2,2′-聯吡啶-3,3′,6,6′-四羧酸過渡金屬配合物的合成、結構及性質
邴穎穎 吳振廷 胡 明
(內蒙古自治區稀土物理與化學重點實驗室,內蒙古大學化學化工學院,呼和浩特010021)
2,2′-聯吡啶-3,3′,6,6′-四羧酸過渡金屬配合物的合成、結構及性質
邴穎穎 吳振廷 胡 明*
(內蒙古自治區稀土物理與化學重點實驗室,內蒙古大學化學化工學院,呼和浩特010021)
以2,2′-聯吡啶-3,3′,6,6′-四羧酸(H4bptc)為配體,通過水熱合成的方法與過渡金屬鹽合成了3個配合物,其分子式分別為[Co2(H2bptc)2(H2O)4]·bpe·9H2O(1),[Ni2(H2bptc)2(H2O)4]·bpe·9H2O(2),[Ni2(H2bptc)2(H2O)4]·0.5bpp·7H2O(3)(bpe=1,2-二(4-吡啶基)乙烯,bpp=1,2-二(4-吡啶基)乙烷)。用紅外光譜,X-射線單晶衍射對化合物的晶體結構進行了表征,并對這3個配合物的熱穩定性進行了測試。化合物1~3為單核結構的配合物,它們均通過分子間氫鍵形成三維超分子結構。
2,2′-聯吡啶-3,3′,6,6′-四羧酸;配合物;合成;晶體結構
0 Introduction
Nowadays,Thedesignandsynthesisof coordination compounds based on multidentate N-donor and/or O-donor ligands have been acquired tremendous attentions,in which numbers of metal coordination compounds with pyridine multi-carboxylic acids ligands have recently been investigated[1-2].The assembly of transition metal ions and pyridine multicarboxylic acids can be used as the construction blocks to give rise to supramolecular architectures with aesthetic structures and unusual properties[3-4]. The1D,2D,3Dandpolynuclearcoordination compounds have been formed owing to the diverse coordinationmodesorduetotheformationof hydrogen bonds and π-π stacking interactions[5-7].Inaddition,hydrogen bonds,π-π and C-H…π interactions are especially considerable in supramolecular construction of coordination compounds,which is important not only from the viewpoint of coordination chemistry,but also for the development of intricate coordination networks[8-10].Furthermore,an effective and controllable route is to introduce auxiliary rigid multidentateN-donorligands.Recently,many supramolecular architectures constructed from small molecules were obtained by hydrogen bonds and π-π stacking interactions[11-14].
Herein,the multidentate 2,2′-bipyridine-3,3′,6,6′-tetracarboxylic acid(H4bptc)was chosen as the building block in favor of its versatile coordination modes in COO-and N donors to design the high dimensional structures[15-16].H4bptc has ten potential coordination sites in four carboxylate groups and two pyridyl N atoms,which can be rotated along the C-C bonds to meet the different coordination environments of metal ions.In this paper,we describe the synthesis, characterization,crystal structures and properties of [Co2(H2bptc)2(H2O)4]·bpe·9H2O(1),[Ni2(H2bptc)2(H2O)4] ·bpe·9H2O(2),[Ni2(H2bptc)2(H2O)4]2·bpp·11H2O(3).
1 Experimental
1.1 Materials and methods
The organic ligand(H4bptc)was synthesized according to the literature procedure[17]and all other reagents for the syntheses were of analytical grade and wereusedasreceivedfromcommercialsources without further purification.Elemental analyses(C,H andN)weredeterminedonPerkin-Elmer2400 analyzer.The IR spectra were recorded as KBr pellets on a Nicolet Avatar-360 spectrometer in the 4 000~400 cm-1region.Thermogravimetric analysis(TGA) was performed on a Perkin-Elmer TG-7 analyzer heated from 25 to 800℃under air atmosphere.
1.2 Syntheses of complexes
1.2.1 Syntheses of complexes 1 and 2
A mixture of MCl2·6H2O(M=Co(1),Ni(2),0.1 mmol),H4bptc(0.05 mmol),bpe(0.1 mmol)and 8 mL H2O was stirred for 30 min at room temperature and sealed in a 23 mL Teflon-lined stainless steel vessel. The mixture was then heated at 160℃for 72 h and followed by slow cooling to the room temperature at a rate of 5℃·h-1.The crystalline products were obtained and collected by filtration and then washed with H2O several times in yield of 35%for 1 and 68%for 2, respectively.Elemental analysis Calcd.for C40H48N6O29Co2(%):C 40.18,H 4.02,N 7.03;Found(%):C 40.19,H 4.05,N 7.01.Elemental analysis Calcd.for C40H48N6O29Ni2(%):C 40.19,H 4.02,N 7.03;Found (%):C 40.21,H 4.01,N 7.04.IR(KBr pellet,cm-1) for Complex 1:3 422(vs),2 512(m),1 988(m),1 710 (s),1 632(vs),1 580(s),1 451(m),1 430(m),1 360 (vs),1 296(s),1 252(s),1 180(s),1 116(m),1 073(s), 844(s),808(vs),780(m),544(m);for 2:3 425(vs),2 516(m),1 991(m),1 709(s),1 628(vs),1 579(s),1 454 (m),1 432(m),1 362(vs),1 295(s),1 253(s),1 179(s), 1 118(m),1 071(s),847(s),809(vs),783(m),547(m).
1.2.2 Synthesis of complex 3
Complex 3 was prepared by the same method as that of 1 except that the auxiliary ligand was changed from bpe to bpp.After being cooled to room temperature at a rate of 5℃·h-1,light green sheet-like crystalline products were obtained by filtration and then washed with H2O several times in 68%yield. Elemental analysis Calcd.for C68H74N10O51Ni4(%):C 39.19,H 3.55,N 6.72;Found(%):C 39.21,H 3.55,N 6.70.IR(KBr pellet,cm-1):3 437(vs),2 619(w),2 501 (w),1 702(s),1 630(s),1 584(vs),1 558(s),1 447(m), 1 420(s),1 368(vs),1 283(s),1 240(s),1 178(s),1 067 (s),864(w),811(vs),654(w),530(m).
1.3 X-ray crystallography
Crystallographic data for compounds 1~3 were collected on a Bruker ApexⅡSmart CCD diffractometer with graphite-monochromated Mo Kα radiation (λ=0.071 073 nm)using the ω-scan technique at room temperature.All the structures were solved by direct methods with SHELXS-97 and refined with the fullmatrix least-squares F2technique using the SHELXL-97 program[18].The position of non-hydrogen atoms were refined with anisotropic displacement parameters. The hydrogen atoms were set in calculated positions and refined as riding atoms with a common isotropic thermal parameter.The crystallographic data for 1~3are listed in Table 1.The selected bond lengths and angles of 1~3 are listed in Table S1.
CCDC:1054309,1;1054310,2;1054311,3.
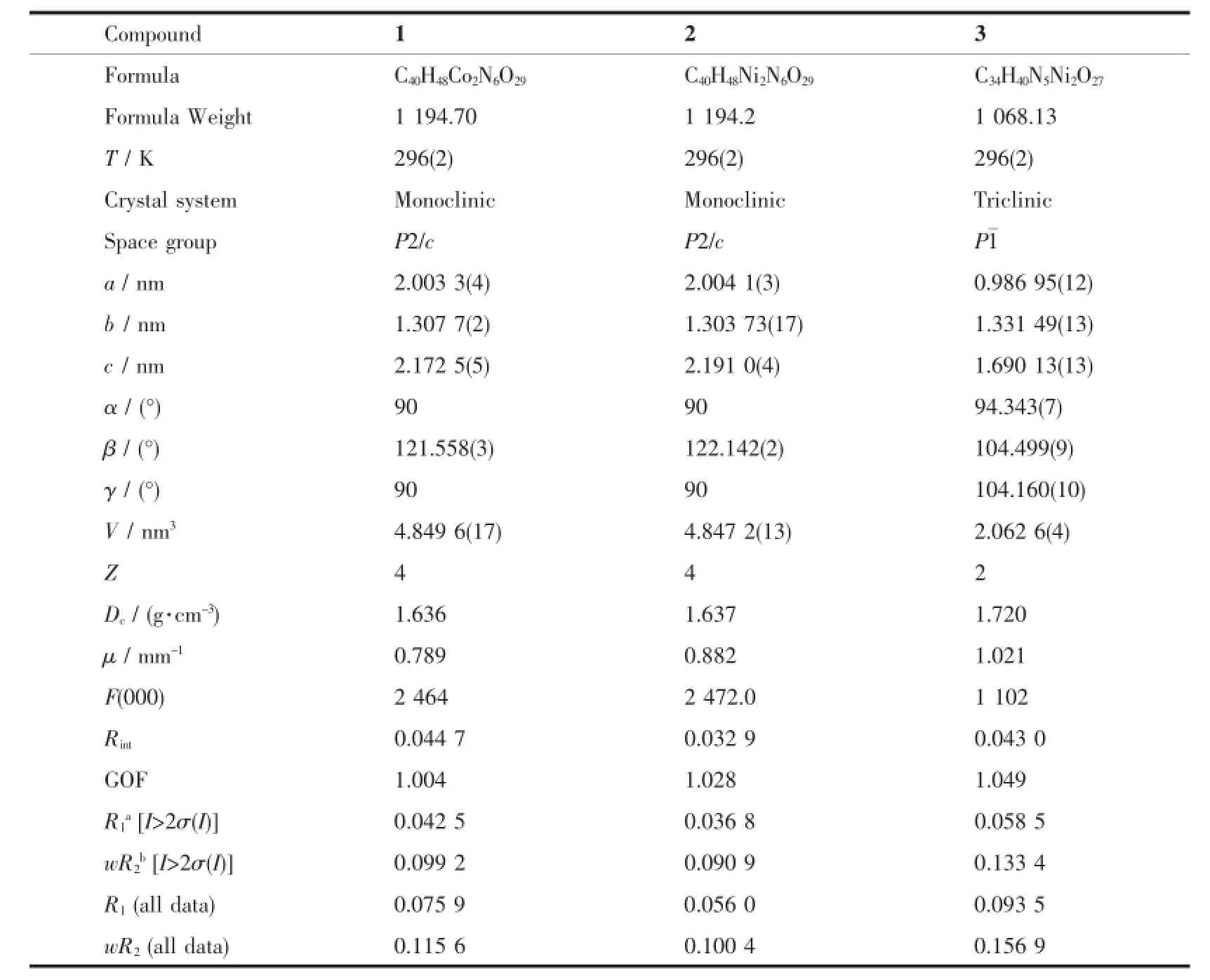
Table 1Crystal data for 1~3

Fig.1(a)Coordinated environment of Co2+ions;(b)3D framework architecture of 1
2 Results and discussion
2.1 Structural description of the compounds
2.1.1 Structural description of the compounds 1 and 2
Thesingle-crystal X-ray diffraction analysis reveals that the structures of complexes 1~2 are isostructural and crystallize in the monoclinic P2/c space group. They all contain two different dinuclear units in which the two metal ions have the same coordination mode but the distances between metal centers are different. The asymmetric unit contains two metal ions,two H2bptc2-ligands,four coordinated water molecules, nine free water molecules and one free bpe molecule (Fig.1a for 1 and Fig.2a for 2).Each metal ion is six coordinated with two N atoms,two O atoms of 6,6′-carboxylate group from two distinct H2bptc ligands and the other two O atoms from water.Here,each metal center adopts a distorted octahedral geometry with the MO4N2(M=Co,Ni)coordination environment. The M-O bond lengths are in the range of 0.204 3(11)~0.212 3(14)nm in Co(Ⅱ)and 0.202 7(19)~0.209 4(2) nm in Ni(Ⅱ),and the M-N bond lengths are in the range of 0.215 6(10)~0.220 8(13)nm in Co(Ⅱ)and 0.210 8(2)~0.216 3(2)nm in Ni(Ⅱ),which are similar to the reported[19].

Fig.2(a)Coordinated environment of ions;(b)3D framework architecture of 2
It is interesting to note that there are two dinuclear units differing in metal center distance, 0.459 3 nm between Co(1)ions and 0.456 6 nm between Co(2)ions.In the same way,the metal center distances in the complex 2 are 0.460 1 and 0.454 2 nm,respectively.From the structure of the ligand,we know C-C bond bridged the pyridyl rings can rotate freely,which contribute to the difference of twist angle between the pyridyl rings planes.In the dinuclear where Co(1) located,the twist angle between two pyridyl rings in one ligand is 74.228°and the twist angle in another dinuclear where Co(2)located is 66.586°.In a similar way,we measured the twist angle in complex 2,they are also different,75.009°and 67.218°,respectively. From the coordination mode of N atoms,we conclude that the longer the twist angle,the greater the metal centerdistance.Therearestrongintermolecular hydrogen-bondinginteractions,whichareformed mainly between 3,3′-COOH groups and coordinated water molecules,free water molecules and coordinated 6,6′-COO-groups.Finally,the two dinuclear units are extended to a 3D supramolecular architecture by hydrogen-bonding interactions in which free bpe fill in the cavity(Fig.1b for 1 and Fig.2b for 2).The selected bond distances and angles of hydrogen bonds for compounds 1 and 2 are listed in Table S2 and S3.
2.1.2Structural description of the compound 3
Single-crystalX-raystructuredetermination reveals that complex 3 crystallize in the triclinic space group P1.The asymmetric unit contains four metal ions,four H2bptc2-ligands,eight coordinated water molecules,seven free water molecules,and one free bpe(Fig.3a).Each Ni(Ⅱ)ion possesses a distorted octahedral geometry with N2O4coordination,in which the nitrogen and oxygen from two different H2bptc2-ligands are involved.The Ni-O bond lengths are in the range of 0.208 0(5)~0.202 8(5)nm and the Ni-N bond lengths are in the range of 0.211 4(5)~0.214 1(5) nm,consistent with literature reports[20].
Thedistancebetweenmetalcentersinthe dinuclear unit is 0.489 6 nm and they are in the same coordinationenvironmentexceptthetwistangle between the pyridyl rings planes of the two ligands (82.141°and 79.359°,respectively).The angles between the pyridyl rings and planes of uncoordinated carboxylate groups are 42.109°,1.133°,14.933°, 18.206°,and coordinated carboxylate groups are 7.003°,3.367°,7.038°,5.523°,respectively.There are strong intermolecular hydrogen-bonding interactions,which are formed mainly between 3,3′-COOH groups and coordinated water molecules,free water molecules and coordinated 6,6′-COO-groups.Finally,the dinuclear units are extended to a 3D supramolecular architecture by hydrogen-bonding interactions in which free bpp fill in the cavity(Fig.3b).The selected bond distances and angles of hydrogen bonds for compounds 3 are listed in Table S4.
2.2 Thermogravimetric Analyses.
To characterize the thermal stability of these complexes,thermogravimetric(TG)analyses were studied in detail(Fig.4).The TGA curves of complex 1 and 2 are very similar for their same structure.As the temperature rises,complexes 1 show the first weight loss of 17.2%,which can be assigned to the loss of free and coordinated water molecules(Calcd. 19.6%).The second weight loss of 64.2%between 275℃and 385℃stems from the losing of free bpe and the decomposition of the frameworks.For 2,the first weight loss of 16.6%is caused by losing of free and coordinated water molecules(Calcd.19.5%)and the second loss of 66.8%between 304℃and 420℃stems from the losing of free bpe and the decomposition of the frameworks.Complex 3 experiences a weight loss of 17.3%between 50~118℃,which corresponds to the liberation of free and coordinated water molecules(Calcd.18.5%).The second weight loss of 8.1%until 284℃(Calcd.8.8%)can be assigned to the losing of free bpp and the frameworks of complex 3 remains stable at approximately 400℃.

Fig.3(a)Coordinated environment of Ni2+ions;(b)3D framework architecture of 3

Fig.4TGA curves of 1~3
3 Conclusions
Insummary,threetransitionmetal-organic coordination compoundsbasedonamultidentate ligand(H4bptc)have been prepared by the hydrothermal method.Complexes 1 and 2 are isostructural and they all contain two different dinuclear units in which metal center distances are different.A comparison of compounds 1 and 2 suggested that the structural diversity could be tuned by altering the auxiliary ligand.The three coordination compounds 1~3 all exhibit three-dimensional supramolecular architectures by hydrogen-bonding interactions.Thermal analytical results show that the compounds 1~3 have higher thermal stability.
Supporting information is available at http://www.wjhxxb.cn
[1]Domasevitch K V,Solntsev P V,Krautscheid H,et al.Chem. Commun.,2012,48:5847-5849
[2]Zhao B,Cheng P,Liao D Z,et al.Inorg.Chem.,2005,44: 911-920
[3]Valencia L,Pérez-Lourido P,Bastida R,et al.Cryst.GrowthDes.,2008,8:2080-2082
[4]LI Xin-Wei(李欣瑋),WANG Peng(王鵬),ZHONG Jing-Wen (鐘靜文),et al.Chinese J.Inorg.Chem.(無機化學學報), 2014,30(6):1361-1366
[5]Urszula D,Florian P P,Radosaw S.Dalton Trans.,2009: 3348-3353
[6]Kochel A,Holynska M.Inorg.Chim.Acta,2013,408:193-198
[7](a)Zheng S L,Tong M L,Fu R W,et al.Inorg.Chem.,2001, 40:3562-3569
(b)YAN Li(閆麗),HAN Pan(韓盼),TIAN Wen-Chao(田文超),et al.Chinese J.Inorg.Chem.(無機化學學報),2014, 30(6):1255-1260
[8]Yu M H,Hu M,Wu Z T,et al.Inorg.Chim.Acta,2013,408: 84-90
[9]Jain P,Ramachandran V,Clark R J.J.Am.Chem.Soc., 2009,131:13625-13627
[10]He Y B,Guo Z Y,Chen B L,et al.Inorg.Chem.,2013,52: 11580-11584
[11]Swastik M,Monika M,Koushik D,et al.Cryst.Growth Des., 2007,7:1716-1721
[12]Li X,Cheng D Y,Lin J L,et al.Cryst.Growth Des.,2008,8: 2854-2861
[13]Houjou H,Ito M,Araki K,et al.Inorg.Chem.,2009,48: 10703-10707
[14]Byun Y,Yun H,Do Y,et al.Inorg.Chem.,1996,35:3981-3989
[15]Ji B M,Deng D S,He X,et al.Inorg.Chem.,2012,51: 2170-2177
[16](a)Hu M,Wu Z T,Yao J Y,et al.Inorg.Chem.Comm., 2013,36:31-34
(b)Bai H,Wu Z T,Hu M,et al.Inorg.Chim.Acta,2015, 427:112-117
[17]Dawid U,Pruchnik F P,Starosta R.Dalton Trans.,2009,38: 3348-3353
[18](a)Sheldrick G M.SHELXL-97,Program for the Solution of Crystal Structures,G?ttingen University,Germany,1997.
(b)Sheldrick G M.SHELXL-97,Program for Crystal Structures Refinement,G?ttingen University,Germany,1997.
[19](a)Chu Q,Su Z,Sun W Y,et al.Cryst.Growth Des.,2011, 11:3885-3894
(b)Huang F P,Liao D Z,Cheng P,et al.CrystEngComm, 2011,13:6538-6548
[20](a)Li X,Wu B L,Hou H W,et al.Inorg.Chem.,2010,49: 2600-2613
(b)Wang X L,Li J,Lin H Y,et al.Cryst.Growth Des.,2011, 11:3456-3462
Syntheses,Crystal Structures and Properties of Three Transition Metal Coordination CompoundsBased on 2,2′-Bipyridine-3,3′,6,6′-tetracarboxylic Acid
BING Ying-YingWU Zhen-TingHU Ming*
(Inner Mongolia Key Laboratory of Chemistry and Physics of Rare Earth Materials,School of Chemistry and Chemical Engineering,Inner Mongolia University,Hohhot 010021,China)
Three transition metal complexes,namely,[Co2(H2bptc)2(H2O)4]·bpe·9H2O(1),[Ni2(H2bptc)2(H2O)4]· bpe·9H2O(2),[Ni2(H2bptc)2(H2O)4]·0.5bpp·7H2O(3)(H4bptc=2,2′-bipyridine-3,3′,6,6′-tetracarboxylic acid,bpe= 1,2-di(4-pyridyl)ethylene,bpp=1,2-di(4-pyridyl)ethane)have been synthesized under hydrothermal conditions and structurally characterized by infrared spectra,thermogravimetric analyses and single crystal X-ray diffraction. Compounds 1~3 are the mononuclear units and extended further to generate the three-dimensional supramolecular architectures by strong hydrogen-bonding interactions,respectively.CCDC:1054309,1;1054310,2; 1054311,3.
2,2′-bipyridine-3,3′,6,6′-tetracarboxylic acid;coordination compound;synthesis;crystal structure
O614.81+2;O614.81+3
A
1001-4861(2015)10-2059-06
10.11862/CJIC.2015.264
2015-03-18。收修改稿日期:2015-07-09。
國家自然科學基金(No.21361017),內蒙古自然科學基金(No.2012MS0214),內蒙古高等學校科學技術研究重點研究(No.NJZZ12012)資助項目。
*通訊聯系人。E-mail:hm988@126.com

