兩個鑭系金屬配合物的水熱合成、晶體結構、熒光、熱穩定性及抑菌活性
宗廣才 任寧 張建軍*, 祁曉霞 高捷 張大海
兩個鑭系金屬配合物的水熱合成、晶體結構、熒光、熱穩定性及抑菌活性
宗廣才1,2任寧*,3張建軍*,1,2祁曉霞1,2高捷1,2張大海3
(1河北師范大學,分析測試中心,石家莊050024)
(2河北師范大學,化學與材料科學學院,石家莊050024) (3邯鄲學院,化學化工與材料學院,邯鄲056005)
水熱法合成了2個鑭系配合物[Ln(3,4-DFBA)3(phen)(H2O)]2(H2O)2(Ln=Sm(1),Ho(2);3,4-DFBA=3,4-二氟苯甲酸根,phen=菲咯啉)。利用X-射線單晶衍射儀測定了配合物的晶體結構。配合物1和2結構相同,配位數為8,屬于三斜晶系,空間群為P1。相鄰的2個雙核分子之間通過分子間相互作用力形成了2D層狀結構。并用元素分析,紅外,紫外,XRD等手段對目標配合物進行了表征。用TG-DTG技術測定了配合物的熱穩定性,同時對配合物1的熒光性能進行了研究。另外,還測定了這兩種配合物對白色念珠菌,革蘭氏陰性菌(大腸桿菌)以及革蘭氏陽性菌(金黃色葡萄球菌)的抑菌活性。
鑭系元素;3,4-二氟苯甲酸;熱分析;熒光;抑菌活性;晶體結構
The lanthanide coordination chemistry has become of increasing significance in the last few years[1].Due to the larger radius of lanthanide ions and higher affinity for hard donor centers,lanthanide ions are easily to construct coordination polymers with organic carboxylic acid ligands and neutral ligands using regular methods[2].Lanthanide complexes with fancy chemical and physical characters have potential applications as fluorescence materials and bioprobes and so on[3].Since a lanthanide organic complex of europium emitted brightly photoluminescence was reported firstly by Weissman[4],the fluorescence materials began to be studied and other lanthanide complexes with metal ions Sm(Ⅱ),Tb(Ⅱ),Dy(Ⅱ)had been involved[5-7].In addition,lanthanide complexes have high sterilization ability and wide inhibition zone,hence,the bacteriostatic activity of complexes is a popular topic over the years in the field ofbiological and other aspects[8-11].
In this paper,two complexes[Ln(3,4-DFBA)3(phen)(H2O)]2(H2O)2(Ln=Sm(1),Ho(2);3,4-DFBA=3, 4-difluorobenzoate,phen=1,10-phenanthroline)have been synthesized by hydrothermal method.Complexes 1 and 2 are binuclear molecules.Two of H2O molecules are coordinated with two metal ions and another two H2O molecules are crystalwater,which are rare in the study of lanthanide carboxylic acid complexes[12-14]. Thermal and luminescent properties,bacteriostatic activities ofthe title complexes are also investigated.
1 Experimental
1.1 Materials and measurement
All reagents and solvents were purchased commercially without further purification.LnCl3·6H2O were prepared by the reaction of Ln2O3and hydrochloric acid in aqueous solution,followed by recrystallization and drying.The obtained complexes were dissolved in DMSO solution with the concentration of 10μmol·L-1and 1 mmol·L-1for the determination of ultraviolet spectra and molar conductivity,respectively.The contents of C,H and N were recorded on FLASH EA 1112 element analyzer (Thermo Fisher).The infrared spectrum were studied with KBr discs technique by using the Bruker TENSOR 27 spectrometer at room temperature over the range of 4 000 to 400 cm-1.Ultraviolet spectra were carried out on a U-3010 Spectrophotometer.The molar conductivity was measured by DDS-307 conductometer(Shanghai Precision&Scientific Instrument CO.LTD)at room temperature.X-ray Diffractions for complexes were tested on X-ray powder(Bruker AXS) diffractometer(Germany,D8 ADVANCE)with a scan speed of 0.1°·s-1,Cu Kαradiation(λ=0.154 18 nm), scan range from 5°to 50°.The thermal stability was investigated by TG/DTG using the NETZSCH STA 449 F3 with a heating rate of 10 K·min-1(Simulated air atmosphere).The fluorescent spectrum of complex 1 was depicted in an F-4500 Hitachispectrophotometer at room temperature.The bacteriostatic activities were investigated by the disk diffusion method using the Mueller-Hinton agar medium.The bacteriostatic effects for escherichia coli and staphylococcus aureus were investigated at the temperature of 310 K,while candida albicans at303 K.
1.2 Synthesis of the complexes
3,4-difluorobenzoic acid(3,4-DFHBA,0.3 mmol) and 1,10-phenanthroline(0.1 mmol)were together dissolved in ethanolsolution(95%)and the pH value of the solution was adjusted to 5~7 by adding 1 mol· L-1NaOH solution.LnCl3·6H2O(Ln=Sm(1),Ho(2)) (0.1 mmol)was dissolved in distilled water.The resulting solution of the ligands was then added dropwise into the aqueous solution of lanthanide metal salts under stirring for 40 min.Subsequently,the mixture solution was transferred to the Teflon-lined autoclave and settled in electrothermal constanttemperature dry box at 120℃for 72 h[15-16].Then the transparent crystals suitable for X-ray diffraction analysis were produced.Anal.Calcd.for C66H42F12N4Sm2O16(%):C,47.31;H,2.53;N,3.34.Found(%): C,47.70;H,2.12;N,3.33.Anal.Calcd.for C66H42F12N4Ho2O16(%):C,46.50;H,2.48;N,3.29.Found(%): C,46.74;H,2.35;N,3.29.
1.3 X-ray crystallography
A suitable crystal of complex 1 with dimensions of 0.40 mm×0.11 mm×0.06 mm was mounted on aSmart-1000(Bruker AXS)diffractometer with monochromated Mo Kαradiation(λ=0.071 073 nm)at 298 K.A total of 8 023 reflections were collected in the range of2.65°≤θ≤25.02°(-7≤h≤9,-16≤k≤16, -17≤l≤17),and 5 452 reflections were independent with Rint=0.054 9,of which 4 297 reflections were observed with I>2σ(I).
A suitable crystal of complex 2 with dimensions of 0.40 mm×0.36 mm×0.18mm was mounted on the diffractometer mentioned above.A total of 7 858 reflections were collected in the range of 2.65°≤θ≤25.02°(-9≤h≤7,-15≤k≤16,-17≤l≤17),and 5 316 reflections were independent with Rint=0.054 9, ofwhich 4 497 reflections were observed with I>2σ(I).
The structures were solved by direct methods using the SHELXS-97 program and refined by fullmatrix least-squares on F2using the SHELXL-97 program[15].The crystal data and structural refinement parameters ofthe complexes are listed in Table 1.
CCDC:1023982,1;1023981,2.
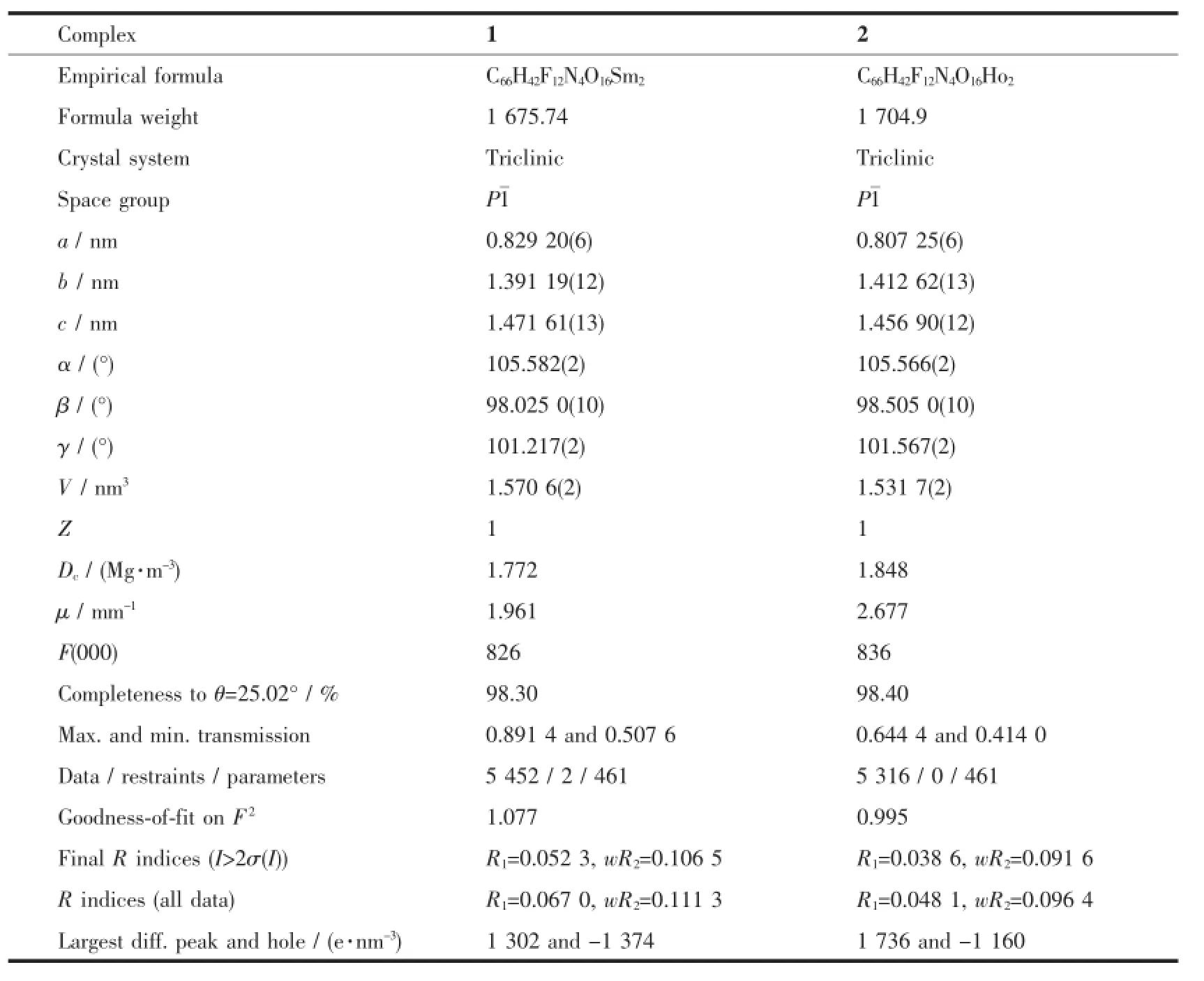
Table 1 Crystal data and structural refinement parameters for complexes 1 and 2
2 Results and discussion
2.1 Infrared spectra
The infrared spectra of the complexes and the ligands are displayed in Table S1.The carboxylic acid ligand exhibits an intense C=O stretching vibration band at 1 694 cm-1.While theνC=Odisappears in the IR spectrum of complexes 1 and 2.The two absorbing bands assigned to the asymmetric(νas(COO-))and symmetric(νs(COO-))vibrations are observed at 1 544~1 547 cm-1and 1 431 cm-1,respectively.Besides,the apparent band at 419 cm-1is attributed to theν(Ln-O)of the two complexes.These all indicate that the oxygen atoms of 3,4-DFBA ligand takes part in coordination to the Ln(Ⅱ)ion[18].As shown in Table S1,theνC=N and δC-Hbands in the IR spectra of complexes show a shift to a low frequency as compared with the free 1,10-phenanthroline ligand,indicating that two nitrogenatoms ofthe ligand are coordinated to the Ln(Ⅱ)ion[19].
2.2 Ultraviolet spectra
UV spectra of the two ligands and the title complexes are recorded in DMSO solution with DMSO as a reference.The data of UV absorption of the ligands and complexes are listed in Table S2.The intense band(255 nm)of 3,4-DFHBA ligand shifts to 265 nm in complexes.This may be explained by the expansion of theπ-conjugated system due to metal coordination[20].The maximum absorption wavelength (λmax)of 1,10-phenanthroline is similar to that in the complexes,suggesting that the formation of Ln-N has no remarkable influence on ultraviolet absorption of 1,10-phenanthroline.The values of Amaxfor complexes 1 and 2 are stronger than that of two ligands.It can be concluded that a larger chelate ring is constructed after coordination to the Ln(Ⅱ)ions[21].
2.3 Crystalstructures
The molecular structures of complexes 1 and 2 with the coordination geometry ofthe Sm(Ⅱ)and Ho(Ⅱ) ions are shown in Fig.1 and Fig.S1.The selected bond lengths of complexes 1 and 2 are listed in Table 2. Because the crystal structures of the two complexes are isostructural,hence complex 1 is taken as an example to discuss in detail.As shown in Fig.1, complex 1 is binuclear and crystallizes in the triclinic P1 space.Each Sm(Ⅱ)ion is eight-coordinated by six O atoms and two N atoms,which resembles a distorted square antiprism geometry.These eight atoms are in three models of bidentate bridging(O5,O6i),bidentate chelating(O3,O4 and N1,N2)and monodentate(O1, O7)to coordinate with the center Sm(Ⅱ)ion.Besides, another two water molecules are not involved in coordination with Sm(Ⅱ)but exist freely in the binuclear crystal structure.It is worth noting that intramolecular hydrogen bond is formed between O7iand O2,O7 and O2i.Further more,the adjacent two binuclear molecules form two-dimensional layered structure by intermolecular forces for complex 1,as shown in Fig.2.The hydrogen bond distances in complexes 1 and 2 are given in Table S3.
The average distance of Sm(1)-O is 0.243 2(3) nm and thatof Ho(1)-O is 0.234 3(7)nm.The average distance of Sm(1)-N and Ho(1)-N are 0.260 7(0)nm and 0.253 2(5)nm,respectively.The length of Ln-O is always shorter than that of Ln-N,which proves the stronger combination ability of Ln to O than N[22].The result can explain why 1,10-phenanthroline ligand is lostfirstly in the decomposition process.
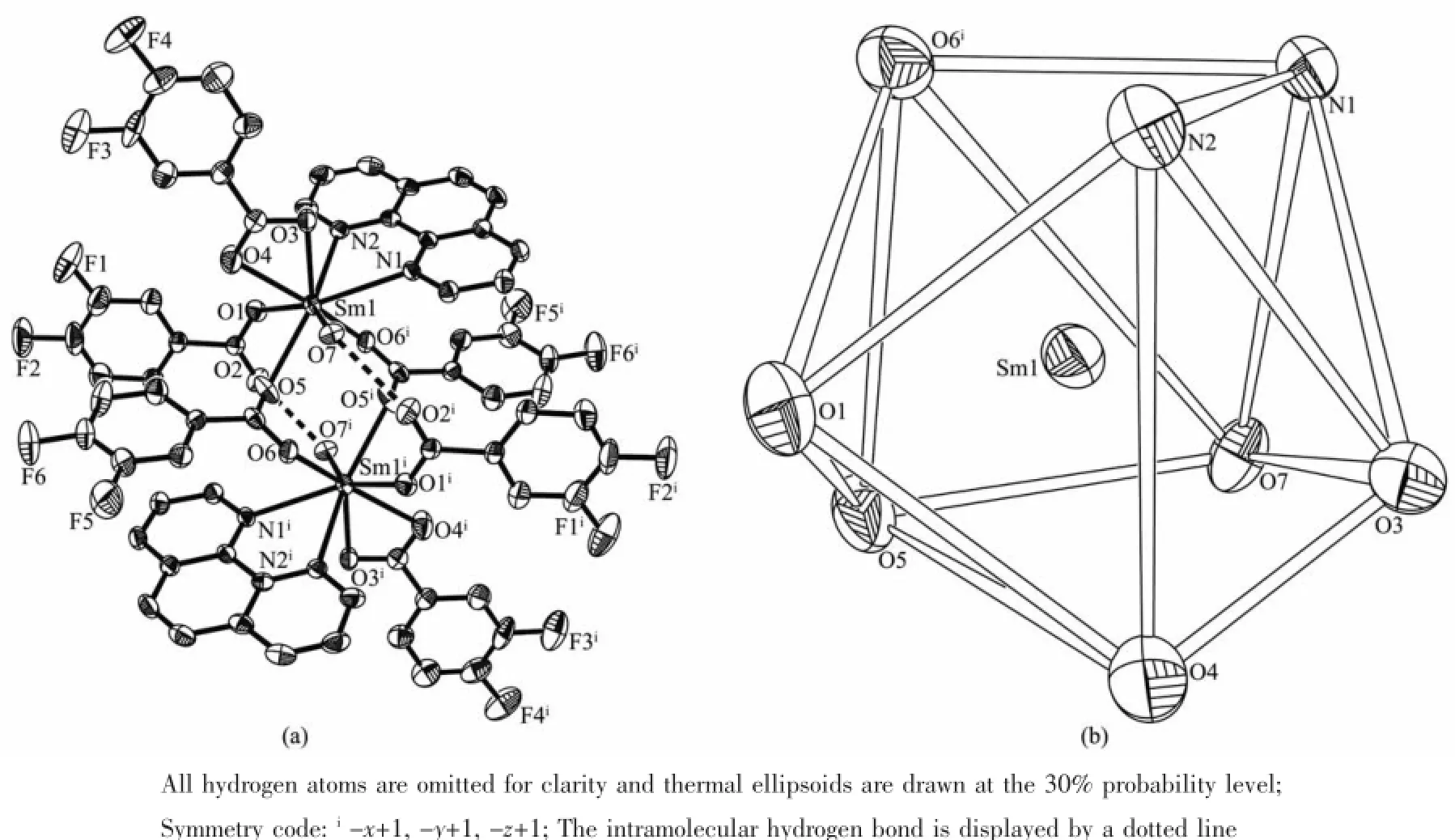
Fig.1(a)Molecular structure of complex 1;(b)Coordination geometry of the Sm(Ⅱ)ion
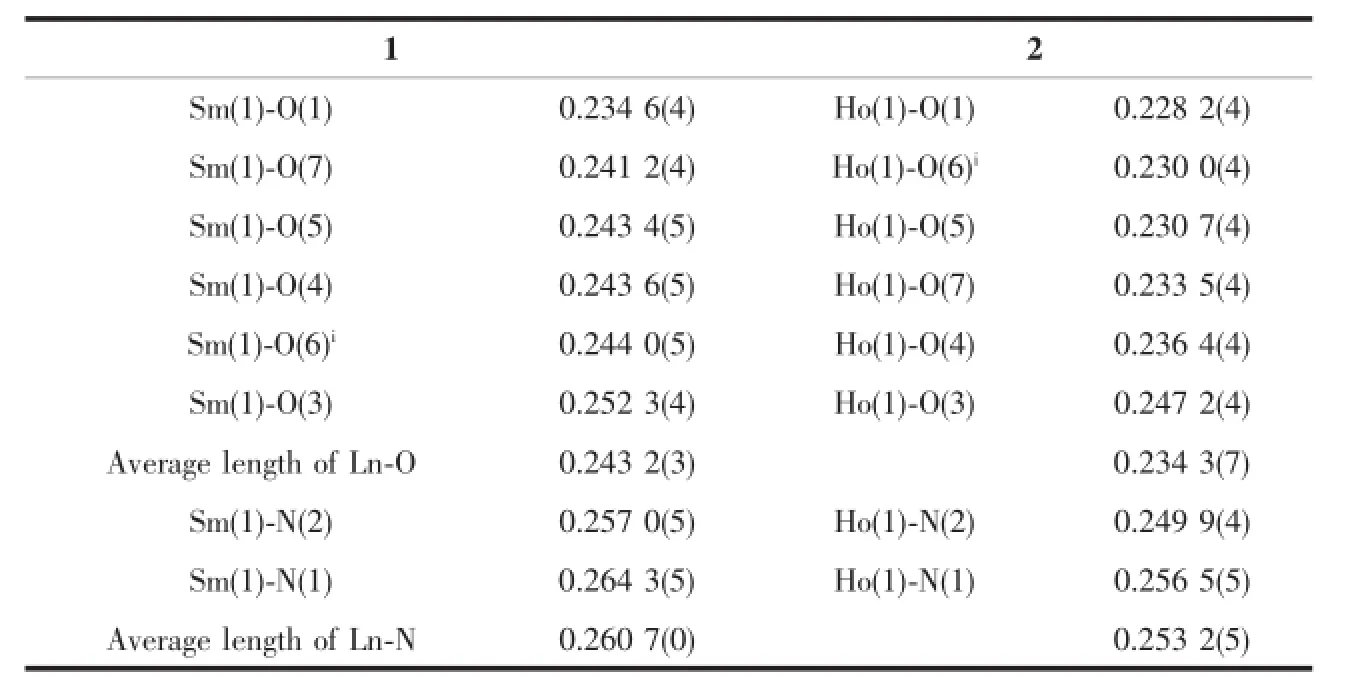
Table 2 Selected bond lengths of the complexes 1 and 2
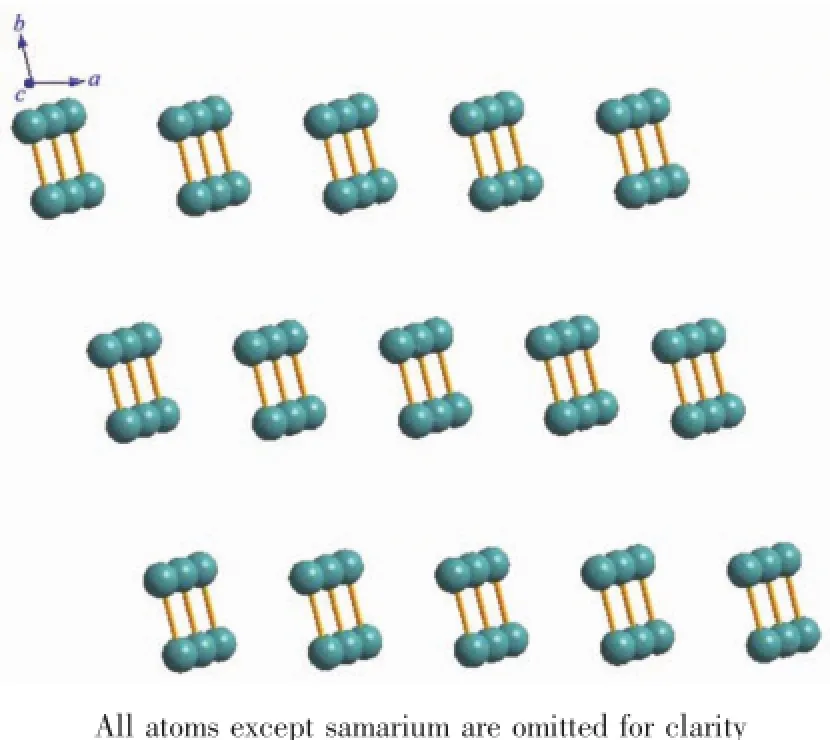
Fig.2 A two-dimensional layered structure of complex 1
2.4 Thermal analysis
TG-DTG curves of the title complexes are shown in Fig.3 and 4.For complex 1,the decomposition process can be divided into three steps.The first step begins at 338.15 K and ends at 441.15 K with a weight loss of 2.48%,assigning to the loss of two crystallization water molecules(Calcd.2.15%).The second degradation stage is in the range of 441.15~659.15 K with weight loss of 22.94%,corresponding to the loss of another two coordinated water molecules and two 1,10-phenanthroline molecules(Calcd. 23.67%).The third weight loss of 52.83%between 676.15 K and 1 363.45 K is attributed to the loss of all 3,4-DFBA ligands.In summary,the total loss rate is 78.25%with a theoretical value of 79.20%if Sm2O3was the finalproducts.For complex 2(Fig.4),to begin with,all water molecules are released from 344.15 K to 459.15 K(Found 4.29%,Calcd.4.22%).The following decomposition belongs to the loss of 1,10-phenanthroline molecules with a value of 21.76% (Calcd.21.14%).To the end,the complex 2 is completely degraded into Ho2O3.
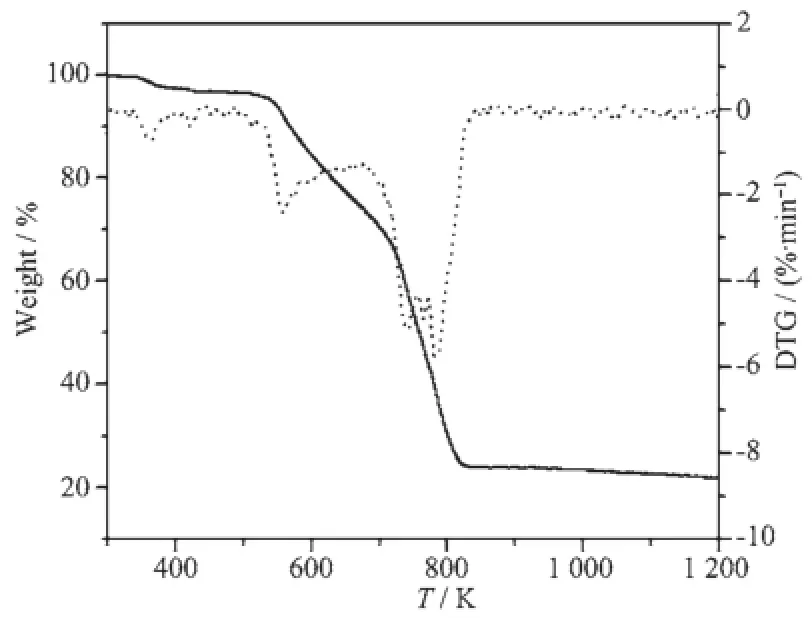
Fig.3 TG-DTG curves of complex 1
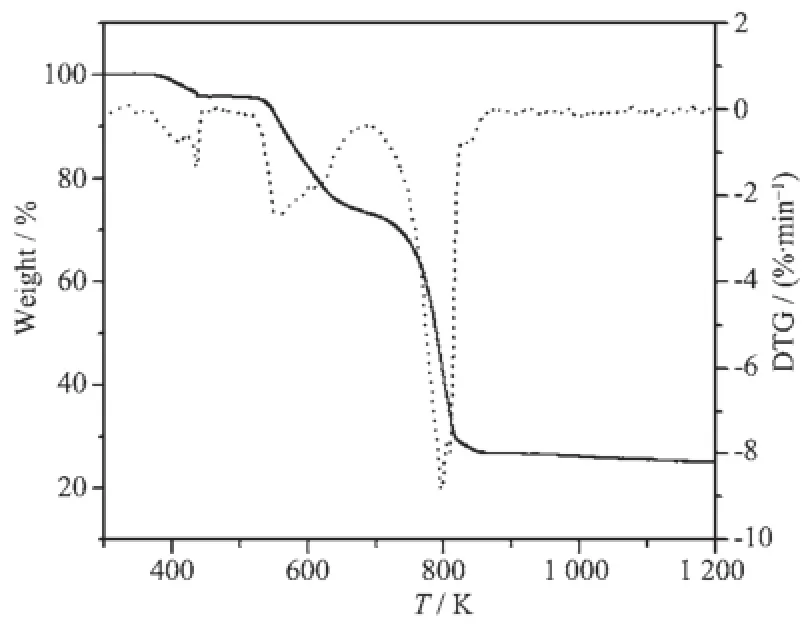
Fig.4 TG-DTG curves of complex 2
2.5 Powder X-ray diffraction
The PXRD patterns of the title complexes were checked atroom temperature,as shown in Fig.S2.The peak positions of the simulated and experimental PXRD patterns are in agreement with each other,which demonstrate the good phase purity of the complexes.
2.6 Fluorescence spectrum
Because of the orange-red emission of Sm(Ⅱ)ion, the complex 1 has been demonstrated to be potential application in full-color fluorescence materials for applications as full-color fluorescence materials.The emission spectrum of complex 1 under excitation at 335 nm is displayed in Fig.5.The characteristic fluorescence of Sm(Ⅱ)ion(at 601 nm)corresponding to the4G5/2→6F7/2are detected[23],and another two emission peaks at 564 nm and 653 nm are attributed to the transitions of4G5/2→6F5/2,4G5/2→6F9/2,respectively.
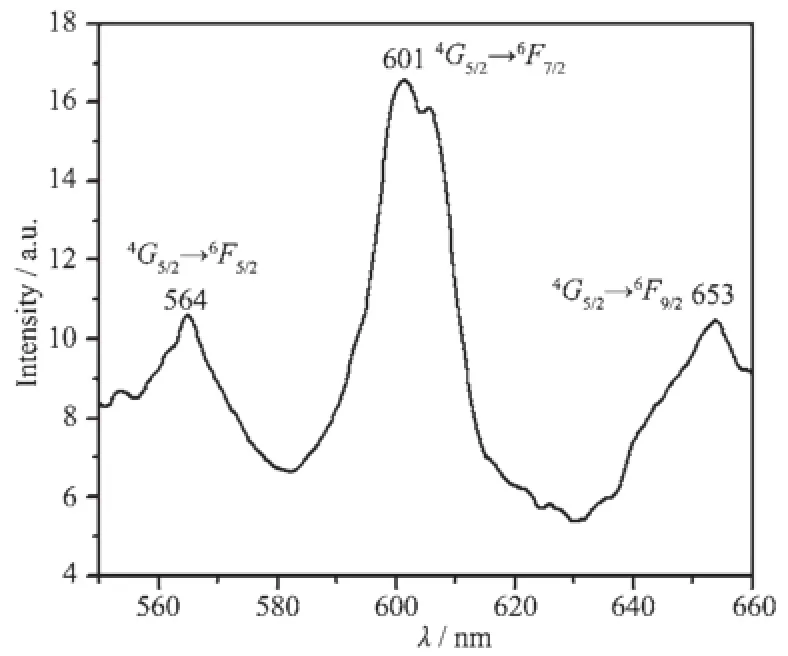
Fig.5 Solid state emission spectra of complex 1 (λex=335 nm)
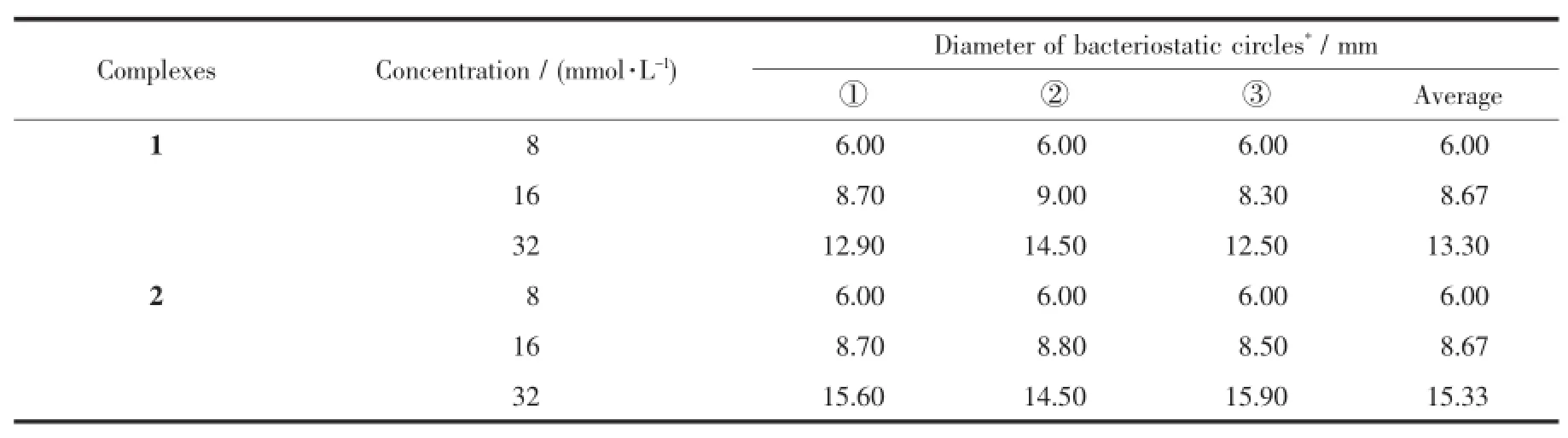
Table 3 Bacteriostatic activities of complexes 1 and 2 to escherichia coli(T=310 K)
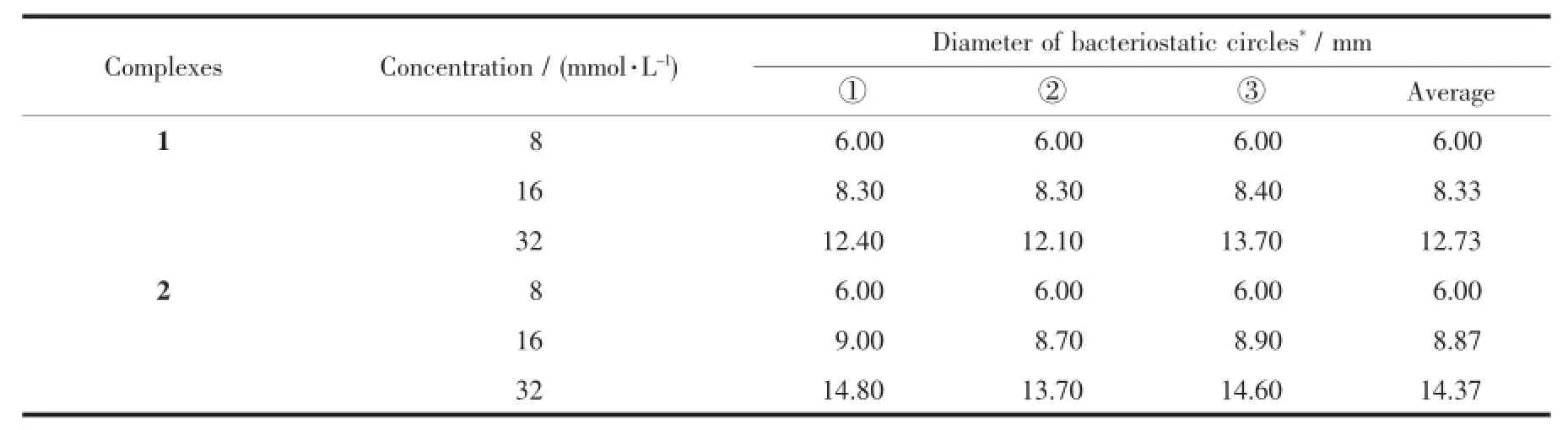
Table 4 Bacteriostatic activities of complexes 1 and 2 to staphylococcus aureus(T=310 K)
2.7 Bacteriostatic activity
The bacteriostatic circles diameter of the complexes 1 and 2 with different concentrations to escherichia coli,staphylococcus aureus and candida albicans are shown in Table 3~5.The temperature of 310 K is optimum for bacteria(escherichia coli, staphylococcus aureus)growth.While 303 K is optimum for fungus(candida albicans)growth.As can be seen from Table 3 and 4,the bacteriostatic circles diameter is 6 mm with the concentration of 8 mmol·L-1.Then with the increase of solution concen-tration, the bacteriostatic circles diameter increases.In other words,the concentration of the complex is higher,the bacteriostatic effectis more obvious.
As shown in Table 5,with a concentration of 8 mmol·L-1,average diameter bacteriostatic circles for complexes 1 and 2 are 11.10 and 13.37 mm,respectively,which is larger than 6 mm of filter diameter itself.This confirms that complexes have remarkable bacteriostatic action to candida albicans even at low concentration.Besides,bacteriostatic actions are moreand more obvious with the increase of concentration, which is similar with escherichia coli and staphylococcus aureus.
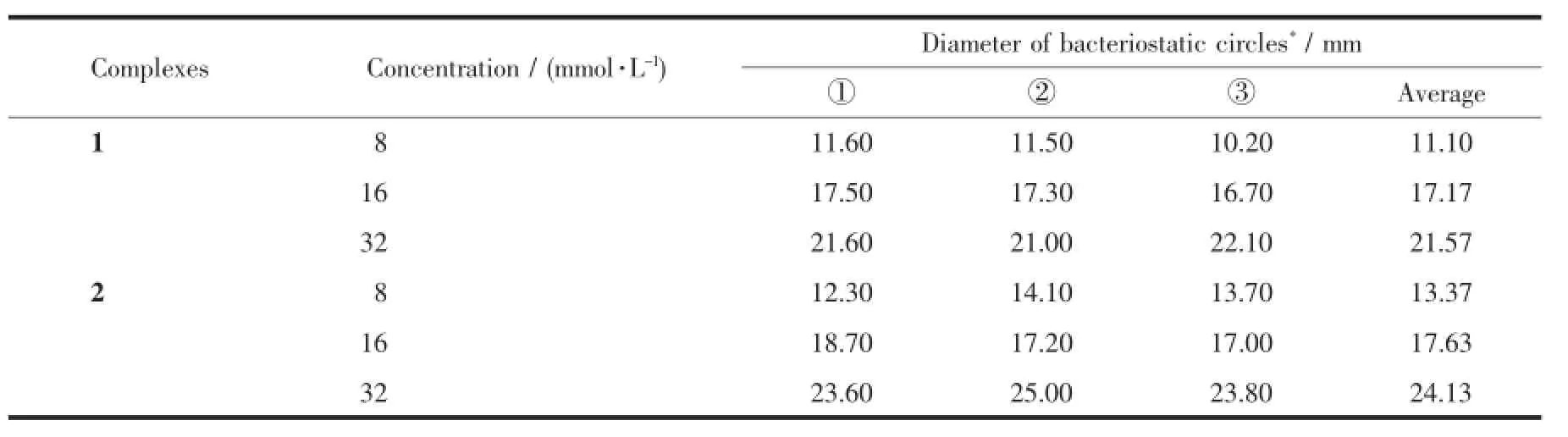
Table 5 Bacteriostatic activities of complexes 1 and 2 to candida albicans(T=303 K)
Lanthanide complexes has certain bacteriostatic actions against microorganism,and it is generally recognized that Ln(Ⅱ)ions are coordinated with the donor atoms of the ligands.This leads to theπelectron delocalization over the chelate ring further to reduces the polarity of the central metal ion.All phenomenon in turn favors permeation to the lipid layer ofthe membrane for lanthanide complexes[24-26].
3 Conclusions
In summary,two complexes[Sm(3,4-DFBA)3(phen)(H2O)]2(H2O)2(1)and[Ho(3,4-DFBA)3(phen) (H2O)]2(H2O)2(2)were synthesized and characterized. Both ofthem were binuclear and isostructural with the space group of P1.The intramolecular hydrogen bond is formed in complexes 1 and 2.Thermaldecomposition processes of the two complexes were divided into three steps.The emission spectrum ofcomplex 1 under excitation at 335 nm indicated the characteristic fluorescence of Sm(Ⅱ)ion corresponding to the 4G5/2→6F7/2.Bacteriostatic activities of these complexes to Gram bacteria bacteria(escherichia coli),Gram negative positive(staphylococcus aureus)and candida albicans have obvious effects athigh concentration.
Supporting Information is available athttp://www.wjhxxb.cn
[1]Hasegawa Y,Wada Y,Yanagida S.J.Photochem.Photobiol. C,2004,5:183-202
[2]Yang L R,Liu L,Wu L Z,et al.Dyes Pigm.,2014,111:176-184
[3]Li W X,Guo F,Zheng Y S,et al.J.Lumin.,2014,153:421-429
[4]Weissman S I.J.Chem.Phys.,1942,10:214-217
[5]Ni Y R,Tao J,Jin J Y,et al.J.Alloys Compd.,2014,612: 349-354
[6]Li L F,Dong D B,Zhang J Y,et al.Mater.Lett.,2014,131: 298-301
[7]Li W X,Feng S Y,Liu Y,et al.J.Lumin.,2013,143:746-753
[8]Lekha L,Raja K K,Rajagopal G,et al.J.Mol.Struct.,2014, 1056-1057:307-313
[9]Kulkarni A,Patil S A,Badami P S.Eur.J.Med.Chem., 2009,44:2904-2912
[10]Subhan M A,Rahman M S,Alam K,et al.Spectrochim. Acta A,2014,118:944-950
[11]Lima L M P,Delgado R,Marques F,et al.Eur.J.Med. Chem.,2010,45:5621-5627
[12]Li X,Zhang Z Y,Zou Y Q.Eur.J.Inorg.Chem.,2005,14: 2909-2918
[13]Li Y,Zheng F K,Liu X,et al.Inorg.Chem.,2006,45:6308-6316
[14]Ma D,Li X,Huo R.J.Mater.Chem.C,2014,43:9073-9076 [15]Wang J F,Li H,Zhang J J,et al.Sci.China Ser.B Chem., 2012,55:2161-2175
[16]Yang Z H,Xu J,Zhang W X,et al.J.Solid State Chem., 2007,180:1390-1396
[17]Sheldrick G M.SHELXTL-97,Programs for Crystal Structure Refinement,University of G?ttingen,Germany,1997.
[18]Zhou X J,Zhao X Q,Wang Y J,et al.Inorg.Chem.,2014, 53:12275-12282
[19]Zheng J R,Ren N,Zhang J J,et al.J.Chem.Thermodyn., 2013,57:169-177
[20]An B L,Gong M L,Li M X,et al.J.Mol.Struct.,2004,687: 1-6
[21]ZHANG Ying-Ying(張瑩瑩),REN Shu-Xia(任書霞),ZHANG Jian-Jun(張建軍),et al.Chinese Sci.Bull.(科學通報),2014,59(27):3398-3405
[22]?yszczek R,Rzaczyńska Z,Kula A,et al.J.Anal.Appl. Pyrolysis,2011,92:347-354
[23]Liu D,Yu H,Wang Z G,et al.Polym.Int.,2010,59:937-94
[24]Hang J,Xu Y H,Chen X K,et al.J.Rare Earths,2012,30: 586-591
[25]Liu J Y,Ren N,Zhang J J,et al.Thermochim.Acta,2013, 570:51-58
[26]Zhao Y F,Chu H B,Bai F,et al.J.Organomet.Chem., 2012,716:167-174
Hydrothermal Syntheses,Crystal Structures,Luminescence, Thermal Stabilities and Bacteriostatic Activities of Two Lanthanide Complexes
ZONG Guang-Cai1,2REN Ning*,3ZHANG Jian-Jun*,1,2QIXiao-Xia1,2GAO Jie1,2ZHANG Da-Hai3
(1Testing and Analysis Center,Hebei Normal University,Shijiazhuang 050024,China)
(2College of Chemistry&Material Science,Hebei Normal University,Shijiazhuang 050024,China)
(3College of Chemical Engineering&Material,Handan College,Handan,Hebei 056005,China)
Two lanthanide complexes[Ln(3,4-DFBA)3(phen)(H2O)]2(H2O)2(Ln=Sm(1),Ho(2);3,4-DFBA=3,4-difluorobenzoate,phen=1,10-phenanthroline)were prepared by hydrothermal method.Their structures were determined by single-crystal X-ray diffraction method.The structures of complexes 1 and 2 are isostructural with the coordination number of eight.They crystallized in the triclinic space group P1.The 2D layered structure is formed by intermolecular forces of adjacent two binuclear molecules.The title complexes were characterized by elemental analysis,IR,UV,XRD,and so on.The thermal stability of the complexes was tested using TG-DTG technology.The luminescentproperty ofcomplex 1 had been explored.Besides,the bacteriostatic activities of the two complexes to candida albicans,Gram negative bacteria(escherichia coli)and Gram positive bacteria (staphylococcus aureus)were also studied.CCDC:1023982,1;1023981,2.
lanthanides;3,4-difluorobenzoic acid;thermal analysis;luminescence;bacteriostatic activity;crystal structure
O614.33+7;O614.343
A
1001-4861(2015)07-1425-08
10.11862/CJIC.2015.176
2015-03-13。收修改稿日期:2015-04-20。
國家自然科學基金(No.21073053、21473049),河北省自然科學基金(No.B2012205022)資助項目。
*通訊聯系人。E-mail:ningren9@163.com,jjzhang6@126.com;會員登記號:S06N6096M1306(任寧),S06N9443M1404(張建軍)。

