Effects of sand burial on survival and growth of Artemisia halodendron and its physiological response
HaLin Zhao ,Hao Qu ,RuiLian Zhou ,JianYing Yun ,Jin Li
1.Cold and Arid Regions Environmental and Engineering Research Institute,Chinese Academy of Sciences,Lanzhou,Gansu 730000,China
2.Faculty of Life Sciences,Ludong University,Yantai,Shandong 264025,China
1 Introduction
In coastal and lake shoreline dunes,as well as in inland dunes and desertified areas,sand burial is a common environmental stress encountered by plants(Maun and Lapierre,1984;Sykes and Wilson,1990).Plants living in these areas may experience various degrees of sand burial,including partial or complete burial(Brown,1997;Maun,1998).Only species with special adaptations to sand burial can survive(Maun and Lapierre,1984;Zhao,2012).Thus,sand burial is considered an important factor controlling the distribution and composition of vegetation in sand dune communities(Shiet al.,2004;Li and Zhao,2006).The seedling stage is the most vulnerable period in a plant's life history,and thus the most vulnerable to the hazards of sand burial(Shietal.,2004;Maet al.,2007).The ability of seedlings to withstand sand burial is crucial for successful establishment and population maintenance by dune vegetation species(Maun,1998;Owenet al.,2004).
There is considerable literature on how sand burial impacts desert plants,which has focused on the effects of sand burial on seed germination,emergence,seedling survival and growth,biological yield,and biomass allocation(Maun and Lapierre,1984;Brown,1997;Li and Zhao,2006;Wanget al.,2011).A number of studies have demonstrated that sand burial may modify the physiology and morphology of plants and affect their survival and growth(Maun and Riach,1981;Maun,1998)because sand burial may change abiotic and biotic conditions such as photosynthetically active radiation,soil temperature,moisture,organic matter,rhizosphere oxygen content,and the activities of soil microorganism(D'Hertefeldt and van der Putten,1998;Zhou,2001;Zhaoet al.,2004).Some studies have indicated that the effects of sand burial on plants may change with the depth of burial(Sykes and Wilson,1990;Brown,1997).Low levels of burial tend to stimulate plant growth,but high levels of burial are inhibitory(Brown,1997;Maun,1998).Therefore,there is a threshold of sand burial that maximizes a plant's vigor.Below a certain threshold level of burial,the growth of plant species is stimulated by sand burial in terms of plant height and biomass(Zhanget al.,2002;Maet al.,2007).As the level of burial increases over the threshold,the response of plants might deteriorate in terms of growth and vigor until survival is impossible(Maun,1998;Yanget al.,2007).However,so far,the physiological adaption mechanisms of plants to sand burial are not clear because few studies have tested for physiological changes in plants affected by sand burial(Wanget al.,2012;Zhao,2012).
Artemisia halodendronis a very important sub-shrub species in the deserts of China and is widely distributed in the country's northern semi-arid regions(Horqin Sand Land,Maowusu Sand Land,Hulunbeir Sand Land,and Songnen Sand Land).A.halodendronis a preferred plant in local biological sand-fixing projects because it is strongly adapted to the dune environment(Zhaoet al.,2004;Zhao,2012).The objectives of this paper are:(1)to examine changes in survival rate,plant height,osmotic regulation substances,protective enzyme activities,and membrane permeability ofA.halodendronat different sand burial depths;and(2)to analyze the ability and mechanism ofA.halodendronas a psammophyte to withstand sand burial.
2 Material and methods
2.1 Study area
The study area is located in Naiman County(42°15'N,120°42'E,345 m a.s.l.),within the Horqin Sandy Land in the eastern part of Inner Mongolia,China.This region is characterized by a temperate continental semi-arid monsoon climate regime in the temperate zone.Mean annual precipitation is 364 mm,mean annual potential evaporation is 1,920 mm,and mean annual temperature is 6.3 °C.The annual frost-free period is approximately 141 days.The average annual wind speed is 3.4–4.5 m/s and mean wind speed in the wind erosion season(spring)is 5.0–6.0 m/s.The landscape in this region is characterized by dunes alternating with lowland areas.Most of the lowland area is reclaimed cropland and the dunes are used for pasture.The soils are identified as degraded sandy Chestnut soils according to the Chinese soil classification system,which are equivalent to Orthi-Sandic Entisols in the FAO-UNESCO system.The sandy soil consists mainly of coarse sand and silt.The natural vegetation consists mainly ofA.halodendron,
Caragana microphylla,Lespedeza davurica,Agriophyllum squarrosum,Setaria viridis,andCorispermum marocarpum(Zhaoet al.,2004).
2.2 Experimental design
A field experiment was conducted from April to August in 2010.The experiment was set up in a water balance field of the Naiman Desertification Research Station(NDRS)in the Chinese Ecosystem Research Network,which consisted of a number of 2m×2m×2m bottomless cement pools filled with sandy soil.Seeds ofA.halodendronwere collected in August 2009 from sand dune areas near NDRS.The seeds were sown in the pools in rows 20 cm apart in April 2010.After germination,the seedlings were thinned out to avoid death due to competition.One hundred seedlings having similar growth were left and marked in each cement pool.The seedlings were kept vertical after thinning,and the average height for the seedlings in mid-May,when they were buried in sand,was 8.0 ± 0.2 cm.There were 10 burial treatments:buried to 0%(CK,no burial),25%(A),50%(B),75%(C),100%(D),125%(E),150%(F),175%(G),200%(H),and 225%(I)of seedling height.Every treatment consisted of four replicates,and there were 40 cement pools in total.Unmarked seedlings that emerged after sand burial were removed.No additional water or fertilizer were added at any time during the experiment,in order to keep the soil condition as close to natural conditions as possible.
2.3 Data collection and analysis
At 10 days of sand burial,leaves from 10 live seedlings were sampled randomly in each treatment to measure enzyme activities(SOD,POD,and CAT),lipid peroxidation,and membrane permeability(measured in terms of MDA content and REL(relative electric conductivity)),as well as content of osmotic substances(soluble sugar and free proline)(Zhang and Zhai,2003).The survival rate,plant height,and aboveground biomass were measured in August,2010.
All data were analyzed using the SPSS program for Windows v.11.5.Multiple-comparison and one-way analysis of variance(ANOVA)procedures were used to compare differences among the treatments.Least significant difference(LSD)tests were performed to determine pairwise significant differences among the treatment means atP<0.05.Pearson correlation coefficients were used to evaluate relationships between the corresponding variables.
3 Results
3.1 Changes in survival rate and seedling height
The survival rates of the seedlings fluctuated and decreased with increasing sand burial depth(Figure 1),decreasing by 48.9%–64.0% in treatments A to C compared with CK,but the differences between them were not significant(P>0.05).The survival rate diminished sharply when the burial depth exceeded 100%of seedling height,decreasing 90.3%–96.9% in treatments E to H compared with CK.Seedling height decreased gradually with increasing sand burial depth,and was significantly lower in all the sand burial treatments compared to CK(P <0.05)except for treatment A.
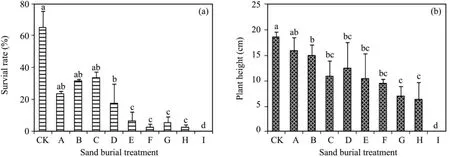
Figure 1 Changes in survival rate(a)and height(b)of A.halodendron seedlings affected by sand burial.Bars represent means ± SD.Values with the same letters are not significantly different at P<0.05
3.2 Changes in RWC,MDA,membrane permeability
Leaf relative water content(RWC)fluctuated and decreased with increasing burial depth,and was lower in all the sand burial treatments than in CK except for treatment F(Figure 2),but the differences between them were not significant(P>0.05)except for treatment E.In comparison with CK,MDA content was significant lower in all the sand burial treatments(P<0.05)except for treatments F and A,where it was higher.Membrane permeability first decreased and then increased with increasing burial depth,being lower in treatments B and C and significantly higher in treatments D to H compared to CK(P <0.05).
3.3 Changes in activities of protective enzymes
The activities of SOD,POD,and CAT all fluctuated violently with increasing sand burial depth(Figure 3).In comparison with CK,SOD activity was lower in all the sand burial treatments,except that it was significantly higher in treatments D and E(P <0.05).POD activity was higher in all the sand burial treatments except for treatment D.CAT activity was significantly higher in treatments C and D and significantly lower in treatment E compared with CK(P <0.05).Although CAT activity was lower in the other sand burial treatments than in CK,the differences were not significant(P >0.05).
3.4 Changes in free proline and soluble sugar
Free proline content fluctuated and increased with increasing depth of sand burial(Figure 4).The free proline content was significantly higher in treatment C and in treatments F to H compared to CK,while the difference was not significantly different from CK in the other treatments(P>0.05).The soluble sugar content also fluctuated with increasing sand burial depth.The differences were not significant in most treatments compared to CK(P>0.05),except for significantly lower levels in treatments B,C,and F.
3.5 Correlation analysis for biological characteristics
The results from the correlation analysis showed that survival rate and seedling height had a positive correlation with RWC and a negative correlation with MDA content and membrane permeability(Table 1),but the correlation only reached a significant level between survival rate,height,and membrane permeability(P<0.05).MDA content had a negative correlation with free proline,soluble sugar,and CAT activity,and a positive correlation with SOD and POD activities,but none of the correlations reached a level of significance(P>0.05).Membrane permeability had a positive correlation with SOD and POD and a negative correlation with CAT,but the correlations again did not reach the level of significance(P>0.05).
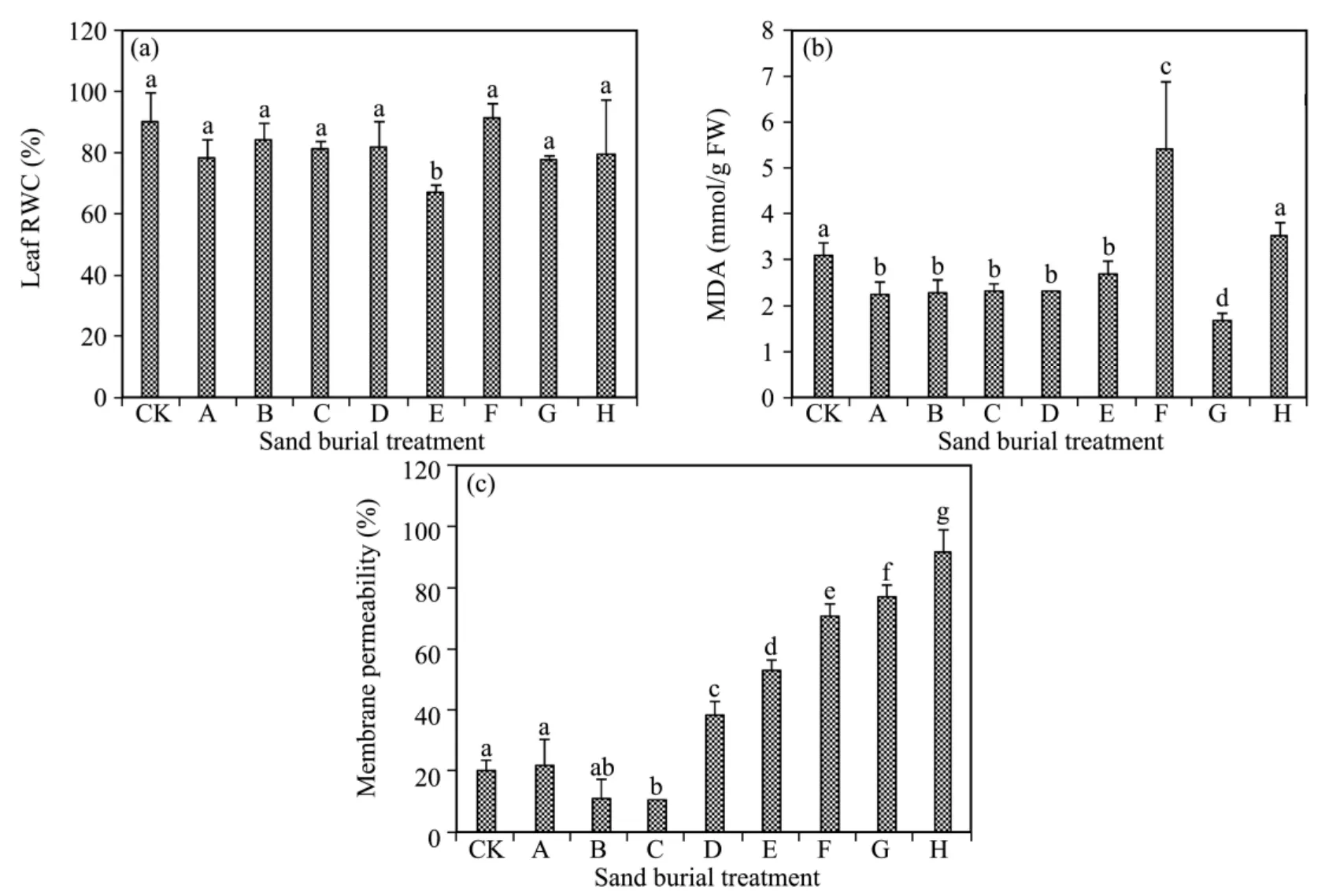
Figure 2 Changes in RWC(a),MDA(b),and membrane permeability(c)of A.halodendron seedlings affected by sand burial.Bars represent means ± SD.Values with the same letters are not significantly different at P<0.05
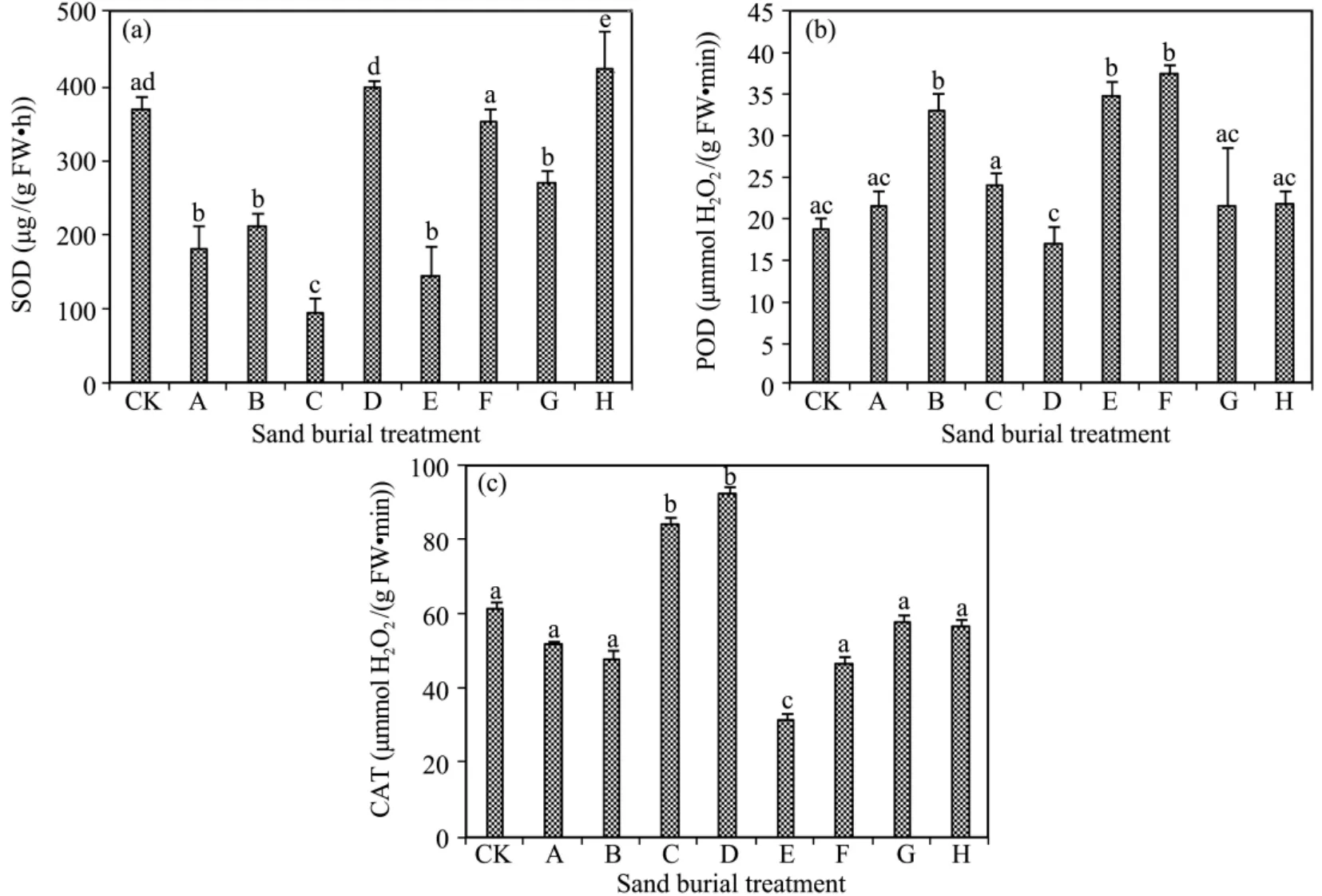
Figure 3 Changes in activity of SOD(a),POD(b),and CAT(c)in A.halodendron seedlings affected by sand burial.Bars represent means ± SD.Values with the same letters are not significantly different at P<0.05
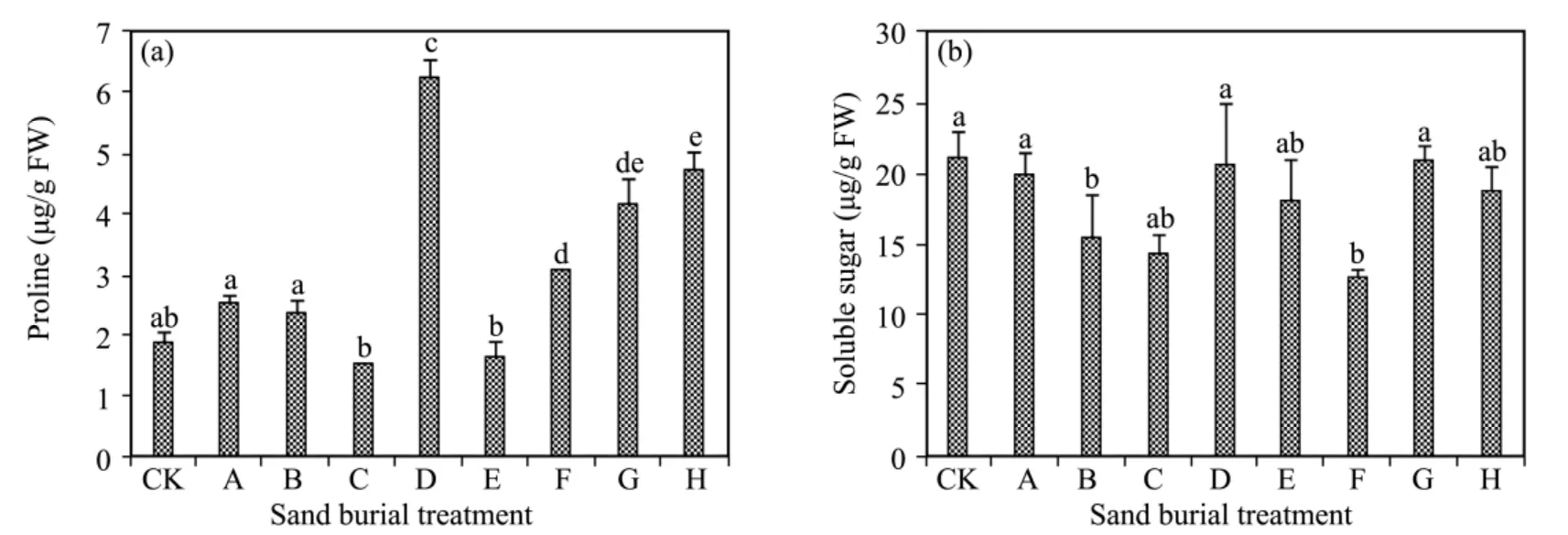
Figure 4 Changes in proline(a)and soluble sugar(b)affected by sand burial.Bars represent means ± SD.Values with the same letters are not significantly different at P<0.05
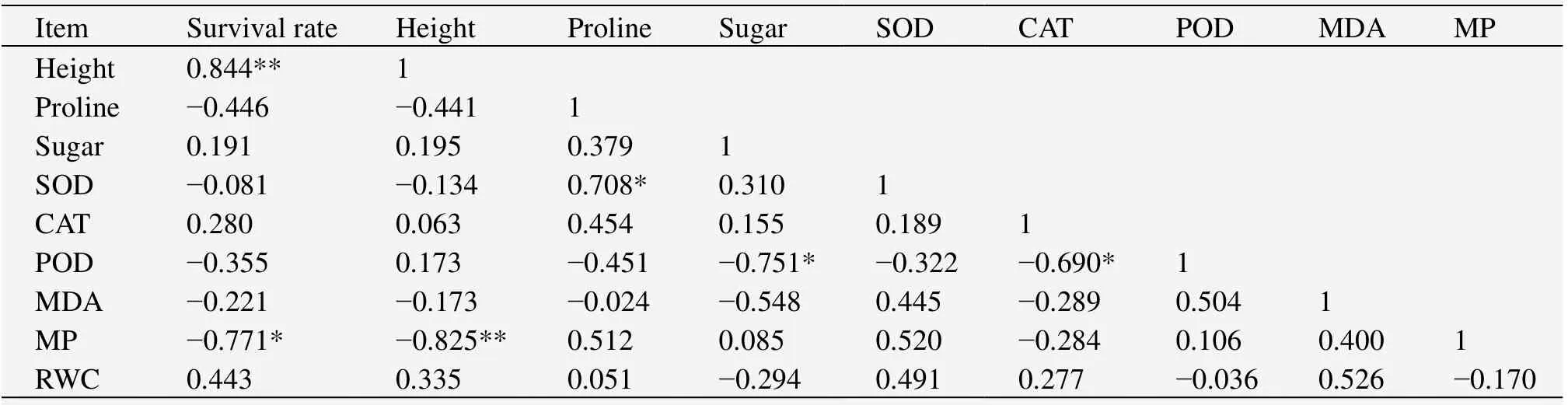
Table 1 Correlation analysis among plant growth properties and physiological indices
4 Discussion and conclusion
4.1 Effects of sand burial on survival and growth of A.halodendron
In desert areas,sand burial is one of the most important factors affecting plant survival and growth,but the abilities of different plants to withstand sand burial have obvious differences(Brown,1997;Li and Zhao,2006).For example,in two studiesSophora moocrofianaandBromus inermisseedlings all died when the sand burial depth was 100% of seedling height(Yanget al.,2007;Wanget al.,2011),whileHedysarum leaveandCaragana microphyllaseedlings all died only when the burial depth was 133% of the seedlings' height(Zhanget al.,2002;Zhaoet al.,2010).In comparison with these plants,A.halodendronseedlings had a stronger ability to withstand sand burial and some seedlings still survived until the burial depth was 225% of seedling height.
The stronger ability ofA.halodendronto withstand sand burial can be attributed to its unusual properties.A.halodendron,as a psammophytic sub-shrub,is a pioneer species in sandy vegetation succession and is naturally distributed in semi-moving sand lands and the lower parts of moving dunes,and has developed characteristics of drought tolerance,barren resistance,and sand resistance in its long-term natural selection and adaptation process to the aeolian environment(Li,1994;Zhaoet al.,2004).However,its responses in terms of seedling survival and growth after sand burial showed significant differences.In comparison with CK,the survival rate decreased by 90.3%–96.9% once the buried depth exceeded 100% of the seedling height,while seedling height only decreased by 44.7%–68.4%.These results suggest that even with the psammophyte,whose survival and growth could be inhibited by severe sand burial,sand burial had a greater effect on survival than height of growth(Shiet al.,2004;Zhaoet al.,2004;Zhao,2012).
Previous studies have shown that with increased depths of sand burial,soil moisture,and soil harnessing increased and soil temperature in the rhizosphere decreased(Zhanget al.,2002;Yanget al.,2007).The present study showed that,unlike drought stress and salt stress,changes in leaf RWC inA.halodendronwere not significant,suggesting that the major mechanism inhibiting the survival and growth of the seedlings after complete sand burial was not water stress(Maun,1998;Yanget al.,2007;Wanget al.,2012).According to the analysis,when subjected to complete burial in sand,increased mortality and growth inhibition of the seedlings resulted mainly from two reasons.First,a deep burial depth may prevent seedlings from re-emerging above the sand because pre-emergence mortality results from a cessation of seedling growth before it can reach the soil surface(Li and Zhao,2006;Wanget al.,2012).Second,even after emerging from complete sand burial,decreased leaf area and reduced photosynthesis may render the substance and energy obtained by the seedlings insufficient to meet their growth needs(Zhanget al.,2002;Yanget al.,2007).
4.2 Physiological response of A.halodendron seedlings to sand burial
Previous studies suggested that when subjected to drought,cold,or high temperature stress,the balance between generating and clearing reactive oxygen was destroyed in the cells,causing overproduction of reactive oxygen and lipid peroxidation,and resulting in MDA accumulation and increased membrane permeability(Monket al.,1989;Zhou and Wang,1999;Zhouet al.,2004).This enhancement in membrane lipid peroxidation was one of the most important mechanisms causing cell membrane oxidative damage and cell death(Baiet al.,2006;Wanget al.,2012).The present results showed that the MDA content was significantly lower in the partial sand burial treatments compared to CK,but membrane permeability did not increase significantly.After being subjected to complete sand burial,MDA content was still lower in most the treatments compared to CK,except for being higher in treatment F,while membrane permeability increased significantly with increased depth of burial.These results suggest that partial burial with sand did not cause lipid peroxidation and cell membrane damage(Zhou,2001;Hernández and Almansa,2002;Chenet al.,2011).Although complete burial with sand did not result in an overt accumulation of MDA,membrane permeability increased significantly and cell membrane damage was exacerbated with increasing burial depth(Shanahanet al.,1990;Zhou and Wang,1999;Wanget al.,2012).
The correlation analysis showed that the survival rate and height growth of the seedlings had a significant negative correlation with changes in membrane permeability,but no significant correlation with changes in MDA content.This suggests that when subjected to sand burial stress,increased mortality and growth inhibition of the seedlings should be attributed to membrane damage instead of to membrane peroxidation(Shanahanet al.,1990;Zhou,2001).Usually,when subjected to drought,salt,and higher temperature stress,membrane permeability and MDA content always increase synchronously;the increased mortality and growth inhibition are seen have a significant correlation with both membrane permeability and MDA content(Hernández and Almansa,2002;Baiet al.,2006;Chenet al.,2011).
The present results were obviously different from past study results on drought,salt,and higher temperature stress.One reason may be that drought,salt,and higher temperature stress normally resulted in water stress,which primarily produced an imbalance between generating and clearing reactive oxygen,with a resulting overproduction of reactive oxygen and lipid peroxidation(Shanahanet al.,1990;Chenet al.,2011).In contrast,plant RWC did not significantly increase when subjected to sand burial stress and sand burial did not produce water stress in the seedlings(Wanget al.,2012;Zhao,2012).According to the analysis,when a plant is subjected to complete sand burial,a major mechanism causing decreased MDA content is that the darkness,hypoxia,and low temperature inhibit production of oxygen free radicals and membrane lipid peroxidation(Wanget al.,2012;Zhao,2012).As to why membrane permeability increases in the absence of membrane lipid peroxidation or MDA accumulation,that feature has yet to be studied further.
Many studies have indicated that plants evolve enzymatic coping mechanisms under conditions of long-term environmental stress.SOD,POD,and CAT,as well as high levels of other anti-oxidative enzymes,may contribute to withstanding environment stress by increasing their capacity to mount a better protection against oxidative damage.Thus,anti-oxidative enzymes are usually considered to be critical factors related to stress tolerance(Zhou and Zhao,2004;Liet al.,2005;Liet al.,2006;Gaoet al.,2008;Wanget al.,2012).The present results showed the activities of three anti-oxidant enzymes all fluctuated.In general,POD activity increased when the activities of the SOD and CAT decreased,and SOD and CAT activities increased when POD activity decreased.Compared with CK,SOD activity was significantly lower in all sand burial treatments except for treatments D and H,POD activity was higher in all sand burial treatments except treatment D,and CAT activity showed no significant changes with any of the sand burial treatments except for being elevated in treatments C and D.
Correlation analysis showed that POD activity had a negative correlation with SOD and CAT activities,with the correlation between POD and CAT reaching a significant level.MDA content had a positive correlation with SOD and POD and a negative correlation with CAT.These results suggest that when subjected to sand burial stress,although there was a complementary interplay among the three enzymes,POD played a central role and SOD and CAT played supporting roles in scavenging active oxygen and minimizing cell membrane damage(Baiet al.,2006;Wanget al.,2012).This result was not in agreement with other study results on drought,salt,and higher temperature stress.When subjected to severe water stress,the activities of SOD,POD,and CAT usually all decrease significantly.However,one kind of enzyme activity is always enhanced when these plants were subjected to severe sand burial stress,which may be an important mechanism by whichA.halodendron,as a psammophyte,inhibits lipid peroxidation under conditions of sand burial stress.
The present results show that when subjected to partial burial with sand,free proline content showed no significant changes and the soluble sugar content decreased significantly compared to CK.After sand burial exceeded the seedling height,the free proline content increased significantly;the difference from CK did not reach a significant level,although the soluble sugar content increased.Osmotic adjustment is an important function in stress resistance(Demiral and Türkan,2005;Trovatoet al.,2008).Free proline and soluble sugar accumulation in leaves under stress conditions is of the utmost importance for plant adaptation during stress(Aliaet al.,2001;Wanget al.,2012).The results suggest that when subjected to the severe stress of sand burial,proline plays a major role and soluble sugar fails to play an effective role in the osmotic adjustment process(Demiral and Türkan,2005;Baiet al.,2006).
The authors are grateful to the anonymous reviewers for their critical review and comments on drafts of this manuscript.This research was funded by the Chinese National Fund Projects(Nos.31270752 and 30972422)and by a Chinese National Support Project of Science and Technology(No.2011BAC07B02-06).
Alia,Mohanty P,Matysik JM,2001.Effect of proline on the production of singlet oxygen.Amino Acids,21(2):195–200.DOI:10.1007/s007260170026.
Bai LP,Sui FG,Ge TD,et al.,2006.Effect of soil drought stress on leaf water status,membrane permeability and enzymatic antioxidant system of maize.Pedosphere,16(3):326–332.DOI:10.1016/S1002-0160(06)60059-3.
Brown JF,1997.Effects of experimental burial on survival,growth,and resource allocation of three species of dune plants.Journal of Ecology,85(2):151–158.
Chen LN,Yin HX,Xu J,et al.,2011.Enhanced antioxidative responses of a salt-resistant wheat cultivar facilitate its adaptation to salt stress.African Journal of Biotechnology,74(10):16887–16896.DOI:10.5897/AJB11.1755.
D'Hertefeldt B,van der Putten WH,1998.Physiological integration of the clonal plantCarex arenariaand its response to soil-borne pathogens.Oikos,81:229–237.DOI:10.2307/ 3547044.
Demiral T,Türkan I,2005.Comparative lipid peroxidation,antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance.Environmental and Experimental Botany,53(3):247–257.DOI:10.1016/j.envexpbot.2004.03.017.
Gao JM,Xiao Q,Ding LP,et al.,2008.Differential responses of lipid peroxidation and antioxidants inAlternanthera philoxeroidesandOryza sativasubjected to drought stress.Plant Growth Regulation,56(1):89–95.DOI:10.1007/s10 725-008-9291-6.
Hernández JA,Almansa MS,2002.Short-term effects of salt stress on antioxidant systems and leaf water relations of pea leaves.Physiologia Plantarum,115(2):251–257.
Li J,1994.Approach to some basic problems of reproduction ofArtemisia halodendron.Journal of Desert Research,14(3):60–65.
Li J,Jiang ZR,Wang JH,et al.,2006.Change of protective enzyme activity of the four species in the genus Atriplex under water stress.Journal of Gansu Agricultural University,41(5):76–80.
Li QY,Zhao WZ,2006.Seedling emergence and growth responses of five desert species to sand burial depth.Acta Ecologica Sinica,26(6):1802–1808.
Li X,Yan XF,Yu T,2005.Effects of water stress on protective enzyme activities and lipid peroxidation inPhellodendron amurenseseedlings.Chinese Journal of Applied Ecology,16(12):2353–2356.
Ma HY,Liang ZW,Yan C,et al.,2007.Effects of sand-burial depth onLeymus chinensisseedlings emergence and growth.Chinese Journal of Ecology,26(12):2003–2007.
Maun MA,1998.Adaptations of plants to burial in coastal sand dunes.Canadian Journal of Botany,76(5):713–738.DOI:10.1139/b98-058.
Maun MA,Lapierre J,1984.The effects of burial by sand onAmmophila breviligulata.Journal of Ecology,72(3):827–839.
Maun MA,Riach S,1981.Morphology of caryopses,seedlings and seedling emergence of the grassCalamovilfa longifoliafrom various depths in sand.Oecologia,49(1):137–142.DOI:10.1007/BF00376911.
Monk SL,Fagerstedt KV,Crawford RMM,1989.Oxygen toxicity and superoxide dismutase as an antioxidant in physiological stress.Physiologia Plantarum,76(3):456–459.DOI:10.1111/j.1399-3054.1989.tb06219.x.
Owen NW,Martin K,Dale MP,2004.Plant species and community responses to sand burial on the machair of the Outer Hebrides,Scotland.Journal of Vegetation Science,15(5):669–678.DOI:10.1111/j.1654-1103.2004.tb02309.x.
Shanahan JF,Edwards IB,Quick JS,et al.,1990.Membrane thermostability and heat tolerance of spring wheat.Crop Science,30:247–251.DOI:10.2135/cropsci1990.00111 83X003000020001x.
Shi L,Zhang ZJ,Zhang CY,et al.,2004.Effects of sand burial on survival,growth,gas exchange and biomass allocation ofUlmus pumilaseedlings in the Hunshandak Sandland,China.Annals of Botany,94(4):553–560.DOI:http://dx.doi.org/10.1093/aob/mch174.
Sykes MT,Wilson JB,1990.An experimental investigation into the response of New Zealand sand dune species to different depths of burial by sand.Acta Botanica Neerlandica,39(2):171–181.
Trovato M,Mattioli R,Costantino P,2008.Multiple roles of proline in plant stress tolerance and development.Rendiconti Lincei,19(4):325–346.DOI:10.1007/s12210-008-0022-8.
Wang J,Zhou RL,Zhao HL,et al.,2012.Growth and physiological adaptation ofMesserschmidia sibiricato sand burial on coastal sandy.Acta Ecologica Sinica,32(14):4291–4299.
Wang WJ,He DH,Tang XQ,et al.,2011.Effects of different temperature and sand burial depths on seed germination and seedling growth ofSophora moorcroftiana.Journal of Desert Research,31(6):1437–1442.
Yang HL,Chao ZP,Dong M,et al.,2007.Effects of sand burying on caryopsis germination and seedling growth ofBromus inermisLeyss.Chinese Journal of Applied Ecology,18(11):2438–2443.
Zhang CY,Yu FH,Dong M,2002.Effects of sand burial on the survival,growth,and biomass allocation in semi-shrubHedysarumleaf seedlings.Acta Botanica Sinica,44(3):337–343.
Zhang ZL,Zhai WJ,2003.Experimental Guide of Plant Physiology.Beijing:Higher Education Press.
Zhao HL,2012.Desert Ecology.Beijing:Science Press.
Zhao HL,He YH,Yue GY,2010.Effects of wind blown and sand burial on the seedling growth and photosynthetic and transpiration rates of desert plants.Chinese Journal of Ecology,29(3):413–419.
Zhao HL,Zhao XY,Zhang TH,2004.Plants Adaption Strategies and Vegetation Stability in the Desertification Process.Beijing:China Ocean Press.
Zhou RL,2001.The physiological mechanism of plant succession in Kerqin Sandy Land.Arid Zone Research,18(3):13–19.
Zhou RL,Wang HO,1999.Correlation between resistance to dehydration and lipid peroxidation of desert plants under atmosphere dehydration and high temperature stresses.Journal of Desert Research,19(1):60–64.
Zhou RL,Zhao HL,2004.Seasonal pattern of antioxidant enzyme system in the roots of perennial forage grsses grown in alpine habitat,related to freezing tolerance.Physiologia Plantarum,121(3):399–408.DOI:10.1111/j.0031-9317.2004.00313.x
 Sciences in Cold and Arid Regions2015年1期
Sciences in Cold and Arid Regions2015年1期
- Sciences in Cold and Arid Regions的其它文章
- Seasonal change mediates the shift between resource and pollen limitation in Hedysarum scoparium(Fabaceae)
- Characteristics of high arsenic groundwater in Hetao Basin,Inner Mongolia,northern China
- The response of Caragana microphylla seedlings to water table changes in Horqin Sandy Land,China
- Effects of sand burial on growth and antioxidant enzyme activities of wheat(Triticum aestivum L.)in northern China
- Photosynthesis of Digitaria ciliarisduring repeated soil drought and rewatering
- Screening of cellulose decomposing fungi in sandy dune soil of Horqin Sandy Land
