zirconium salt solved in nano-reactors.The stability and microstructure of the lamellar liquid crystal are investigated.The effect of concentrations of zirconium oxychloride(ZrOCl2·8H2O)and ammonia water(NH3·H2O)on the size of ZrO2particles is discussed.
2.Experimental
2.1.Materials
Triton X-100(polyoxyethylene tert-octylphenyl ether with an average number of EO units of 9.5,>99%),ZrOCl2·8H2O(purity 99.5%),ethanol and NH3·H2O were purchased from Sinopharm Chemical Reagent Limited Corporation,and SDS was purchased from Tianjing Fine Chemical Factory(China).The chemicals were used as received without further puri fication.Water used was deionized and distilled.
2.2.Characterization
The birefringence properties of samples were recorded with polarizing optical microscope(POM)(Axio Scope.A1,Ceiss,Germany).Structural analysis was carried out by X-ray diffraction using(Bruker D8 Advance,Germany)equipped with Ni- filtered Cu-Kαradiation(a scan rate of 3(°) · min-1and a step size of 0.02°)and Fourier Transform Infrared(FT-IR)Spectroscopy(Thermo Scienti fic,Nicolet iS10,USA).Particle size and particle size distribution were determined by NanoZS90(Malvern Instruments Ltd.,England).The morphology of prepared ZrO2powder was examined by a transmission electron microscope(TEM)(JEM-2010,Hitachi Ltd.,Japan).Surface morphology of ZrO2nanostructures was examined by a field emission scanning electron microscope(SEM)(S-3400,Hitachi Ltd.,Japan).TG-DTA was measured on a thermoanalyzer(PT1600,Linseis,Germany)in air from room temperature to around 1000 °C at a speed of 10 °C · min-1.
2.3.Experimental method
2.3.1.Determination of lamellar liquid crystal region
The lamellar liquid crystal templates were prepared by mixing Triton X-100,SDS,and H2O solutions.The ternary diagram was determined by titration of the non-ionic surfactant(Triton X-100)into anionic surfactant SDS aqueous solution.The samples were mixed vigorously using a vortex mixer and stored in a thermostat for 24 h at 298 K.Phase boundary of the lamellar liquid crystal region was determined by using a polarizing microscope,under which anisotropic phases exhibit typical microscopic textures.For example,a lamellar liquid crystalline phase shows a cruciate flower or oily streaks.On the other hand,isotropic phases such as micellar and cubic phases do not present a particular texture but a dark background under the polarizing microscope.
Now it happened that three tailors had met together, and the two elder thought, that after having successfully put in so many fine and strong stitches with never a wrong one amongst them, they were certain to do the right thing here too
2.3.2.Preparation of zirconia nano-powder
Lamellar liquid crystal samples were prepared by substituting zirconia and NH3·H2O aqueous solution for water in the Triton X-100/SDS/H2O system.All the samples were prepared by weight in the sealed glass vials.Mixtures were then vigorously stirred at room temperature(25°C)until the formation of a homogenous and highly viscous gel.Samples were centrifuged at 4000 r·min-1for 2 min to remove air bubbles.When the lamellar phase reached equilibrium state,the sample became transparent with birefringence features under the polarizing microscope.The lamellar phase templates were removed using absolute ethanol and deionized water.The resulting nanoparticles were separated by centrifugation and washed with deionized water and alcohol alternatively.The precursor was calcined at 600°C in the air.
3.Results and Discussion
3.1.Determination of the lamellar liquid crystal region
Apartialphase diagram ofthe ternary system SDS/Triton X-100/H2O is shown in Fig.1.L phase occupies minor partofthe phase diagram,extending in the range of 3%-66%SDS,4%-61%Triton X-100,and 47.6%-78.9%water.
3.2.Stability of the lamellar liquid crystalline templates
For successful template of nanostructured materials,the template should be appropriate to de fine the synthesis.It is necessary to prove that the composition of inorganic precursors does not disrupt the long-distance order of lamellar liquid crystalline.Therefore,it is important to determine the stable region of lamellar phase.
3.2.1.Stability of the lamellar liquid crystal formed by Triton X-100/SDS/NH3·H2O system
Texture oflamellar phase.The optical textures revealthatthe microstructure of the lamellar liquid crystal template does not rupture with NH3·H2O.When the content of NH3·H2O increases to 25%(Fig.2D),lamellarliquid crystalturns fromtransparentto white-turbid in appearance,indicating that the long-distance order of the lamellar liquid crystal is disrupted.
3.2.2.Stability of the lamellar liquid crystal formed by Triton X-100/SDS/ZrOCl2·8H2O system
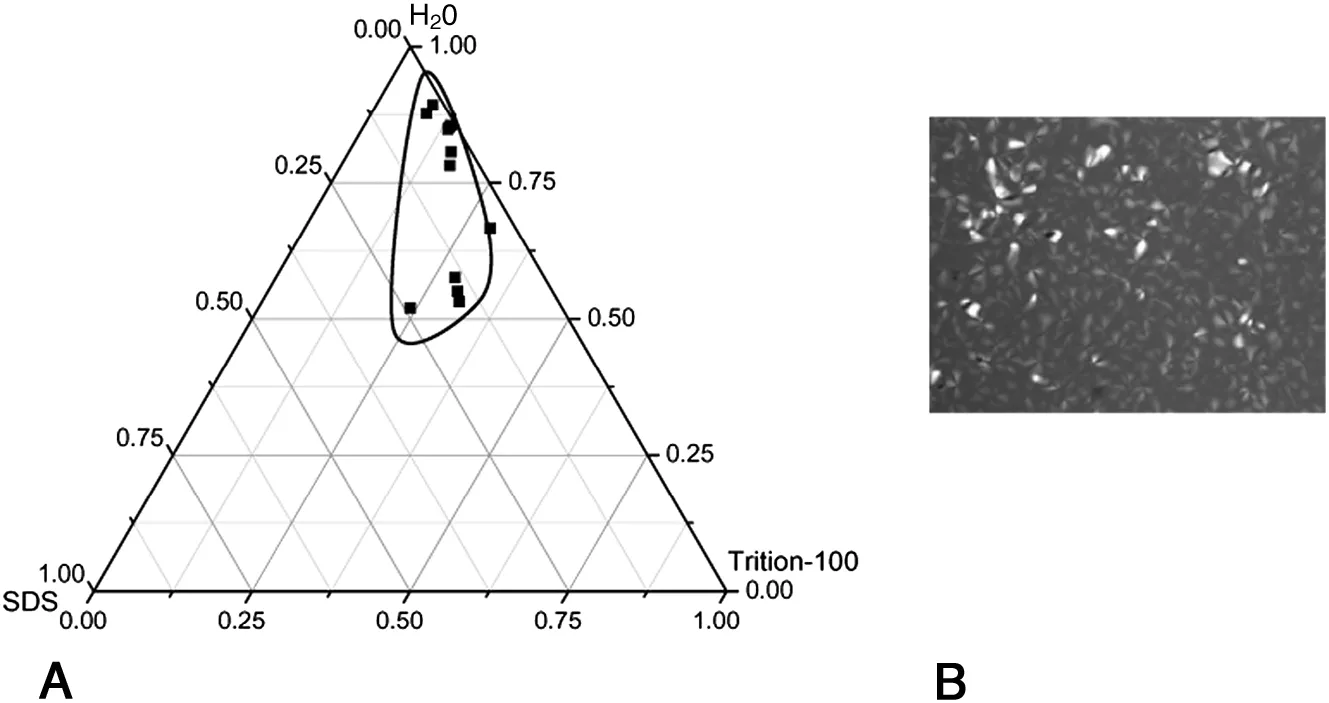
Fig.1.Partial phase diagram of Triton X-100/SDS/H2O system(A)and typical POM picture of lamellar liquid crystalline phase(B)at 298 K.
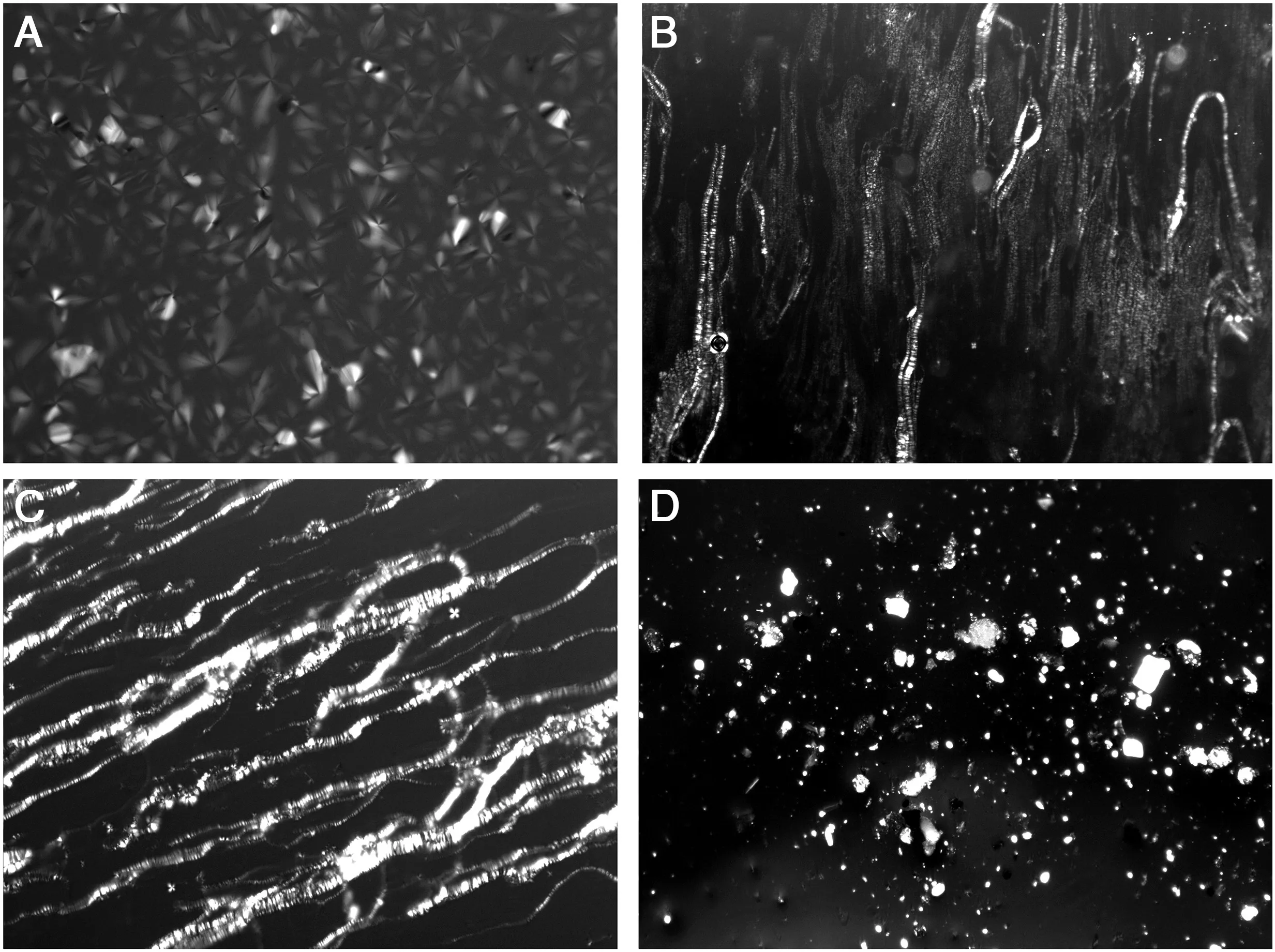
Fig.2.POM images(×200)of lyotropic liquid crystal of Triton X-100/SDS/NH3·H2O at different contents of NH3·H2O.(A)0;(B)6%;(C)12.5%;(D)25%.
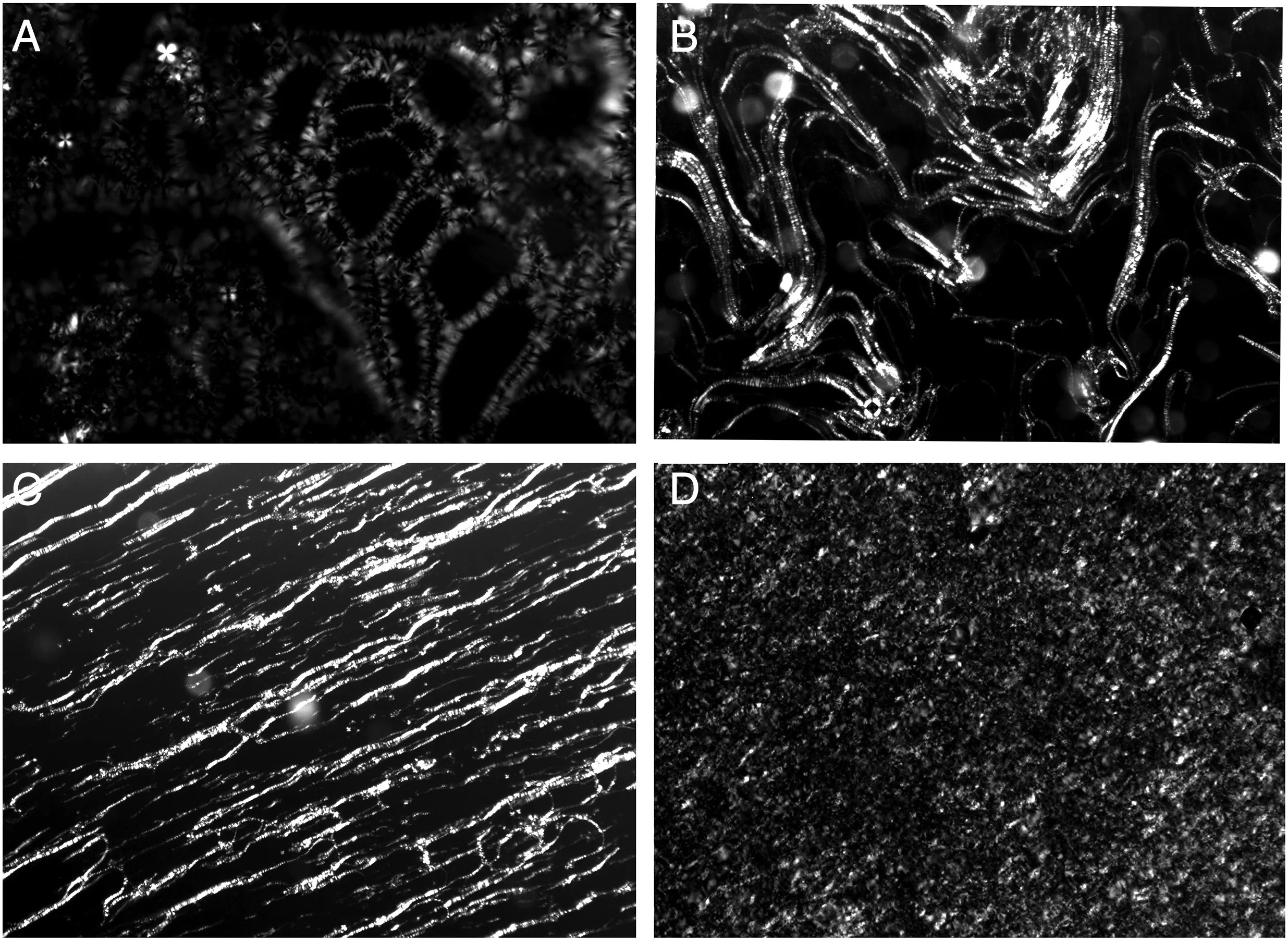
Fig.3.POM images(×200)of lyotropic liquid crystal of Triton X-100/SDS/NH3·H2O at different ZrOCl2·8H2O concentrations.(A)0.5 mol·L-1;(B)1.0 mol·L-1;(C)1.5 mol·L-1;(D)2 mol·L-1.
Fig.3 shows POM images of lyotropic liquid crystal of Triton X-100/SDS/ZrOCl2·8H2O.The samples with different ZrOCl2·8H2O concentrations also show cruciate flower and oil stripping texture of lamellar phase.The optical textures reveal that the microstructure of the liquid crystal template does not break after introduce ZrOCl2·8H2O.
3.3.Effect of ZrOCl2·8H2O concentration on the size of zirconia powder
For examining the effect of ZrOCl2·8H2O concentration on the size of zirconia powder,the weight ratio of Triton X-100/SDS/H2O is fixed at 15/38/47.The amount of ammonia is 2 ml(12.5%).In Fig.4,the size of precursor particle increases with the concentration of ZrOCl2·8H2O.The growth process ofprecursor is composed oftwo stages.As the concentration of zirconium oxychloride in the solvent increases from 0.1 mol·L-1to 1.5 mol·L-1,the size ofprecursorparticle increasesslowly(from 50 to 100 nm),while it increases sharply as ZrOCl2·8H2O concentration approaches 2 wt%.This phenomenon indicates that the zirconium oxychloride concentration has a remarkable effecton the size ofprecursor particles,because the thickness ofthe solventlayer increases with the zirconium oxychloride concentration.
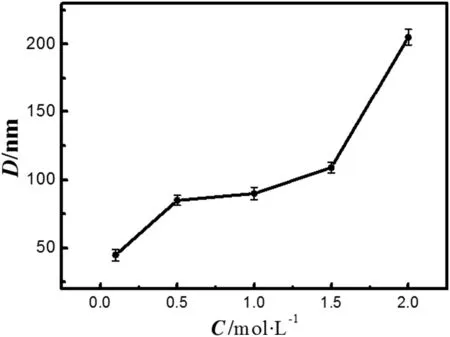
Fig.4.Effect of ZrOCl2·8H2O concentration on the size of zirconia nanoparticle.
3.4.Effect of NH3·H2O concentration on the size of zirconia powder
Fig.5 shows the effect of NH3·H2O concentration on the size of zirconia nanoparticles.The samples are prepared by substituting 1 mol·L-1ZrOCl2·8H2O solutions for water in the Triton X-100/SDS/H2O system.The particle size of ZrO2increases with the concentration of NH3·H2O greatly.
We also study the stability of the lamellar liquid crystal after adding into ammonia water.The microstructure of lamellar liquid crystal template does not rupture when ammonia water concentration ranges from 6%to 12.5%.An increase in the range of particle may be due to the large thickness of the solvent layer so that the particle size is not strictly restricted owing to the flexibility of surfactant bilayers[25].It is clear thatthe mesophases collapse(Fig.2D)increases particle size remarkably.
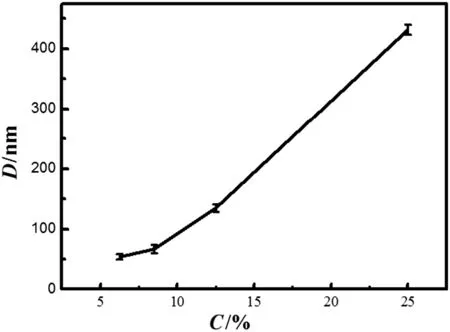
Fig.5.Effect of NH3·H2O concentration on the size of zirconia nanoparticle.
3.5.Characterization of ZrO2 powder
Fig.6 shows the DSC-TGpro files ofsample Afrom lamellar liquid crystal template methods.The DSC curve presents two major peaks.The first one is endothermic and the other is exothermic.The broad endothermic peak appears atabout113°C,which corresponds to the elimination ofresidual water and solvent.The peak at 550 °C named “glow endotherm”is attributed to the crystallization of amorphous zirconia[26],which is confirmed by the following XRDresults.Exothermic peaks appeared at313°C may be attributed to incomplete dehydration of hydrous oxide[27].
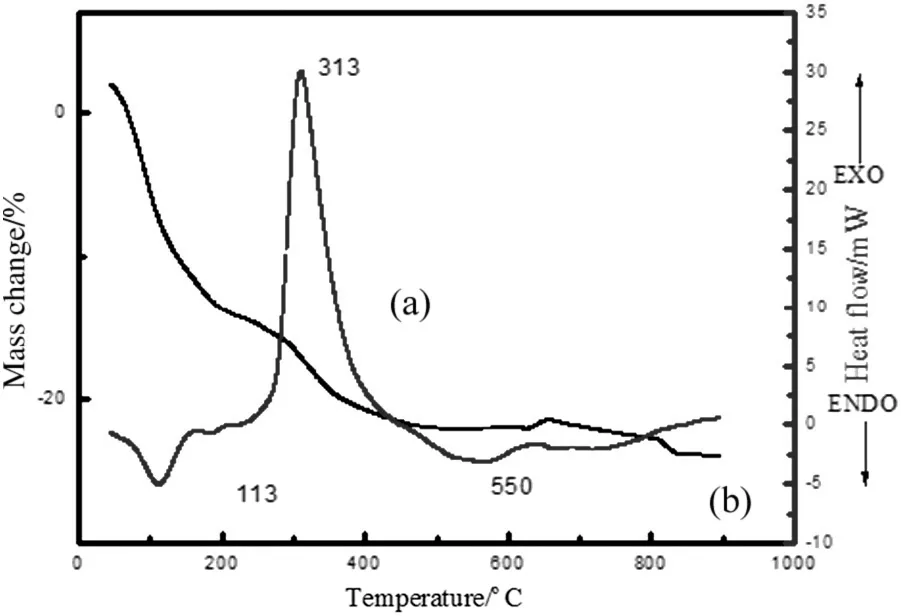
Fig.6.DSC(a)and TG(b)curves of ZrO2 precursor in a static air atmosphere with heating rate of 10°C·min-1.
The FT-IR spectra of precursor and calcined samples are shown in Fig.7.Absorption peak at 883 cm-1in curve A is typical for cubic zirconia[28].Curve B shows two absorption bands at 3441 cm-1and 1600 cm-1,which can be attributed to-OH group stretching and bending vibrations,and sharp band at 1051 cm-1describes Zr=O group stretching vibrations,indicating that at this stage Zr=O bonds do not form Zr-O bonds.
Fig.8 illustrates XRDpatternsofzirconia precursorcalcined atdifferenttemperatures.The sample is amorphous atthe calcination temperature below 310 °C.The sample calcinated at 600 °C presents cubic zirconia.Fig.8 recon firms that the endothermic peak of Fig.6,spanning 550°C,is due to the formation of cubic zirconia nano-particles.
In order to determine the crystallite size of sample,Debye-Scherrer formula is applied[28],
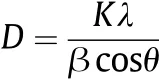
where K=0.89 is the shape factor,λ=0.15406 nm represents the wavelength of Cu Kαradiation,β and θ are the half width and half angle of each diffraction peak,respectively.The distinguishing characteristic peaks for cubic occur at 2θ =30.2°,34.9°,50.32°,and 60.1°.The crystallite size is determined as 20 nm.
Fig.9 illustrates the typical scanning electron micrograph(SEM)of the zirconia powder calcined at 600°C.Aggregated nano-crystalline cubic ZrO2particles have a spherical-like morphology.
Fig.10 shows the TEM micrographs of the sample.In spite of agglomeration of particles,the particle size is between 10 and 30 nm,which is narrow and well consistent with the XRD results.The average particle size is 10-20 nm.
3.6.Synthetic mechanism of zirconia nanoparticles
The structure of the lamellar liquid crystaland synthetic mechanism of zirconia nanoparticles are illustrated in Fig.11.In an aqueous solution,amphiphile molecules form a conic building block,which aggregates to plate shape at high concentration.A possible mechanism for zirconia nanocrystal growth comprises mainly two processes:(i)formation of zirconia in lamellar liquid crystal aqueous layers as the nascent crystal nuclei;(ii)subsequent anisotropic growth from these nuclei.ZrOCl2·8H2O and NH3·H2O exist mostly in the solvent layer of lamellar liquid crystal.When ZrOCl2·8H2O reacts with OH-released from NH3·H2O,zirconium hydroxide particles precipitate.At the initial stage,the dominant process is zirconia nucleation.During crystal growth,because the thickness of the solvent layer is less than 10 nm[29],the size of zirconia particles is controlled and limited by this thickness.However,the size of zirconia particles precipitated in lamellar liquid crystals is slightly larger than the thickness of solvent layer,indicating that the interface of the solvent layer is out of shape[30].
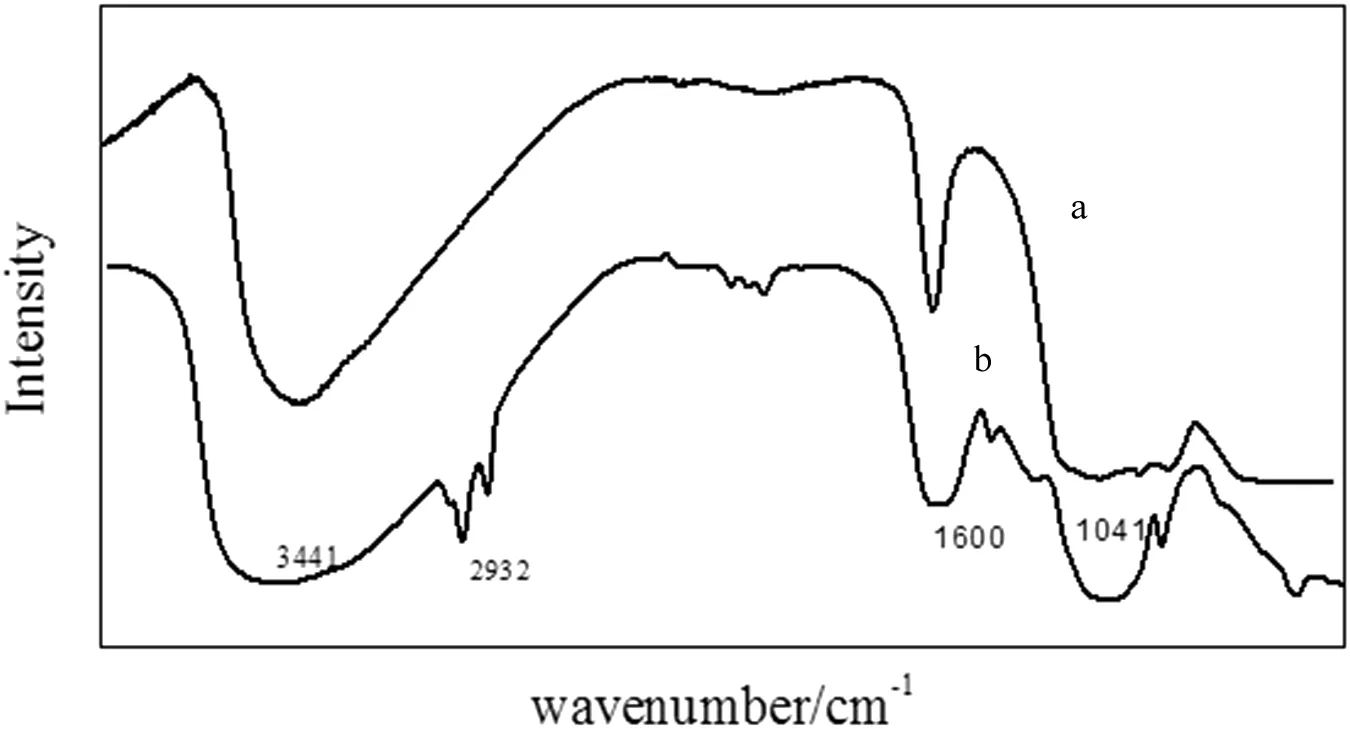
Fig.7.FT-IR spectra for zirconia particles.(a)Calcined at 600°C for 1 h;(b)zirconia precursor.
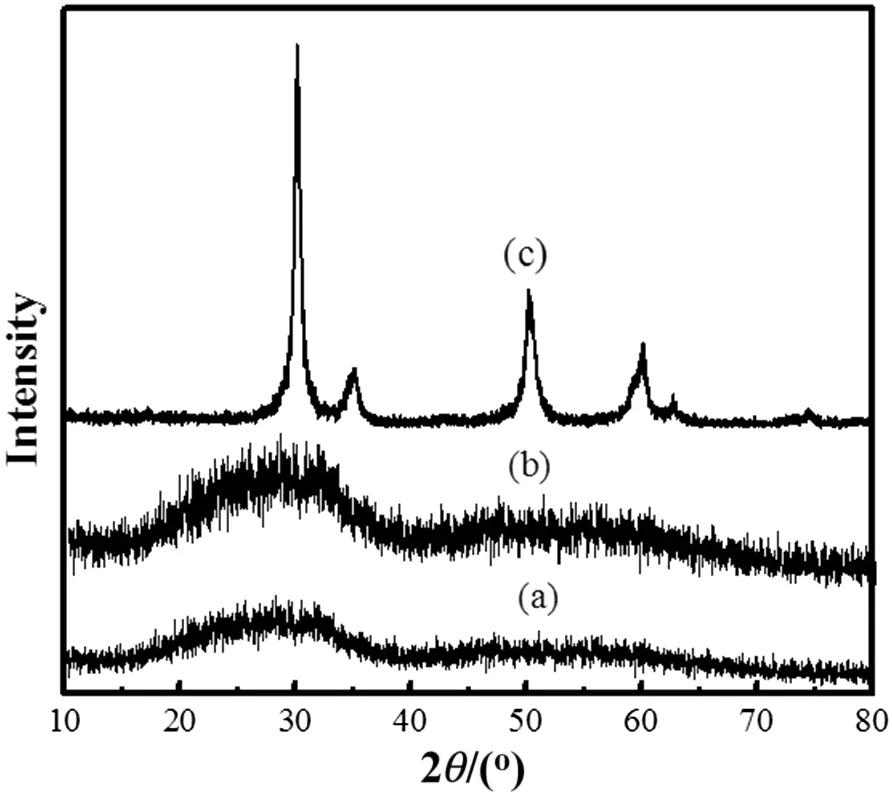
Fig.8.XRD patterns of zirconia precursor calcined at different temperatures.(a)Uncalcined;(b)calcined at 310 °C;(c)calcined at 600 °C.

Fig.9.SEM photograph of ZrO2 powder calcined at 600°C for 1 h.
4.Conclusions
Spherical zirconia nanoparticles were synthesized by a direct template route in the lamellar liquid crystal formed by Triton X-100/SDS/H2Osystem.The stability ofthe lamellarliquid crystalis notably affected by the concentration of NH3·H2O.ZrO2powder with an average size of 15-20 nm is obtained under the con finement of lamellar structure.
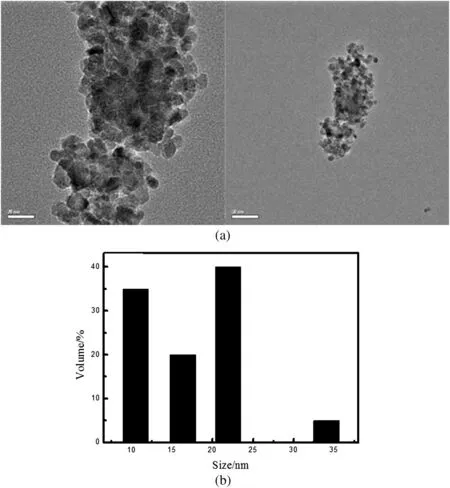
Fig.10.TEM micrograph of zirconia nanoparticles calcined at 600°C for 1 h(a)and their particle size distribution(b).
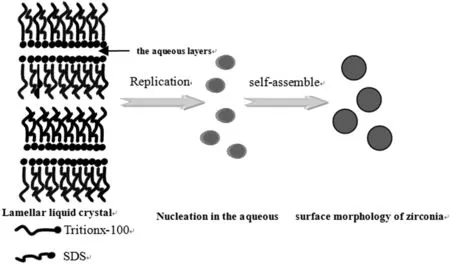
Fig.11.Schematic diagram of zirconia in lamellar liquid crystals formed by SDS/Triton X-100/H2O.
[18]Y.Chang,X.-B.Li,Preparation of ZrO2spherical nanometer powders via emulsion processing route,Trans.Nonferrous Metals Soc.China 16(2006)33.
[19]C.R.Abreu,M.Lazzari,Emulsions with structured continuous phases,Curr.Opin.Colloid Interface Sci.13(2008)198-205.
[20]M.Signoretto,A.Breda,F.Somma,F.Pinna,G.Cruciani,Mesoporous sulphated zirconia by liquid-crystal templating method,Microporous Mesoporous Mater.91(2006)23-32.
[21]D.U.Lee,D.Pradhan,R.Mouawia,D.H.Oh,N.F.Heinig,K.T.Leung,E.Prouzet,ZnO nanostructures grown onto polypyrrole films prepared in swollen liquid crystals via integrative chemistry,Chem.Mater.22(2009)218-225.
[22]Y.Ding,L.Chen,R.Guo,Preparation of zinc gluconate nanostructures with different shapes by lamellar liquid crystal template,Colloids Surf.A Physicochem.Eng.Asp.295(2007)85-90.
[23]F.Freitas,V.Sarmento,C.Santilli,S.Pulcinelli,Controlling the growth of zirconia needles precursor from a liquid crystal template,Colloids Surf.A Physicochem.Eng.Asp.353(2010)77-82.
[24]E.Pena dos Santos,C.V.Santilli,S.H.Pulcinelli,E.Prouzet,Zirconia needles synthesized inside hexagonal swollen liquid crystals,Chem.Mater.16(2004)4187-4192.
[25]E.Stig Fribergt,J.-I.-W.Anthony,Dynamic structure of a nonaqueous lamellar liquid crystal:Comparison with the aqueous case,Langmuir 3(1987)735-131.
[26]Q.Chang,J.-E.Zhou,Y.Wang,G.Meng,Preparation and characterization of unique zirconia crystals within pores via a sol-gel-hydrothermal method,Adv.Powder Technol.20(2009)371-374.
[27]B.Xia,L.Duan,Y.Xie,ZrO2nanopowders prepared by low-temperature vapor-phase hydrolysis,J.Am.Ceram.Soc.83(5)(2000)77-80.
[28]N.Chandra,D.K.Singh,M.Sharma,Synthesis and characterization of nano-sized zirconia powder synthesized by single emulsion-assisted direct precipitation,J.Colloid Interface Sci.342(2010)327-332.
[29]J.-J.Guo,X.-Y.Li,Template-directed synthesis of lamellar polystyrene nanomaterial,Polym.J.39(11)(2007)1120-1121.
[30]N.Cheng,Q.-Z.Hu,Y.-H.Bi,W.Xu,Y.-J.Gong,L.Yu,Gels and lyotropic liquid crystals:Using an imidazolium-based catanionic surfactant in binary solvents,Langmuir 30(2014)9076-9084.
 Chinese Journal of Chemical Engineering2015年10期
Chinese Journal of Chemical Engineering2015年10期
- Chinese Journal of Chemical Engineering的其它文章
- Synthesis and characterization of switchable ionic compound based on DBU,CH3OH,and CO2☆
- Preparation and simulation of a taro flavor☆
- Effect of PSA tin plating process on trace lead in tin coating☆
- Biological treatment of high wastewater using an ammonia-tolerant photosynthetic bacteria strain(ISASWR2014)☆
- Adsorption and degradation of nor floxacin by a novel molecular imprinting magnetic Fenton-like catalyst☆
- TG-FTIR analysis of pyrolusite reduction by major biomass components☆
